Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chuanping Zhang | -- | 1626 | 2022-10-27 10:42:04 | | | |
| 2 | Camila Xu | Meta information modification | 1626 | 2022-10-28 10:05:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, L.; Zhang, C.; Zhang, Z.; Wang, H.; Wang, S. Tire Rubber and Its Degradation Behavior. Encyclopedia. Available online: https://encyclopedia.pub/entry/31693 (accessed on 02 March 2026).
Zhang L, Zhang C, Zhang Z, Wang H, Wang S. Tire Rubber and Its Degradation Behavior. Encyclopedia. Available at: https://encyclopedia.pub/entry/31693. Accessed March 02, 2026.
Zhang, Lu, Chuanping Zhang, Zhen Zhang, Hanbing Wang, Shifeng Wang. "Tire Rubber and Its Degradation Behavior" Encyclopedia, https://encyclopedia.pub/entry/31693 (accessed March 02, 2026).
Zhang, L., Zhang, C., Zhang, Z., Wang, H., & Wang, S. (2022, October 28). Tire Rubber and Its Degradation Behavior. In Encyclopedia. https://encyclopedia.pub/entry/31693
Zhang, Lu, et al. "Tire Rubber and Its Degradation Behavior." Encyclopedia. Web. 28 October, 2022.
Copy Citation
The use of ground tire rubber (GTR) for modifying asphalt is very promising and is a sustainable development strategy. The addition of GTR to asphalt shows many improvements in the physical, chemical and mechanical properties of the rubber asphalt binder, such as enhanced stiffness, increased skid resistance, extended service life, mitigated fatigue cracking and so on.
devulcanized rubber
rubberized asphalt
colloidal structure
1. Introduction
One of the key challenges in waste management, especially in the disposal of end-of-life tires, has always been the task of monitoring in the 21st century [1]. The most widespread disposal methods for end-of-life tires are landfills and incineration [2], causing much environmental pollution due to the nonbiodegradability, flammability and chemical characteristic of scrap tires, such as the emission of toxic gases and other harmful substances [3][4]. On the other hand, scrap tires are mainly composed of vulcanized rubber, reinforcing filler and a variety of materials, which is not only an environmental pollutant but also a great economic loss. Therefore, recycling and reuse of these waste materials are of great significance considering the world is facing a lack of resources and a shortage of energy.
The use of ground tire rubber (GTR) for modifying asphalt is very promising and is a sustainable development strategy [5]. The addition of GTR to asphalt shows many improvements in the physical, chemical and mechanical properties of the rubber asphalt binder, such as enhanced stiffness, increased skid resistance, extended service life, mitigated fatigue cracking and so on [6][7][8]. However, the multiple cross-linking network structure of GTR leads to not only high energy consumption and pollution emissions but also results in the poor compatibility and bad stability of rubber asphalt. The above problems limit the efficient utilization and cause the unscientific application of rubber asphalt. In view of this, devulcanization or degradation of the cross-linking network is urgent for reutilization with high efficiency.
In order to obtain stable rubber asphalt with good performance, the compatibility between asphalt and rubber should be improved. Tire rubber is usually composed of cross-linked rubber, carbon black (CB), silica and so on [9]. Devulcanization or degradation destroys the cross-linked network and rubber body carbon chains to be short chains and other separated small substances such as CB. Most previous studies focused on the devulcanization of rubber and the reduced molecular weight of sol fraction (Table 1); less is reported about the decreased size of CB (from several micrometers to micro-nanometers, Figure 1A–C) with the increase in degradation degree [10][11]. These degraded substances are well dispersed in the asphalt, as shown in Figure 1D [12]. Therefore, deeply degraded rubber provides a new strategy for preparing rubberized asphalt from a different perspective.
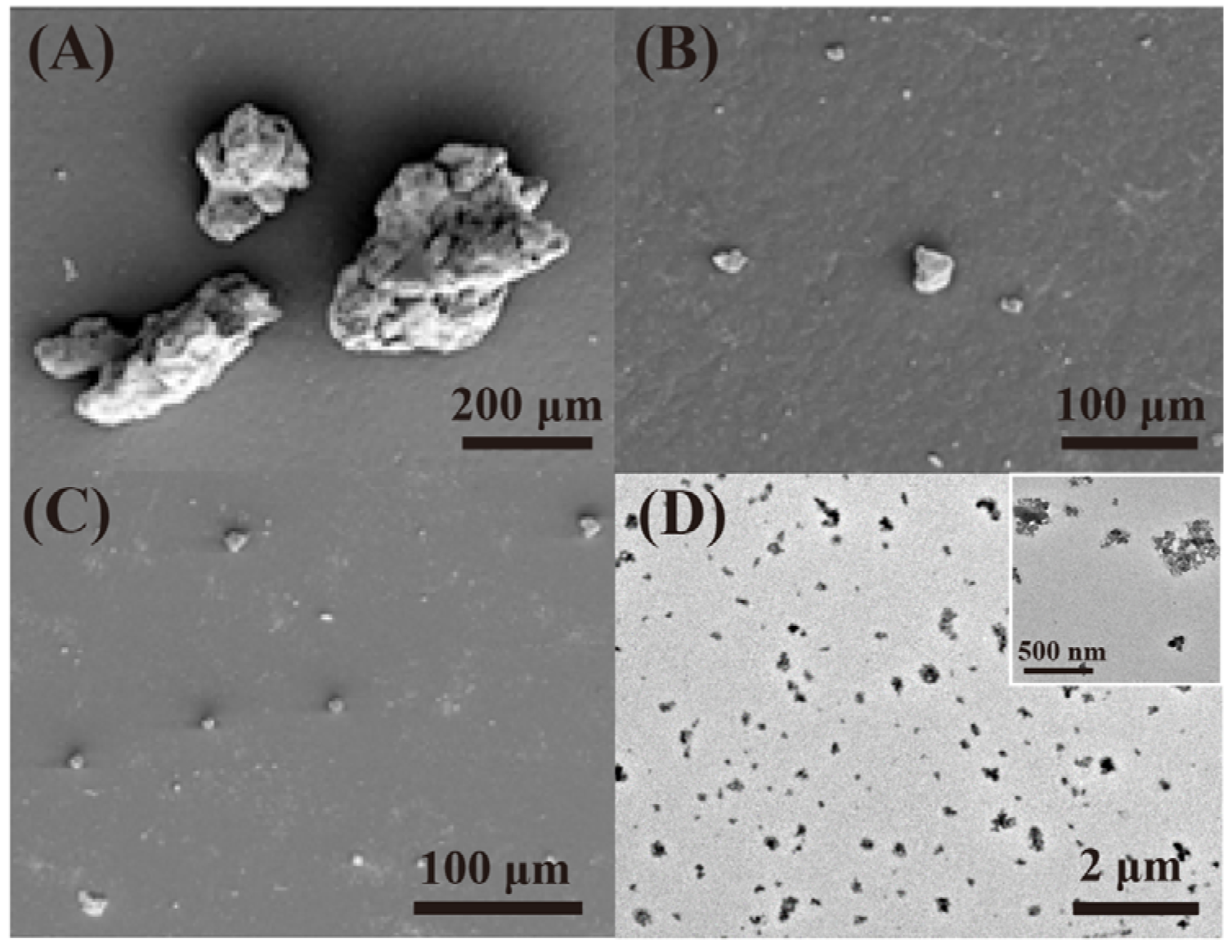
Table 1. Molecular weight and its distribution of sol fraction [11].
| Temperature/°C | M¯¯¯¯n(104 g/mol) | M¯¯¯¯w(104 g/mol) | M¯¯¯¯w/M¯¯¯¯n |
|---|---|---|---|
| 220 | 1.19 | 6.12 | 5.12 |
| 240 | 1.25 | 6.01 | 4.79 |
| 260 | 1.42 | 6.89 | 4.84 |
| 280 | 1.34 | 6.29 | 4.70 |
| 300 | 0.41 | 2.28 | 5.57 |
The DR mainly consisted of micro-nano CB, low molecular weight of sol fraction and inorganic substances, denoted as micro-nano rubber. The DR showed much better dispersibility and reinforcement compared with GTR, thus usually used for the modification of asphalt with high content. Therefore, the DR was more and more used as the potential alternative for asphalt in pavement, which significantly improved the anti-cracking and anti-aging performance [13][14][15]. Although DRMA demonstrates much improved properties compared with both base asphalt or GTR modified asphalt, the standardized applications of rubber asphalt have always been a challenge for the lack of a deep understanding of the mixing mechanism, especially on a colloidal scale.
2. Tire Rubber and Its Degradation Behavior
2.1. Chemical Composition of Tire Rubber
Tire rubber has a crucial role in many fields such as industry, agriculture and national defense. It is reported that the global tire output is about 1.5 billion each year and that of waste tires is more than 17 million tons each year. With the increased demand for tires in developing countries, the output of waste tires grows more rapidly. Due to the cross-linking and reinforcing network structure of waste tires, they are difficult to degrade under natural conditions, which results in a large accumulation. Serious “black pollution” formed by wasted rubber severely threatens the health and life of human beings.
Tires mainly consist of vulcanized rubber and a series of additives [16]. The composition of a typical tire is shown in Figure 2 (by weight). It is mainly composed of natural rubber (NR) which comprises about 40% of the weight of truck tire rubber, making the tire full of elasticity and durability even under heavy loads [17][18]. The proportion of NR has increased nowadays, possibly due to the expansion in the use of radial tires and heavy-duty tires. It should be pointed out that the properties of NR can largely affect the workability of materials. For example, the molecular weight and gel content of NR may affect compound viscosity and filler dispersion in rubber mixing. In addition, the tendency of NR to crystallize upon stretching, even before cross-linking, has contributed to the strength of uncured rubber compounds.
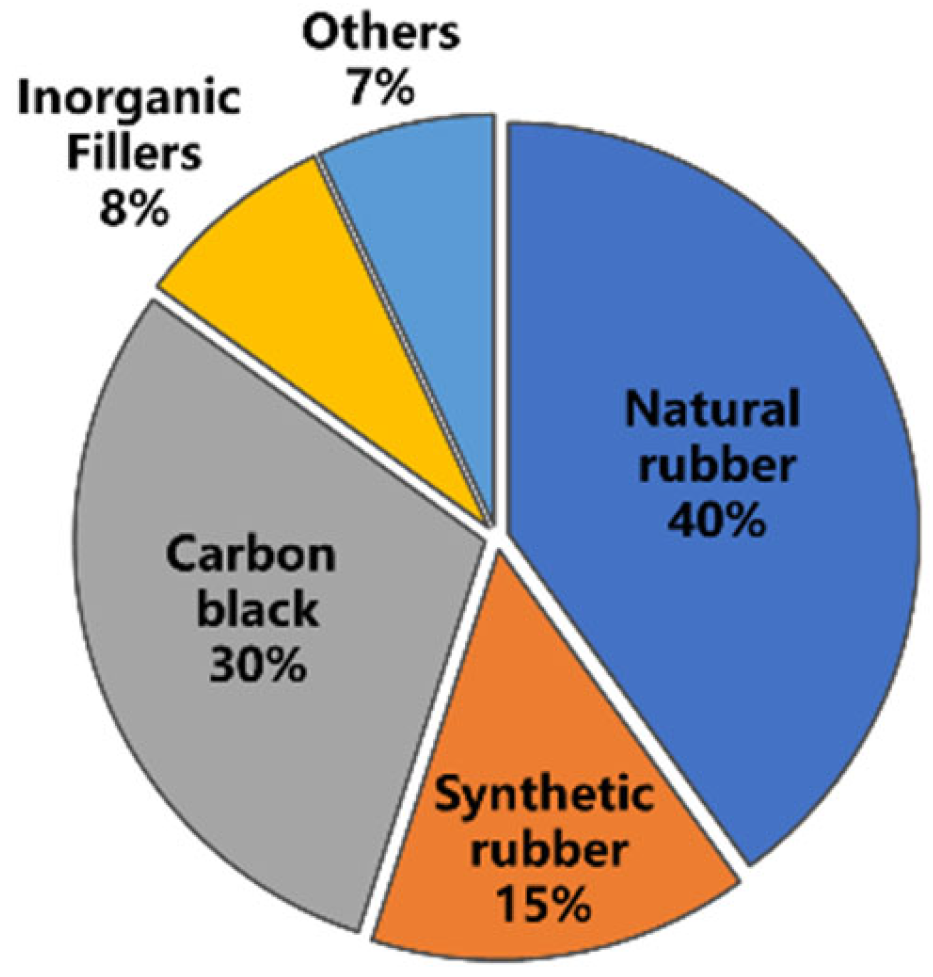
Figure 2. The composition of the GTR from truck tire [16].
Adding fillers to NR can improve interfacial adhesion and promote uniform dispersion, thus enhancing a series of properties of products, including stiffness, hardness, wear resistance, etc. Nanostructured CB and silica (SiO2) have a large surface area, allowing them to be dispersed in the rubber on a microscale.
CB is a proven reinforcing filler and has been widely applied in rubber, due to the improvement of mechanical properties, aging resistance, etc. Ayippadath et al. reported a more than 10-fold increase in tensile strength for SBR after adding N330 CB filler [19]. However, CB from petroleum is non-renewable and contaminative; therefore, recycling and reusing CB are quite necessary [20]. Silica is another widely used reinforcing filler. Different from CB, it is independent of oil resources and shows great advantages in low rolling resistance [21]. Idrus et al. evaluated the functions SiO2 plays in vulcanized NR [22]. They proved that irregularly shaped silica with ultra-fine particle size can better improve torque values. Many other chemicals are also used in rubber, such as cross-linking agents, antioxidants, plasticizers, etc.[23].
Different tires have distinguishable compositions; even different parts of the whole tire are different. However, their basic formulations and main components do not vary much: 50% rubber and 30% filler, as far as researchers know.
2.2. Degradation Behavior of Tire Rubber
Due to the irreversible vulcanization of tire rubber, conventional GTR with three-dimensional chemical cross-linking is an insoluble and infusible thermoset material, making it difficult to recycle and reuse. Therefore, the reclamation of vulcanized rubbers is urgent.
Reclamation is a procedure where the vulcanized rubber is converted into a product that can be vulcanized, processed and mixed again by using thermal energy and chemicals. The mechanism of this process is devulcanization or degradation [24]. In degradation, the breakage of chemical bonds occurs in the network, such as the C-S bond and S-S bond, and the chains of rubber are shortened as a result. While for devulcanization, the C-S bond, S-S bond and C-C bond are all broken without the scission of the main chains of rubber. Once the reclamation is increased seriously, the CB will separate from the rubber, and lightly pyrolyzed tire rubber will occur.
Song et al. reported the sol fractions and core–shell CB nanoparticles (Figure 3) in the process of reactive extrusion or warm aging [25]. They also reported a heterogeneous degradation of covalently cross-linked networks of GTR to core–shell CB nanoparticles [26]. Li et al. achieved the separation of CB from GTR by melt-extrusion pyrolysis at 300 °C [10]. Terminal blend technology can induce devulcanization and degradation of GTR at a high temperature of over 260 °C in asphalt but gives off a huge fume [11]. Seghar et al. summarized the previous works of rubber reclamation, desulfurization and regeneration for GTR in detail [18][27][28][29].
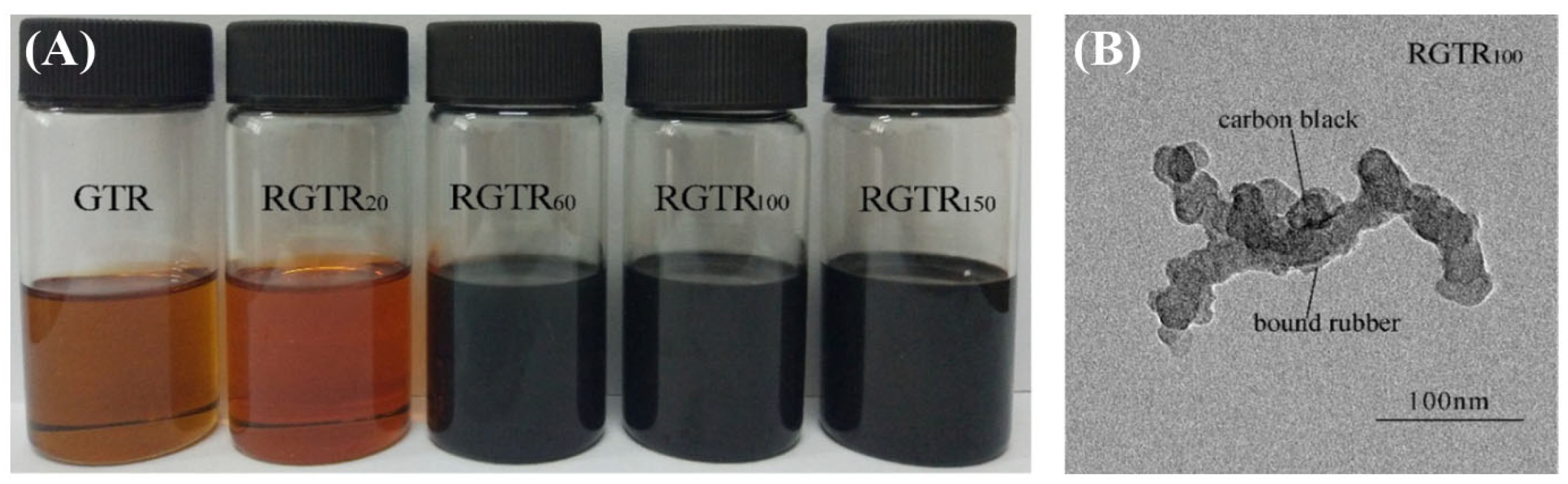
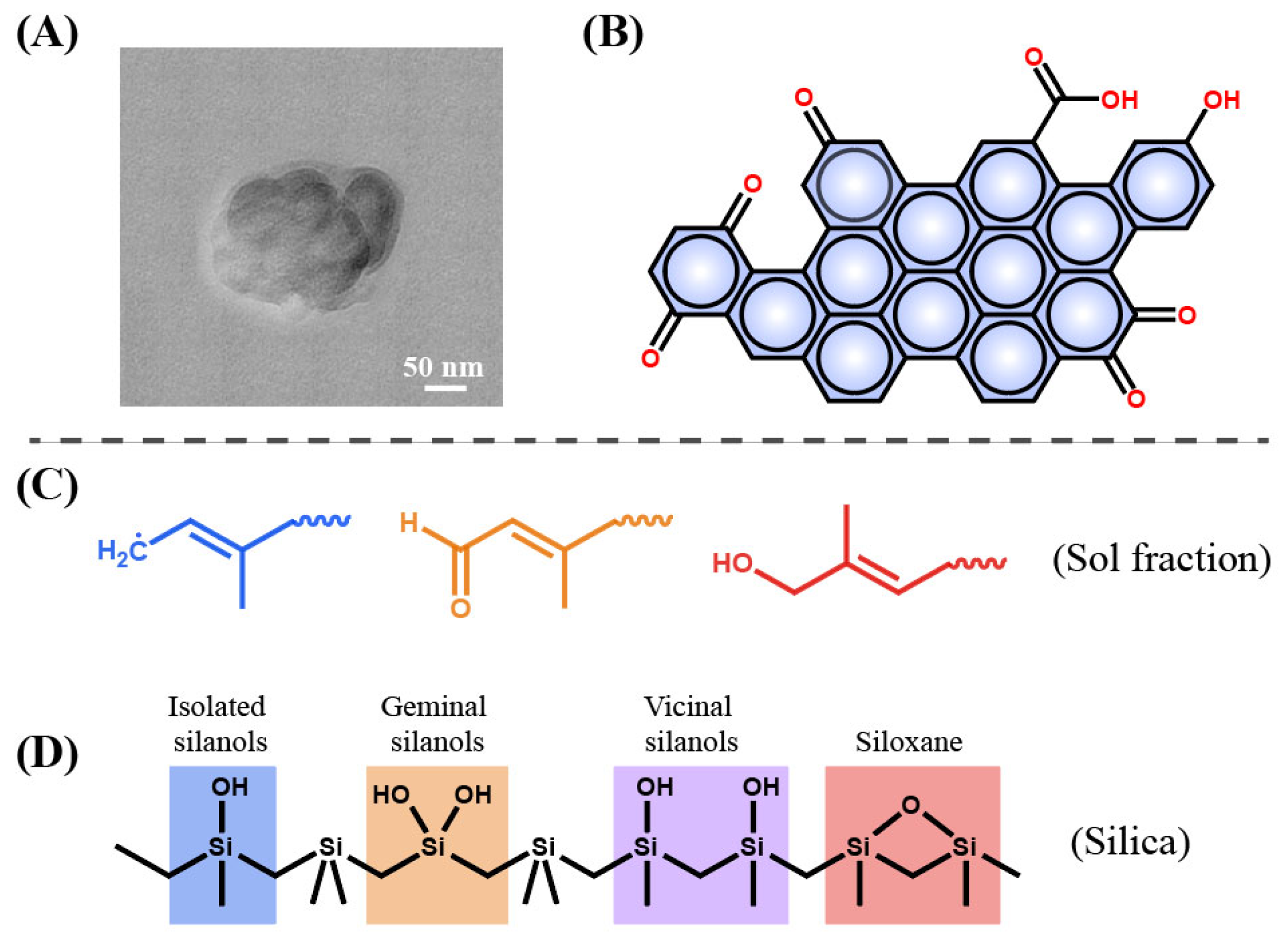
Figure 3. (A) Sol fractions obtained from degraded rubber with types of soybean oil under 150 °C for 4 h; (B) TEM image of CB from RGTR100 [26].
Wang et al. developed a new method named “thermal-oxidative reclamation” for devulcanizing GTR (Figure 4) [30]. This environmentally friendly method induces the completed breakage of the cross-link and part breakage of the main chain, leading to a decreased molecular weight of the sol with high content. The CB with micro-nano size can be released from the network of GTR.
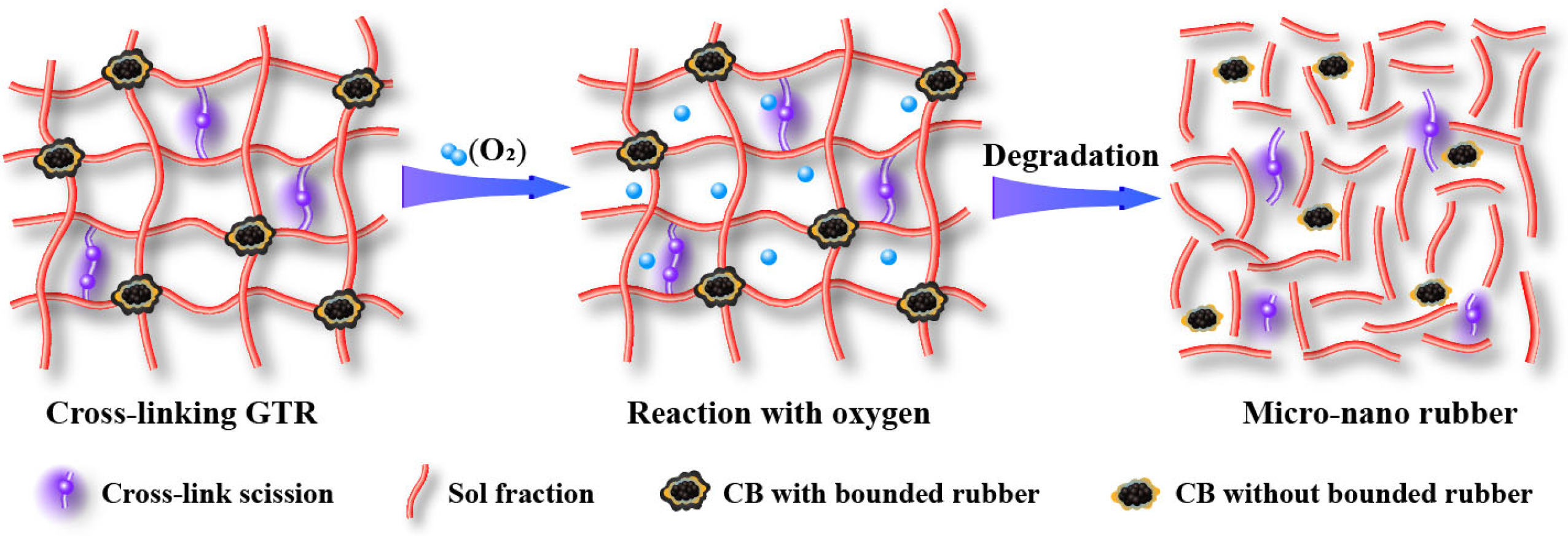
Figure 4. Schematic diagram of the dynamic thermal-oxidative reclaiming process [30].
The DR produced by the light pyrolysis process mainly consists of CB, sol fraction and inorganic species such as ZnO, silica, ZnS and so on. The CB has a very thin layer of tightly bound rubber, resulting in a core–shell structure (Figure 5A) [25]. With a crystal morphology between graphene crystal and amorphous, CB is usually less than 10 μm in diameter. Importantly, many polar functional groups including hydroxyl (-OH), carboxyl (-COOH), and amino groups (-NH2) are exposed on the surface of CB (Figure 5B), which provides an active site for the chemical reaction with asphalt [31]. In addition, sol fraction shows a relatively low molecular weight and is expected to be an alternative to asphalt because of its asphalt-like properties (Figure 5C) [30]. The inorganic products mainly contain ZnO, silica and ZnS. Silica is mainly composed of SiO2, and it can be expressed as SiO2·nH2O. nH2O in the SiO2·nH2O formula exists in the form of isolated silanols, vicinal silanols and geminal silanols (Figure 5D) [32]. These hydroxyls in silanols provide the possibility of intermolecular chemical reaction with other species such as asphalt. Previous reports found that DR could significantly improve the crack resistance of asphalt, providing an important foundation for the application and analysis of rubber asphalt [33].
References
- Dina Czajczyńska; Renata Krzyżyńska; Hussam Jouhara; Nik Spencer; Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121-1131, 10.1016/j.energy.2017.05.042.
- Nelson Flores Medina; Reyes Garcia; Iman Hajirasouliha; Kypros Pilakoutas; Maurizio Guadagnini; Samar Raffoul; Composites with recycled rubber aggregates: Properties and opportunities in construction. Construction and Building Materials 2018, 188, 884-897, 10.1016/j.conbuildmat.2018.08.069.
- Andrew Turner; Lynsey Rice; Toxicity of tire wear particle leachate to the marine macroalga, Ulva lactuca. Environmental Pollution 2010, 158, 3650-3654, 10.1016/j.envpol.2010.08.001.
- Anna Wik; Göran Dave; Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environmental Pollution 2009, 157, 1-11, 10.1016/j.envpol.2008.09.028.
- Chui-Te Chiu; Use of ground tire rubber in asphalt pavements: Field trial and evaluation in Taiwan. Resources, Conservation and Recycling 2008, 52, 522-532, 10.1016/j.resconrec.2007.06.006.
- Hainian Wang; Zhanping You; Julian Mills-Beale; Peiwen Hao; Laboratory evaluation on high temperature viscosity and low temperature stiffness of asphalt binder with high percent scrap tire rubber. Construction and Building Materials 2012, 26, 583-590, 10.1016/j.conbuildmat.2011.06.061.
- Miao Yu; Zhanping You; Guoxiong Wu; Lingyun Kong; Chaochao Liu; Junfeng Gao; Measurement and modeling of skid resistance of asphalt pavement: A review. Construction and Building Materials 2020, 260, 119878, 10.1016/j.conbuildmat.2020.119878.
- Hainian Wang; Zhengxia Dang; Lian Li; Zhanping You; Analysis on fatigue crack growth laws for crumb rubber modified (CRM) asphalt mixture. Construction and Building Materials 2013, 47, 1342-1349, 10.1016/j.conbuildmat.2013.06.014.
- N. Rattanasom; T. Saowapark; C. Deeprasertkul; Reinforcement of natural rubber with silica/carbon black hybrid filler. Polymer Testing 2007, 26, 369-377, 10.1016/j.polymertesting.2006.12.003.
- Shuo Li; Chaoying Wan; Xiaoyu Wu; Shifeng Wang; Core-shell structured carbon nanoparticles derived from light pyrolysis of waste tires. Polymer Degradation and Stability 2016, 129, 192-198, 10.1016/j.polymdegradstab.2016.04.013.
- Xiaoyu Wu; Shifeng Wang; Ruikun Dong; Lightly pyrolyzed tire rubber used as potential asphalt alternative. Construction and Building Materials 2016, 112, 623-628, 10.1016/j.conbuildmat.2016.02.208.
- Hongru Yao; Shuai Zhou; Shifeng Wang; Structural evolution of recycled tire rubber in asphalt. Journal of Applied Polymer Science 2015, 133, #-#, 10.1002/app.42954.
- Naipeng Tang; Zhiyu Zhang; Ruikun Dong; Hongzhou Zhu; Weidong Huang; Emission behavior of crumb rubber modified asphalt in the production process. Journal of Cleaner Production 2022, 340, 130850, 10.1016/j.jclepro.2022.130850.
- Xingyu Yi; Ruikun Dong; Chenguang Shi; Jun Yang; Zhen Leng; The influence of the mass ratio of crumb rubber and waste cooking oil on the properties of rubberised bio-rejuvenator and rejuvenated asphalt. Road Materials and Pavement Design 2022, #, 1-14, 10.1080/14680629.2022.2041070.
- Lei Lyu; Peter Mikhailenko; Zhengyin Piao; Elham H. Fini; Jianzhong Pei; Lily D. Poulikakos; Unraveling the modification mechanisms of waste bio-oils and crumb rubber on asphalt binder based on microscopy and chemo-rheology. Resources, Conservation and Recycling 2022, 185, 106447, 10.1016/j.resconrec.2022.106447.
- Pan Song; Shuo Li; Shifeng Wang; Interfacial interaction between degraded ground tire rubber and polyethylene. Polymer Degradation and Stability 2017, 143, 85-94, 10.1016/j.polymdegradstab.2017.06.020.
- Lucia Asaro; Michel Gratton; Saïd Seghar; Nourredine Aït Hocine; Recycling of rubber wastes by devulcanization. Resources, Conservation and Recycling 2018, 133, 250-262, 10.1016/j.resconrec.2018.02.016.
- Enes Akca; Ali Gursel; Nuri Sen; A review on devulcanization of waste tire rubber. Periodicals of Engineering and Natural Sciences (PEN) 2018, 6, 154-160, 10.21533/pen.v6i1.167.
- Jineesh Gopi; Sanjay Kumar Patel; Arup Kumar Chandra; Deba Kumar Tripathy; SBR-clay-carbon black hybrid nanocomposites for tire tread application. Journal of Polymer Research 2011, 18, 1625-1634, 10.1007/s10965-011-9567-9.
- Hélène Angellier; Sonia Molina-Boisseau; And Laurent Lebrun; Alain Dufresne; Processing and Structural Properties of Waxy Maize Starch Nanocrystals Reinforced Natural Rubber. Macromolecules 2005, 38, 3783-3792, 10.1021/ma050054z.
- K. W. Stöckelhuber; A. S. Svistkov; A. G. Pelevin; G. Heinrich; Impact of Filler Surface Modification on Large Scale Mechanics of Styrene Butadiene/Silica Rubber Composites. Macromolecules 2011, 44, 4366-4381, 10.1021/ma1026077.
- Suhaida S. Idrus; H. Ismail; Samayamutthirian Palaniandy; Study of the effect of different shapes of ultrafine silica as fillers in natural rubber compounds. Polymer Testing 2011, 30, 251-259, 10.1016/j.polymertesting.2010.10.002.
- Richard B. Simpson. Rubber basics; Richard B. Simpson, Eds.; iSmithers Rapra Publishing: Shawbury, 2002; pp. 117.
- Franta, I.. Elastomers and Rubber Compounding Materials, Manufacture, Properties and Application; Franta, I., Eds.; Elvevier: New York: Oxford, 1989; pp. 1.
- Pan Song; Xinyu Zhao; Xiangyun Cheng; Shuo Li; Shifeng Wang; Recycling the nanostructured carbon from waste tires. Composites Communications 2018, 7, 12-15, 10.1016/j.coco.2017.12.001.
- Pan Song; Chaoying Wan; Yanling Xie; Krzysztof Formela; Shifeng Wang; Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Management 2018, 78, 238-248, 10.1016/j.wasman.2018.05.054.
- Saïd Seghar; Lucia Asaro; Morena Rolland-Monnet; Nourredine Aït Hocine; Thermo-mechanical devulcanization and recycling of rubber industry waste. Resources, Conservation and Recycling 2019, 144, 180-186, 10.1016/j.resconrec.2019.01.047.
- Ming Chao Luo; Xiao Xue Liao; Shuang Quan Liao; Yan Fang Zhao; Review on the Broken Three-Dimensional Network Modification Methods of Waste Rubber Powder. Advanced Materials Research 2012, 554-556, 181-186, 10.4028/www.scientific.net/amr.554-556.181.
- Erich Markl; Maximilian Lackner; Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246, 10.3390/ma13051246.
- Yuxin Zhang; Zhen Zhang; Alan Matheson Wemyss; Chaoying Wan; Yongtao Liu; Pan Song; Shifeng Wang; Effective Thermal-Oxidative Reclamation of Waste Tire Rubbers for Producing High-Performance Rubber Composites. ACS Sustainable Chemistry & Engineering 2020, 8, 9079-9087, 10.1021/acssuschemeng.0c02292.
- J Leblanc; Rubber–filler interactions and rheological properties in filled compounds. Progress in Polymer Science 2002, 27, 627-687, 10.1016/s0079-6700(01)00040-5.
- Adeel Ahmad Hassan; Anees Abbas; Tahir Rasheed; Muhamad Bilal; Hafiz M.N. Iqbal; Shifeng Wang; Development, influencing parameters and interactions of bioplasticizers: An environmentally friendlier alternative to petro industry-based sources. Science of The Total Environment 2019, 682, 394-404, 10.1016/j.scitotenv.2019.05.140.
- Raqiqa Tur Rasool; Shifeng Wang; Yong Zhang; Yue Li; Guangtai Zhang; Improving the aging resistance of SBS modified asphalt with the addition of highly reclaimed rubber. Construction and Building Materials 2017, 145, 126-134, 10.1016/j.conbuildmat.2017.03.242.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
01 Nov 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No