Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Chuanping Zhang and Version 2 by Camila Xu.
The use of ground tire rubber (GTR) for modifying asphalt is very promising and is a sustainable development strategy. The addition of GTR to asphalt shows many improvements in the physical, chemical and mechanical properties of the rubber asphalt binder, such as enhanced stiffness, increased skid resistance, extended service life, mitigated fatigue cracking and so on.
- devulcanized rubber
- rubberized asphalt
- colloidal structure
1. Introduction
One of the key challenges in waste management, especially in the disposal of end-of-life tires, has always been the task of monitoring in the 21st century [1]. The most widespread disposal methods for end-of-life tires are landfills and incineration [2], causing much environmental pollution due to the nonbiodegradability, flammability and chemical characteristic of scrap tires, such as the emission of toxic gases and other harmful substances [3][4]. On the other hand, scrap tires are mainly composed of vulcanized rubber, reinforcing filler and a variety of materials, which is not only an environmental pollutant but also a great economic loss. Therefore, recycling and reuse of these waste materials are of great significance considering the world is facing a lack of resources and a shortage of energy.
The use of ground tire rubber (GTR) for modifying asphalt is very promising and is a sustainable development strategy [5]. The addition of GTR to asphalt shows many improvements in the physical, chemical and mechanical properties of the rubber asphalt binder, such as enhanced stiffness, increased skid resistance, extended service life, mitigated fatigue cracking and so on [6][7][8]. However, the multiple cross-linking network structure of GTR leads to not only high energy consumption and pollution emissions but also results in the poor compatibility and bad stability of rubber asphalt. The above problems limit the efficient utilization and cause the unscientific application of rubber asphalt. In view of this, devulcanization or degradation of the cross-linking network is urgent for reutilization with high efficiency.
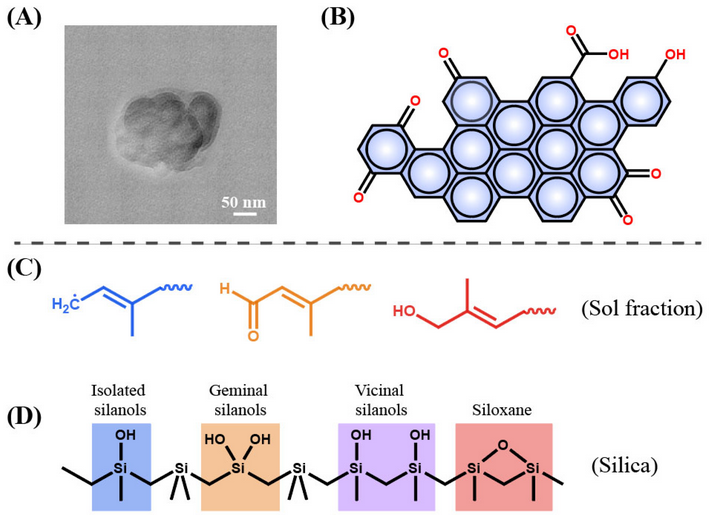
In order to obtain stable rubber asphalt with good performance, the compatibility between asphalt and rubber should be improved. Tire rubber is usually composed of cross-linked rubber, carbon black (CB), silica and so on [9]. Devulcanization or degradation destroys the cross-linked network and rubber body carbon chains to be short chains and other separated small substances such as CB. Most previous studies focused on the devulcanization of rubber and the reduced molecular weight of sol fraction (Table 1); less is reported about the decreased size of CB (from several micrometers to micro-nanometers, Figure 1A–C) with the increase in degradation degree [10][11]. These degraded substances are well dispersed in the asphalt, as shown in Figure 1D [12]. Therefore, deeply degraded rubber provides a new strategy for preparing rubberized asphalt from a different perspective.
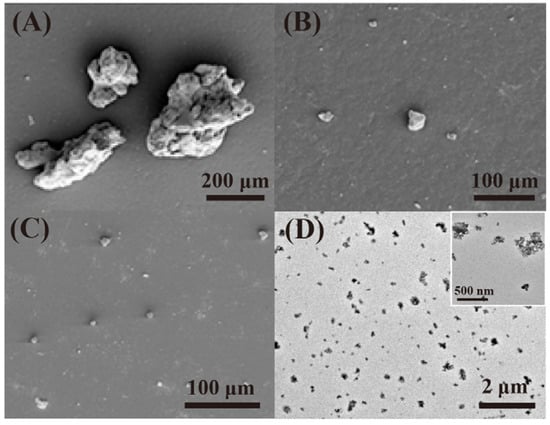
Table 1.
Molecular weight and its distribution of sol fraction
[11]
.
| Temperature/°C | M¯¯¯¯n | (10 | 4 | g/mol) | M¯¯¯¯w | (10 | 4 | g/mol) | M¯¯¯¯w/M¯¯¯¯n |
|---|
| 220 | 1.19 | 6.12 | 5.12 |
| 240 | 1.25 | 6.01 | 4.79 |
| 260 | 1.42 | 6.89 | 4.84 |
| 280 | 1.34 | 6.29 | 4.70 |
| 300 | 0.41 | 2.28 | 5.57 |
The DR mainly consisted of micro-nano CB, low molecular weight of sol fraction and inorganic substances, denoted as micro-nano rubber. The DR showed much better dispersibility and reinforcement compared with GTR, thus usually used for the modification of asphalt with high content. Therefore, the DR was more and more used as the potential alternative for asphalt in pavement, which significantly improved the anti-cracking and anti-aging performance [13][14][15]. Although DRMA demonstrates much improved properties compared with both base asphalt or GTR modified asphalt, the standardized applications of rubber asphalt have always been a challenge for the lack of a deep understanding of the mixing mechanism, especially on a colloidal scale.
2. Tire Rubber and Its Degradation Behavior
2.1. Chemical Composition of Tire Rubber
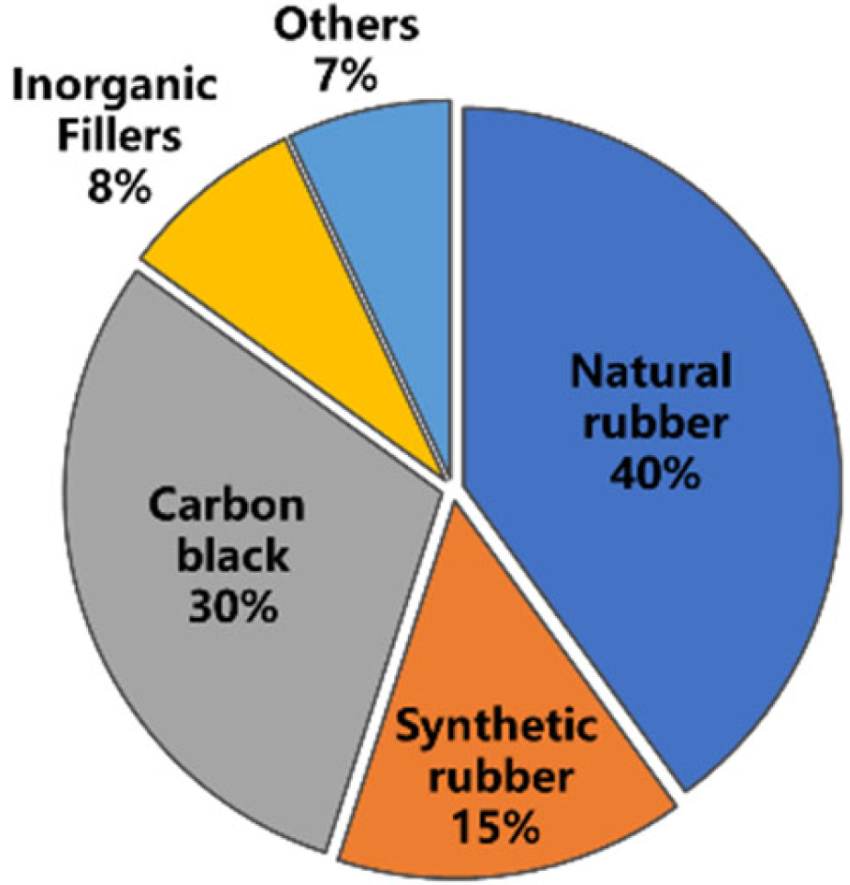
Tire rubber has a crucial role in many fields such as industry, agriculture and national defense. It is reported that the global tire output is about 1.5 billion each year and that of waste tires is more than 17 million tons each year. With the increased demand for tires in developing countries, the output of waste tires grows more rapidly. Due to the cross-linking and reinforcing network structure of waste tires, they are difficult to degrade under natural conditions, which results in a large accumulation. Serious “black pollution” formed by wasted rubber severely threatens the health and life of human beings. Tires mainly consist of vulcanized rubber and a series of additives [16]. The composition of a typical tire is shown in Figure 2 (by weight). It is mainly composed of natural rubber (NR) which comprises about 40% of the weight of truck tire rubber, making the tire full of elasticity and durability even under heavy loads [17][18]. The proportion of NR has increased nowadays, possibly due to the expansion in the use of radial tires and heavy-duty tires. It should be pointed out that the properties of NR can largely affect the workability of materials. For example, the molecular weight and gel content of NR may affect compound viscosity and filler dispersion in rubber mixing. In addition, the tendency of NR to crystallize upon stretching, even before cross-linking, has contributed to the strength of uncured rubber compounds.2.2. Degradation Behavior of Tire Rubber
Due to the irreversible vulcanization of tire rubber, conventional GTR with three-dimensional chemical cross-linking is an insoluble and infusible thermoset material, making it difficult to recycle and reuse. Therefore, the reclamation of vulcanized rubbers is urgent. Reclamation is a procedure where the vulcanized rubber is converted into a product that can be vulcanized, processed and mixed again by using thermal energy and chemicals. The mechanism of this process is devulcanization or degradation [24]. In degradation, the breakage of chemical bonds occurs in the network, such as the C-S bond and S-S bond, and the chains of rubber are shortened as a result. While for devulcanization, the C-S bond, S-S bond and C-C bond are all broken without the scission of the main chains of rubber. Once the reclamation is increased seriously, the CB will separate from the rubber, and lightly pyrolyzed tire rubber will occur. Song et al. reported the sol fractions and core–shell CB nanoparticles (Figure 3) in the process of reactive extrusion or warm aging [25]. They also reported a heterogeneous degradation of covalently cross-linked networks of GTR to core–shell CB nanoparticles [26]. Li et al. achieved the separation of CB from GTR by melt-extrusion pyrolysis at 300 °C [10]. Terminal blend technology can induce devulcanization and degradation of GTR at a high temperature of over 260 °C in asphalt but gives off a huge fume [11]. Seghar et al. summarized the previous works of rubber reclamation, desulfurization and regeneration for GTR in detail [18][27][28][29].
Figure 3.
(
A
) Sol fractions obtained from degraded rubber with types of soybean oil under 150 °C for 4 h; (
B
) TEM image of CB from RGTR
100
[26]
.
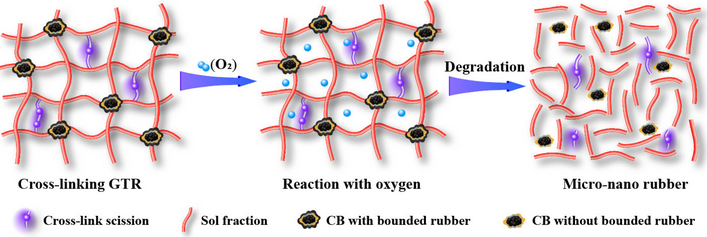
Figure 4.
Schematic diagram of the dynamic thermal-oxidative reclaiming process
[30].
.
References
- Dina Czajczyńska; Renata Krzyżyńska; Hussam Jouhara; Nik Spencer; Use of pyrolytic gas from waste tire as a fuel: A review. Energy 2017, 134, 1121-1131, 10.1016/j.energy.2017.05.042.
- Nelson Flores Medina; Reyes Garcia; Iman Hajirasouliha; Kypros Pilakoutas; Maurizio Guadagnini; Samar Raffoul; Composites with recycled rubber aggregates: Properties and opportunities in construction. Construction and Building Materials 2018, 188, 884-897, 10.1016/j.conbuildmat.2018.08.069.
- Andrew Turner; Lynsey Rice; Toxicity of tire wear particle leachate to the marine macroalga, Ulva lactuca. Environmental Pollution 2010, 158, 3650-3654, 10.1016/j.envpol.2010.08.001.
- Anna Wik; Göran Dave; Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environmental Pollution 2009, 157, 1-11, 10.1016/j.envpol.2008.09.028.
- Chui-Te Chiu; Use of ground tire rubber in asphalt pavements: Field trial and evaluation in Taiwan. Resources, Conservation and Recycling 2008, 52, 522-532, 10.1016/j.resconrec.2007.06.006.
- Hainian Wang; Zhanping You; Julian Mills-Beale; Peiwen Hao; Laboratory evaluation on high temperature viscosity and low temperature stiffness of asphalt binder with high percent scrap tire rubber. Construction and Building Materials 2012, 26, 583-590, 10.1016/j.conbuildmat.2011.06.061.
- Miao Yu; Zhanping You; Guoxiong Wu; Lingyun Kong; Chaochao Liu; Junfeng Gao; Measurement and modeling of skid resistance of asphalt pavement: A review. Construction and Building Materials 2020, 260, 119878, 10.1016/j.conbuildmat.2020.119878.
- Hainian Wang; Zhengxia Dang; Lian Li; Zhanping You; Analysis on fatigue crack growth laws for crumb rubber modified (CRM) asphalt mixture. Construction and Building Materials 2013, 47, 1342-1349, 10.1016/j.conbuildmat.2013.06.014.
- N. Rattanasom; T. Saowapark; C. Deeprasertkul; Reinforcement of natural rubber with silica/carbon black hybrid filler. Polymer Testing 2007, 26, 369-377, 10.1016/j.polymertesting.2006.12.003.
- Shuo Li; Chaoying Wan; Xiaoyu Wu; Shifeng Wang; Core-shell structured carbon nanoparticles derived from light pyrolysis of waste tires. Polymer Degradation and Stability 2016, 129, 192-198, 10.1016/j.polymdegradstab.2016.04.013.
- Xiaoyu Wu; Shifeng Wang; Ruikun Dong; Lightly pyrolyzed tire rubber used as potential asphalt alternative. Construction and Building Materials 2016, 112, 623-628, 10.1016/j.conbuildmat.2016.02.208.
- Hongru Yao; Shuai Zhou; Shifeng Wang; Structural evolution of recycled tire rubber in asphalt. Journal of Applied Polymer Science 2015, 133, #-#, 10.1002/app.42954.
- Naipeng Tang; Zhiyu Zhang; Ruikun Dong; Hongzhou Zhu; Weidong Huang; Emission behavior of crumb rubber modified asphalt in the production process. Journal of Cleaner Production 2022, 340, 130850, 10.1016/j.jclepro.2022.130850.
- Xingyu Yi; Ruikun Dong; Chenguang Shi; Jun Yang; Zhen Leng; The influence of the mass ratio of crumb rubber and waste cooking oil on the properties of rubberised bio-rejuvenator and rejuvenated asphalt. Road Materials and Pavement Design 2022, #, 1-14, 10.1080/14680629.2022.2041070.
- Lei Lyu; Peter Mikhailenko; Zhengyin Piao; Elham H. Fini; Jianzhong Pei; Lily D. Poulikakos; Unraveling the modification mechanisms of waste bio-oils and crumb rubber on asphalt binder based on microscopy and chemo-rheology. Resources, Conservation and Recycling 2022, 185, 106447, 10.1016/j.resconrec.2022.106447.
- Pan Song; Shuo Li; Shifeng Wang; Interfacial interaction between degraded ground tire rubber and polyethylene. Polymer Degradation and Stability 2017, 143, 85-94, 10.1016/j.polymdegradstab.2017.06.020.
- Lucia Asaro; Michel Gratton; Saïd Seghar; Nourredine Aït Hocine; Recycling of rubber wastes by devulcanization. Resources, Conservation and Recycling 2018, 133, 250-262, 10.1016/j.resconrec.2018.02.016.
- Enes Akca; Ali Gursel; Nuri Sen; A review on devulcanization of waste tire rubber. Periodicals of Engineering and Natural Sciences (PEN) 2018, 6, 154-160, 10.21533/pen.v6i1.167.
- Jineesh Gopi; Sanjay Kumar Patel; Arup Kumar Chandra; Deba Kumar Tripathy; SBR-clay-carbon black hybrid nanocomposites for tire tread application. Journal of Polymer Research 2011, 18, 1625-1634, 10.1007/s10965-011-9567-9.
- Hélène Angellier; Sonia Molina-Boisseau; And Laurent Lebrun; Alain Dufresne; Processing and Structural Properties of Waxy Maize Starch Nanocrystals Reinforced Natural Rubber. Macromolecules 2005, 38, 3783-3792, 10.1021/ma050054z.
- K. W. Stöckelhuber; A. S. Svistkov; A. G. Pelevin; G. Heinrich; Impact of Filler Surface Modification on Large Scale Mechanics of Styrene Butadiene/Silica Rubber Composites. Macromolecules 2011, 44, 4366-4381, 10.1021/ma1026077.
- Suhaida S. Idrus; H. Ismail; Samayamutthirian Palaniandy; Study of the effect of different shapes of ultrafine silica as fillers in natural rubber compounds. Polymer Testing 2011, 30, 251-259, 10.1016/j.polymertesting.2010.10.002.
- Richard B. Simpson. Rubber basics; Richard B. Simpson, Eds.; iSmithers Rapra Publishing: Shawbury, 2002; pp. 117.
- Franta, I.. Elastomers and Rubber Compounding Materials, Manufacture, Properties and Application; Franta, I., Eds.; Elvevier: New York: Oxford, 1989; pp. 1.
- Pan Song; Xinyu Zhao; Xiangyun Cheng; Shuo Li; Shifeng Wang; Recycling the nanostructured carbon from waste tires. Composites Communications 2018, 7, 12-15, 10.1016/j.coco.2017.12.001.
- Pan Song; Chaoying Wan; Yanling Xie; Krzysztof Formela; Shifeng Wang; Vegetable derived-oil facilitating carbon black migration from waste tire rubbers and its reinforcement effect. Waste Management 2018, 78, 238-248, 10.1016/j.wasman.2018.05.054.
- Saïd Seghar; Lucia Asaro; Morena Rolland-Monnet; Nourredine Aït Hocine; Thermo-mechanical devulcanization and recycling of rubber industry waste. Resources, Conservation and Recycling 2019, 144, 180-186, 10.1016/j.resconrec.2019.01.047.
- Ming Chao Luo; Xiao Xue Liao; Shuang Quan Liao; Yan Fang Zhao; Review on the Broken Three-Dimensional Network Modification Methods of Waste Rubber Powder. Advanced Materials Research 2012, 554-556, 181-186, 10.4028/www.scientific.net/amr.554-556.181.
- Erich Markl; Maximilian Lackner; Devulcanization Technologies for Recycling of Tire-Derived Rubber: A Review. Materials 2020, 13, 1246, 10.3390/ma13051246.
- Yuxin Zhang; Zhen Zhang; Alan Matheson Wemyss; Chaoying Wan; Yongtao Liu; Pan Song; Shifeng Wang; Effective Thermal-Oxidative Reclamation of Waste Tire Rubbers for Producing High-Performance Rubber Composites. ACS Sustainable Chemistry & Engineering 2020, 8, 9079-9087, 10.1021/acssuschemeng.0c02292.
- J Leblanc; Rubber–filler interactions and rheological properties in filled compounds. Progress in Polymer Science 2002, 27, 627-687, 10.1016/s0079-6700(01)00040-5.
- Adeel Ahmad Hassan; Anees Abbas; Tahir Rasheed; Muhamad Bilal; Hafiz M.N. Iqbal; Shifeng Wang; Development, influencing parameters and interactions of bioplasticizers: An environmentally friendlier alternative to petro industry-based sources. Science of The Total Environment 2019, 682, 394-404, 10.1016/j.scitotenv.2019.05.140.
- Raqiqa Tur Rasool; Shifeng Wang; Yong Zhang; Yue Li; Guangtai Zhang; Improving the aging resistance of SBS modified asphalt with the addition of highly reclaimed rubber. Construction and Building Materials 2017, 145, 126-134, 10.1016/j.conbuildmat.2017.03.242.
More