
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benjamin David Mercier | -- | 2441 | 2022-10-28 01:40:28 | | | |
| 2 | Lindsay Dong | Meta information modification | 2441 | 2022-10-28 03:29:14 | | |
Video Upload Options
Chemotherapy and radiotherapy are first-line treatments in the management of advanced solid tumors. While these treatments are directed at mitigating the growth of and potentially eliminating cancer cells, they cause significant adverse effects that can be detrimental to a patient’s quality of life or even life-threatening. Diet is a modifiable risk factor that has been shown to affect cancer risk, recurrence, and treatment toxicity, but little information is known how diet interacts with cancer treatment modalities. Dietary interventions, such as caloric restriction, intermittent fasting and ketogenic diets, have shown promise in clinical pilot studies and pre-clinical mammal model studies by reducing the toxicity and increasing the efficacy of chemotherapeutics and radiotherapy treatment. However, further clinical trials on a wider scale and much more evidence are required to before any changes to clinical practice can be advised.
1. Introduction
2. Dietary Interventions
2.1. Caloric Restriction and Fasting-Mimicking Diets Assist in Further Attenuating Tumor Growth with CT/RT Treatment Regimens
As noted previously, CR represents the reduction in the gross number of calories that are consumed daily, which is generally to less than 20–30% of the normal caloric intake [16]. This diet has a well-established effect of decreasing the overall presence of ROS among the somatic cells, likely resulting from the activation of the eNOS pathway via SIRT1 upregulation [6]. CR has been associated with tumor growth attenuation and progression, with experiments in rodent models indicating that CR can reduce the vascular density of tumors [17].
2.2. Intermittent Fasting Is a Safe and Feasible Way of Increasing the Efficacy of CT/RT Treatment as Well as Decreasing Treatment Toxicity
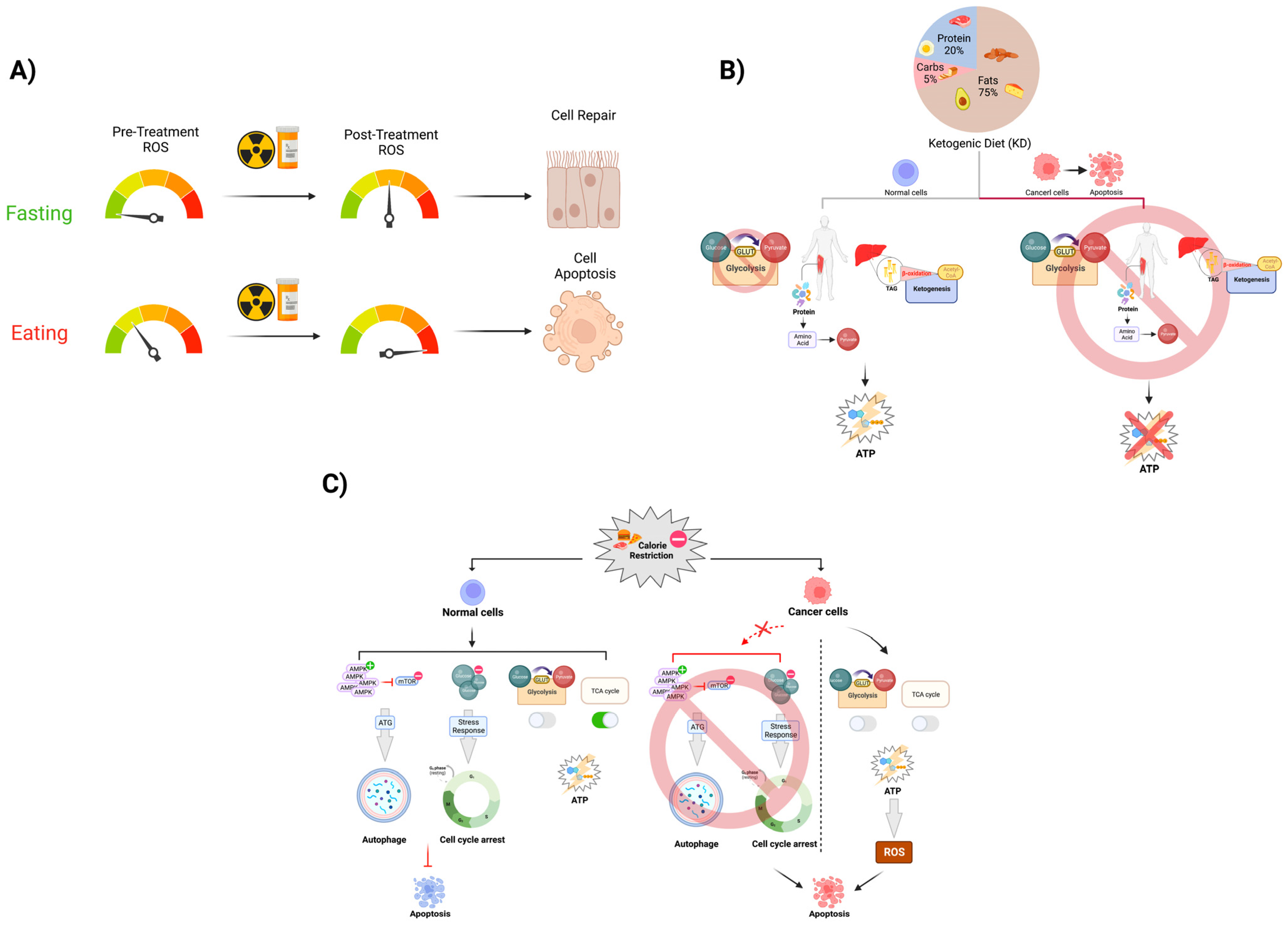
2.3. Ketogenic Diet Has Been Shown to Maintain Non-Fat Body Mass and Increase CT/RT Treatment Efficacy in Murine Models and Human Cancer Patients
3. Conclusions
References
- DeVita, V.T., Jr.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653.
- Morrison, W.b. Cancer Chemotherapy: An Annotated History. J. Vet. Intern. Med. 2010, 24, 1249–1262.
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and Radiation Therapy: Current Advances and Future Directions. Int. J. Med. Sci. 2012, 9, 193–199.
- Hajdu, S.I.; Vadmal, M. A note from history: Landmarks in history of cancer, Part 6. Cancer 2013, 119, 4058–4082.
- Givens, D.J.; Karnell, L.H.; Gupta, A.K.; Clamon, G.H.; Pagedar, N.A.; Chang, K.E.; Van Daele, D.J.; Funk, G.F. Adverse Events Associated with Concurrent Chemoradiation Therapy in Patients with Head and Neck Cancer. Arch. Otolaryngol. Neck Surg. 2009, 135, 1209–1217.
- Man, A.W.C.; Li, H.; Xia, N. Impact of Lifestyles (Diet and Exercise) on Vascular Health: Oxidative Stress and Endothelial Function. Oxid. Med. Cell Longev. 2020, 2020, 1496462.
- Bianchi, G.; Martella, R.; Ravera, S.; Marini, C.; Capitanio, S.; Orengo, A.; Emionite, L.; Lavarello, C.; Amaro, A.; Petretto, A.; et al. Fasting induces anti-Warburg effect that increases respiration but reduces ATP-synthesis to promote apoptosis in colon cancer models. Oncotarget 2015, 6, 11806–11819.
- Kritchevsky, D. Caloric restriction and cancer. J. Nutr. Sci. Vitaminol. 2001, 47, 13–19.
- Manukian, G.; Simone, B.; DeAngelis, T.; Kivolowitz, C.; Ko, K.; Simone, N.L. Caloric Restriction Curtails Tumor Infiltrating Regulatory T Cells and Enhances Effector T Cell Activity after Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S164.
- O’Farrell, A.M.; Abrams, T.J.; Yuen, H.A.; Ngai, T.J.; Louie, S.G.; Yee, K.W.H.; Wong, L.M.; Hong, W.; Lee, L.B.; Town, A.; et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003, 101, 3597–3605.
- Hursting, S.D.; Dunlap, S.M.; Ford, N.A.; Hursting, M.J.; Lashinger, L.M. Calorie restriction and cancer prevention: A mechanistic perspective. Cancer Metab. 2013, 1, 10.
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631.
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121.
- Coppola, G.; Veggiotti, P.; Cusmai, R.; Bertoli, S.; Cardinali, S.; Dionisi-Vici, C.; Elia, M.; Lispi, M.L.; Sarnelli, C.; Tagliabue, A.; et al. The Ketogenic Diet in Children, Adolescents and Young Adults with Refractory Epilepsy: An Italian Multicentric Experience. Epilepsy Res. 2002, 48, 221–227.
- Marsh, E.B.; Freeman, J.M.; Kossoff, E.H.; Vining, E.P.G.; Rubenstein, J.E.; Pyzik, P.L.; Hemingway, C. The Outcome of Children with Intractable Seizures: A 3- to 6-Year Follow-up of 67 Children Who Remained on the Ketogenic Diet Less than One Year. Epilepsia 2006, 47, 425–430.
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2017, 39, 36–45.
- Powolny, A.A.; Wang, S.; Carlton, P.S.; Hoot, D.R.; Clinton, S.K. Interrelationships between dietary restriction, the IGF-I axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats. Mol. Carcinog. 2008, 47, 458–465.
- Farazi, M.; Nguyen, J.; Goldufsky, J.; Linnane, S.; Lukaesko, L.; Weinberg, A.D.; Ruby, C.E. Caloric restriction maintains OX40 agonist-mediated tumor immunity and CD4 T cell priming during aging. Cancer Immunol. Immunother. 2014, 63, 615–626.
- Sukumar, M.; Roychoudhuri, R.; Restifo, N.P. Nutrient Competition: A New Axis of Tumor Immunosuppression. Cell 2015, 162, 1206–1208.
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. In Metabolism in Cancer; Recent Results in Cancer Research; Cramer, T., Schmitt, C.A., Eds.; Springer: Cham, Switzerland, 2016; Volume 207, pp. 241–266.
- Nikolai, S.; Pallauf, K.; Huebbe, P.; Rimbach, G. Energy restriction and potential energy restriction mimetics. Nutr. Res. Rev. 2015, 28, 100–120.
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236.
- Riedinger, C.J.; Kimball, K.J.; Kilgore, L.C.; Bell, C.; Heidel, E.; Boone, J.D. Water-only fasting and its effect on chemotherapy administration in gynecologic malignancies. Gynecol. Oncol. 2020, 159, 13.
- Kozubík, A.; Pospíšil, M. Adaptation to intermittent fasting as a factor modifying the radiation resistance of mice. Experientia 1982, 38, 958–959.
- Murata, Y.; Uehara, Y.; Hosoi, Y. Activation of mTORC1 under nutrient starvation conditions increases cellular radiosensitivity in human liver cancer cell lines, HepG2 and HuH6. Biochem. Biophys. Res. Commun. 2015, 468, 684–690.
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851.
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When less may be more: Calorie restriction and response to cancer therapy. BMC Med. 2017, 15, 106.
- Icard, P.; Ollivier, L.; Forgez, P.; Otz, J.; Alifano, M.; Fournel, L.; Loi, M.; Thariat, J. Perspective: Do Fasting, Caloric Restriction, and Diets Increase Sensitivity to Radiotherapy? A Literature Review. Adv. Nutr. 2020, 11, 1089–1101.
- Raffaghello, L.; Lee, C.; Safdie, F.M.; Wei, M.; Madia, F.; Bianchi, G.; Longo, V.D. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 8215–8220.
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.W.A.; Jochems, A.; Hoursma, D.; Putter, H.; van der Hoeven, J.J.M.; Nortier, J.W.R.; et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: A randomized pilot study. BMC Cancer 2015, 15, 652.
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796.
- Champ, C.E.; Palmer, J.D.; Volek, J.S.; Werner-Wasik, M.; Andrews, D.W.; Evans, J.J.; Glass, J.; Kim, L.; Shi, W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J. Neurooncol. 2014, 117, 125–131.
- Klement, R.J.; Pazienza, V. Impact of Different Types of Diet on Gut Microbiota Profiles and Cancer Prevention and Treatment. Medicina 2019, 55, 84.
- Zahra, A.; Fath, M.A.; Opat, E.; Mapuskar, K.A.; Bhatia, S.K.; Ma, D.C.; Rodman, S.N.; Snyders, T.P.; Chenard, C.A.; Eichenberger-Gilmore, J.M.; et al. Consuming a Ketogenic Diet while Receiving Radiation and Chemotherapy for Locally Advanced Lung Cancer and Pancreatic Cancer: The University of Iowa Experience of Two Phase 1 Clinical Trials. Radiat Res. 2017, 187, 743–754.




