Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MANUEL LISARDO Sanchez SANCHEZ | -- | 6387 | 2022-10-26 11:43:46 | | | |
| 2 | Lindsay Dong | -3 word(s) | 6384 | 2022-10-27 03:33:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sánchez, M.L.; Coveñas, R. The Galaninergic System. Encyclopedia. Available online: https://encyclopedia.pub/entry/31390 (accessed on 07 February 2026).
Sánchez ML, Coveñas R. The Galaninergic System. Encyclopedia. Available at: https://encyclopedia.pub/entry/31390. Accessed February 07, 2026.
Sánchez, Manuel Lisardo, Rafael Coveñas. "The Galaninergic System" Encyclopedia, https://encyclopedia.pub/entry/31390 (accessed February 07, 2026).
Sánchez, M.L., & Coveñas, R. (2022, October 26). The Galaninergic System. In Encyclopedia. https://encyclopedia.pub/entry/31390
Sánchez, Manuel Lisardo and Rafael Coveñas. "The Galaninergic System." Encyclopedia. Web. 26 October, 2022.
Copy Citation
Peptidergic systems play an important role in cancer progression. The galaninergic system (the peptide galanin and its receptors: galanin 1, 2 and 3) is involved in tumorigenesis, the invasion and migration of tumor cells and angiogenesis and it has been correlated with tumor stage/subtypes, metastasis and recurrence rate in many types of cancer. Galanin exerts a dual action in tumor cells: a proliferative or an antiproliferative effect depending on the galanin receptor involved in these mechanisms. Galanin receptors could be used in certain tumors as therapeutic targets and diagnostic markers for treatment, prognosis and surgical outcome.
galanin
galanin receptor
galanin receptor antagonist
galanin receptor agonist
1. Introduction
The GLOBOCAN 2020 database (World Health Organization (WHO)) states that of the 7,794,798,844 inhabitants of our planet, 19,292,789 of them were diagnosed with some type of cancer and 9,958,133 died, with prevalence cases at 5 years of 50,550,287. Female breast cancer is the most diagnosed cancer and the leading cause of cancer death is lung cancer (1.8 million deaths) [1]. In 2040, 28.4 million patients suffering from cancer are expected in the world [1]. These data are sufficiently representative of the health problem that cancer represents today. Cells, escaping from normal behavior, acquire distinctive characters (evading growth suppressors, maintaining proliferative signaling, allowing replicative immortality, resisting cell death, activating invasion/metastasis, inducing angiogenesis) that make them cancerous [2] (Figure 1). Moreover, the reprogramming of energy metabolism and evasion of immune destruction have also been added to the previous hallmarks of cancer [2]. These behaviors arise from the instability of the genome that produces genetic diversity, and inflammatory mechanisms that promote the multiple actions described above (Figure 1). Tumors are not currently considered as simple masses of cancer cells; they are more complex in that they contain a repertoire of apparently normal recruited cells that contribute to the acquisition of distinctive features by regulating the tumor microenvironment [2]. The full knowledge of the previously mentioned hallmarks will help to develop new therapeutic strategies against cancer.
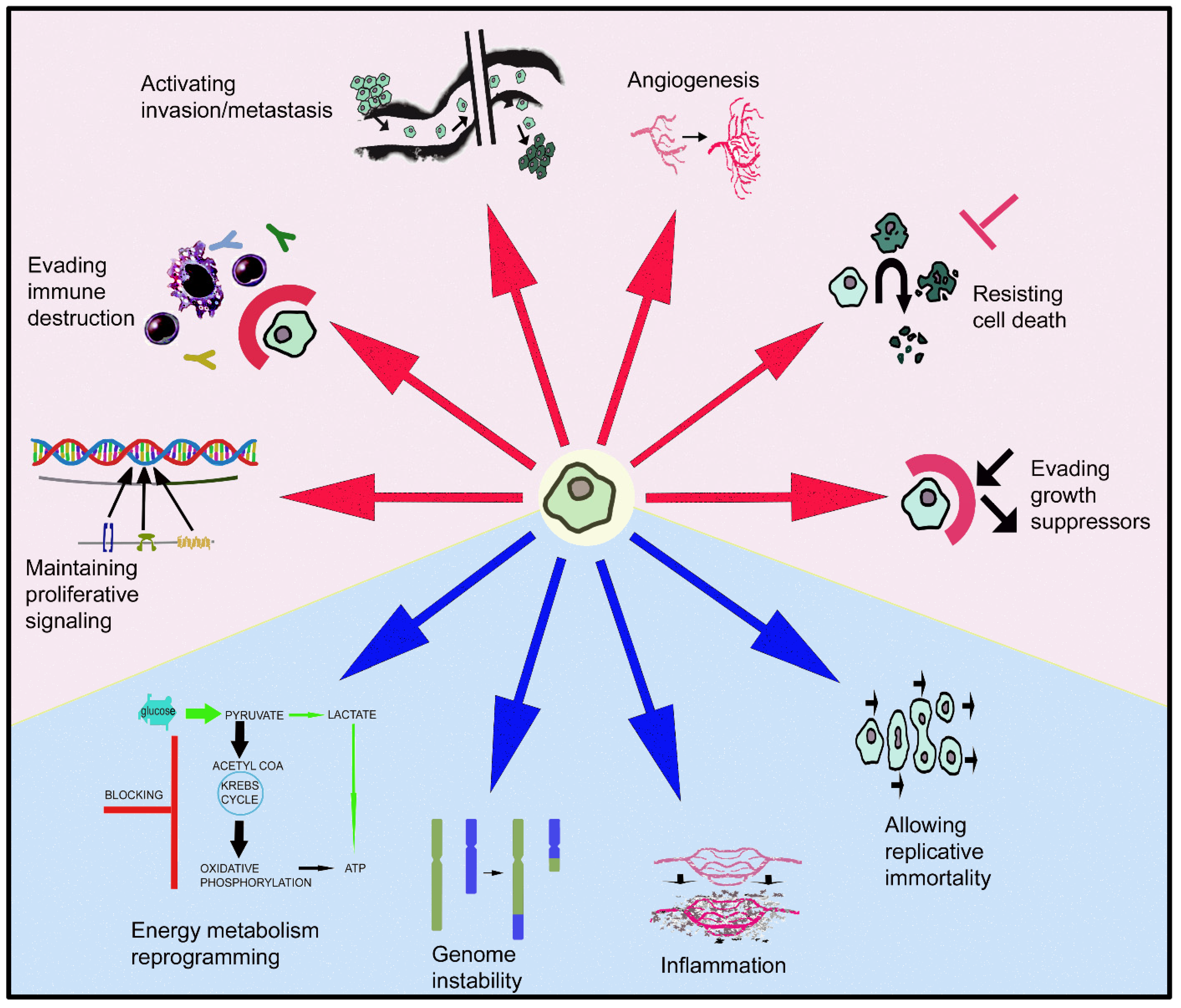
Figure 1. Ten keys of cellular/tissue behavior that make a cell a cancer cell, contrary to its normal biological destiny, leading to the formation of a primary tumor and later a secondary one. Red arrows show the involvement of the galaninergic system in these mechanisms: note that GAL is involved in six of them.
2. The Galaninergic System: Galanin and Its Receptors
GAL was discovered in porcine intestinal extracts and contains 29 amino acids [3]; however, in humans, the peptide contains 30 amino acid residues (Figure 2) and, unlike porcine GAL, the carboxy-terminus is not amidated [4][5][6]. The amino acid sequence of GAL is highly conserved among species (almost 90%) [7]. The C-terminus of GAL is involved in its receptor-binding affinity and the N-terminus is crucial for its biological activity [8]; the fifteen N-terminal residues of GAL are highly conserved throughout evolution [9]. GAL and other peptides (GAL message-associated peptide (GMAP), GAL-like peptide (GALP), alarin) belong to the GAL family of peptides. In addition, the peptide spexin (neuropeptide Q, 14 amino acids) is the most recently discovered member of this family; spexin has been shown to be involved in reproduction, nociception, renal function and energy homeostasis [10]. GALP, an endogenous ligand that activates the three known types of GALRs, was isolated from the porcine hypothalamus, contains 60 amino acids and is involved in reproduction and energy homeostasis [11][12]. Alarin (25 amino acids) is a splice variant of GALP mRNA [13]. The human chromosome 11q13.3-q13.5 contains the pre-pro-GAL gene-encoding GAL, which shows five introns and six exons, which in turn are translated into a pre-prohormone (123 amino precursor) containing the signal peptide, GAMP and GAL [6][14] (Figure 2). Some oncogenes have been located in the abovementioned region, which is also the breakpoint for the translocation t (11; 14) (q13; q32) in diffuse B-cell lymphoma and chronic lymphocytic leukemia [15]. The gene spans 6.5 kb and its first exon only encodes the 5′ untranslated sequence. In the pre-pro-GAL gene, its 5-prime flanking sequence shows a TATA box preceded by binding sites for transcription factors (e.g., NF-κB) and contains a CT-rich region that is flanked by two Alu repeats-, 2.3 kb upstream of the transcriptional start site; the region (500 bp) preceding this site contains 79% CG [16]. GALP and alarin are encoded by the pre-pro-GALP gene, which is located on the human chromosome 19q13.43 and comprises six exons [17]. The region encoding GALP is contained in exons 2–5 and alarin is formed when post-transcriptional splicing leads to the exclusion of exon 3, resulting in a frame shift and a novel precursor peptide [13].
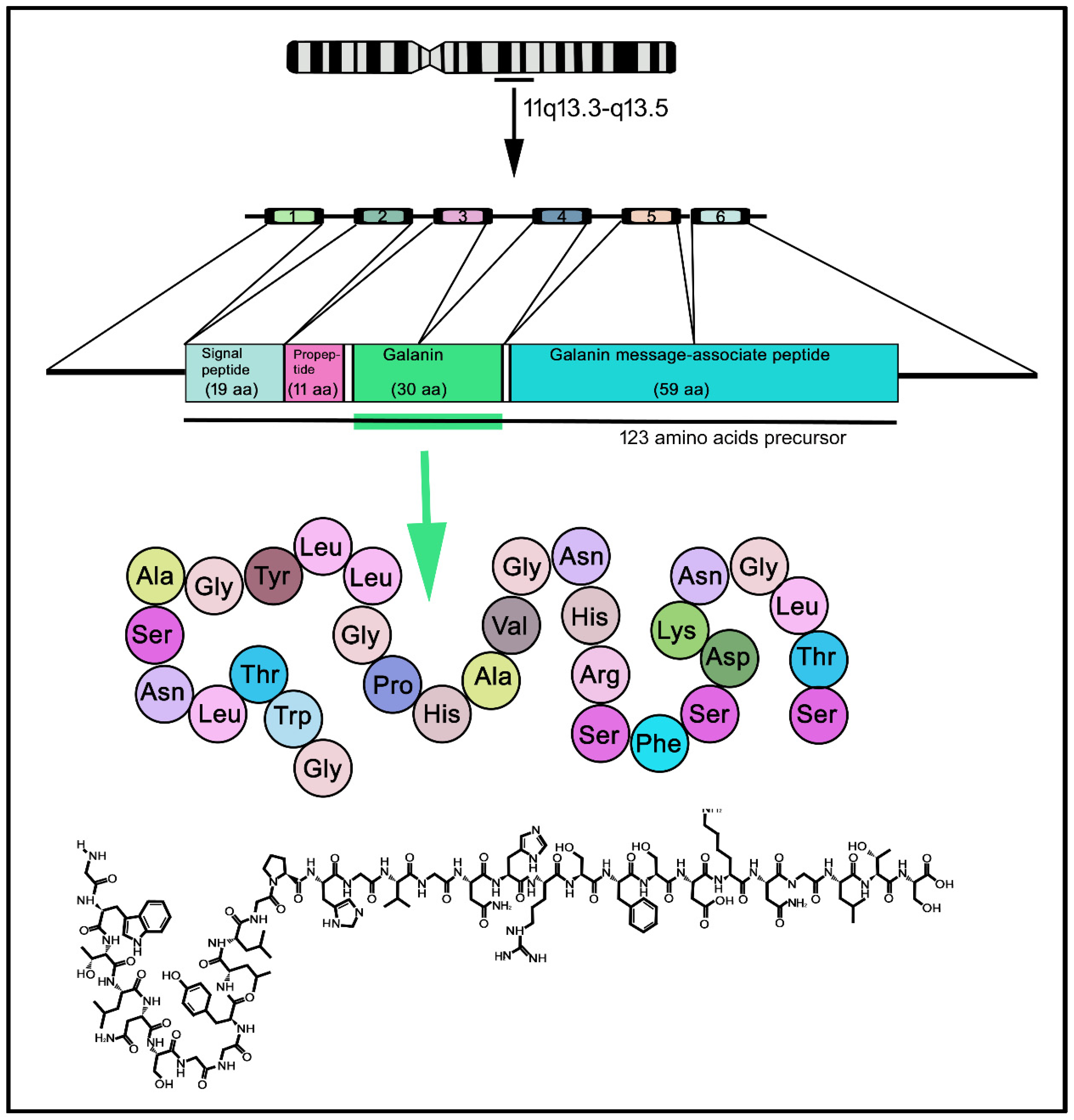
Figure 2. Transcription–maturation–translation processing of GAL, from human chromosome 11. Human GAL contains 30 amino acids residues. 1–6: exons; aa: amino acids.
The galaninergic system (GAL and GAL receptors (GALRs)) is widely distributed by the mammalian gastrointestinal tract, testis, ovary, uterus, kidney and heart, and by the immune, endocrine, peripheral and central nervous systems (e.g., endocrine pancreas, pituitary gland, paravertebral sympathetic ganglia, myenteric plexus, glial cells, dorsal root ganglion, spinal cord, brainstem, thalamus, hypothalamus, hippocampus, amygdala) [14][18][19][20][21][22][23][24][25]. The half-life of GAL in plasma is about five minutes and GAL coexists with many other neuroactive substances (e.g., enkephalin, vasopressin, calcitonin gene-related peptide, substance P, neuropeptide Y, cholecystokinin, growth hormone, luteinizing hormone-releasing hormone, dopamine, glutamate, noradrenalin, serotonin, acetylcholine) [18][26][27][28][29][30][31][32][33]. In general, GMAP in the rat central nervous system showed a similar profile of expression to GAL; however, GALP and alarin showed a more restricted expression than GAL [34]. Due to the widespread distribution of the galaninergic system by the whole body, GAL has been involved in many physiological actions after binding to specific G protein-coupled receptors: smooth muscle contraction, acetylcholine release inhibition, energy metabolism, food and water intake, hyperglycemia, osmotic and metabolic homeostasis, spinal reflexes, injury response, nociception, reproduction, memory, cognition, learning, arousal, sleep, neural growth, glucose-induced insulin release inhibition and respiratory, cardiovascular, neuroendocrine and gastrointestinal mechanisms [3][7][9][14][18][22][27][35][36][37][38][39][40]. Moreover, GAL regulates the level of growth hormone, prolactin, dopamine, pancreatic peptide, luteinizing hormone, luteinizing hormone-releasing hormone, somatostatin and insulin [7][31][41][42][43]. GAL acts as a neurotransmitter and neuromodulator in the central nervous system and the peptide has been involved in several diseases (e.g., anxiety, depression, stroke, alcoholism, Alzheimer’s disease, Parkinson’s disease, epilepsy); the galaninergic system also plays an important role in inflammatory bowel diseases and diabetes [7][9][14][44][45][46][47][48].
GALRs (GAL 1 receptor (GAL1R), GAL 2 receptor (GAL2R), GAL 3 receptor (GAL3R)) belong to the rhodopsin-like (class A) G protein-couple receptor family (seven transmembrane receptors or 7TM) [49]. They contain three extracellular loops, three intracellular loops, an extracellular N-terminus and three intercellular loops [49][50]. The helix 8 acts as a conformational switch at the C-terminus [51]. GALRs have sequence homologies in the transmembrane region: GAL1R-GAL3R (33%) and GAL2R-GAL3R (54%) [9], whereas human GAL3R and GAL2R respectively show 89% and 92% sequence homology with their receptor homologs present in the rat [52]. Human GAL has tens of nanomolar affinity at GAL3R, subnanomolar to nanomolar affinity at GAL2R and subnanomolar affinity at GAL1R [53]. Although the structure of GALRs is quite similar, different binding characteristics and intracellular signaling pathways have been reported after the activation of these receptors by ligands [49][50]. Thus, the lengths of the N-terminus (which plays an important role in the binding of ligands) and C-terminus are different in GALRs (C-terminus: GAL1R, 37 residues; GAL2R, 30; GAL3R, 13; N-terminus: GAL1R, 47 residues; GAL2R, 80; GAL3R, 62) [49]. The physiological actions of GAL are mediated by GAL1R, GAL2R and GAL3R; several signaling pathways are activated after the binding of GAL to these receptors: the stimulation of phospholipase C (PLC, mediated by GAL2R) or the inhibition of cyclic adenosine monophosphate (cAMP)/PKA (mediated by GAL1R/GAL3R) [15].
GAL1R was isolated from a human melanoma cell line [54]. It is coupled to Gβγ/Gαi signaling pathways and promotes, via a PKC-independent mechanism, the activation of mitogen-activated protein kinases (MAPKs) [6][55]. Moreover, the activation of GAL1R inhibited AC activity via an interaction with G-proteins (Gαi/αo), leading to G protein-coupled inwardly-rectifying potassium (GIRK) channels opening [21][54][56]. GAL1R activation can also inhibit the transcription factor cAMP regulatory element binding protein (CREB)-dependent signaling pathway [57], and the expression of GAL1R (but not that of GAL2R or GAL3R) was controlled by cAMP via CREB [58][59]. The GAL1R gene (located in chromosome 18q23) in humans shows three exons that are translated into a long protein containing 349 amino acids; GAL1R homology is high between species (e.g., in mouse, 93% of the residues are identical to those observed in humans) [60]. GAL1R has been located in the central (e.g., cortex, amygdala, hippocampus, thalamus, hypothalamus, locus coeruleus, medulla oblongata, spinal cord) and peripheral (e.g., dorsal root ganglion) nervous systems [22][23] and in the gastrointestinal tract [54][61].
GAL2R was first identified in the rat central nervous system [24][62][63] and was cloned in rat hypothalamic cells for the first time [24]. GAL2R contains His252/His253 (transmembrane domain 6) and Phe264/Tyr271 (extracellular loop 3) residues, which play a crucial role in the binding of ligands and in the activation of the receptor [64]. The sequence of human GAL2R shows a high homology with that observed in the rat (85–92%) and it was 39% identical to human GAL1R [22][52][65]. In the rat, GAL2R shows 38% amino acid identity with GAL1R [24]. In comparison with GAL1R, the distribution of GAL2R is more widespread since it has been observed in the nervous system (piriform cortex, dentate gyrus, amygdala, hypothalamus, mammillary nuclei, spinal cord), skeletal muscle, liver, testis, ovary, uterus, spleen, heart, kidney, lung, gastrointestinal tract and pituitary gland [22][24][52][66][67]. GAL2R mRNA expression has been reported in the neocortex, dentate gyrus, hypothalamus, cerebellar cortex, substantia nigra, vestibular complex and dorsal root ganglion [67][68][69].
GAL3R was first isolated from rat hypothalamic cDNA libraries [70]. Human GAL3R (368 amino acids long) shows 36% amino acids identity with human GAL1R, 58% with human GAL2R and 90% with rat GAL3R [52]. The distribution of GAL3R (olfactory cortex, hippocampus, hypothalamus, medulla oblongata) is more restricted than that reported in the brain for GAL1R or GAL2R [22][52][64][70][71][72]. GAL3R mRNA has been located in the amygdala, periaqueductal gray, locus coeruleus, brainstem reticular formation, spinal cord, pancreas, adrenal gland and testis [52][72]. GAL3R promotes the activation of Gαi/αo, blocking AC activity and opening GIRK channels [52][71]. Spexin binds to human GAL2/3Rs (not to GAL1R), exerting a higher potency toward GAL3R than GAL [10][73].
GAL agonists or antagonists (e.g., galantide, M35, M40, C7) have been used for the treatment of several disorders: GAL antagonists have been administered for the treatment of food intake disorders and Alzheimer’s disease, whereas GAL agonists have been used for the treatment of chronic pain [7][74]. Some fragments of GAL (GAL1-15; GAL1-16, GAL1-29), exerting physiological actions through GALRs (e.g., mood or cardiovascular regulation, alcohol intake), have been reported [75][76][77][78]. The conformational changes observed in GAL1R lead to a higher affinity of this receptor for GAL1-15 than for GAL, increasing the signaling (mediated by Gi/o) and decreasing AC activity and CREB level [79]. GALRs may form heteromers with each other and with other types of G protein-coupled receptors in the central nervous system [80]. Thus, the GAL1R/GAL2R heteroreceptor complex [79] and heteromers of GALRs with alpha2-adrenoceptors and 5-hydroxytryptamine (HT), dopamine 1, neuropeptide Y1 or Y2 receptors have been reported [9]. The formation of the heterotrimer GAL1R-GAL2R-5-HT1A receptor complex could explain why GAL1-15, but not GAL1-29, antagonistically moderated the serotonin receptor [80]. In addition, this heterotrimer has been suggested as a potential target to reverse the actions mediated by fluoxetine on memory mechanisms [75][81]. Thus, heteromers can alter the recognition of GAL ligands, and they are promising new targets for therapeutic interventions.
3. The Galaninergic System and Cancer
Peptides and their receptors are one of the molecular bases for the therapeutic targeting of tumors [82]. The galaninergic system is expressed in normal tissues and, in cancer cells, is involved in tumorigenesis, invasion and migration (metastasis) [19][25][28][82][83][84][85][86][87][88][89][90][91][92][93], although in some tumors, GAL and GALRs are silenced [94]. This system has been observed in neuroendocrine (e.g., phaeochromocytoma, pituitary adenoma, gangliocytoma, paraganglioma, neuroblastoma) and non-neuroendocrine (e.g., glioblastoma and other brain tumors, melanoma, basal cell carcinoma, head and neck squamous cell carcinoma, embryonic carcinoma, colon cancer, breast cancer, gastrointestinal cancer, prostate cancer) tumors [19][25][28][62][82][83][84][85][86][87][88][89][90][91][92][93][95][96][97][98][99][100][101][102]. For example, in squamous cell carcinoma, GAL1R was involved in tumor suppression and GAL2R favored tumor development and decreased survival [103][104]. GAL exerted a tumor-reducing effect in experimental murine models (gastrointestinal cancer), but in other models (adenoma formation), GAL promoted cell proliferation and tumor formation [82]. Thus, GAL can promote or inhibit the development of tumors; this is an important characteristic of the galaninergic system: to exert both proliferative and antiproliferative actions on tumor cells. Importantly, GAL/GALR expression has been correlated with tumor subtypes (colon carcinoma, squamous cell carcinoma, neuroblastic tumors, pituitary adenoma) or with tumor stage [82] and the activation of GAL1R was generally antiproliferative, whereas the activation of GAL2R showed antiproliferative or proliferative effects [82]. The stage and tumor size in colon cancer have been related to the GAL mRNA level: the higher the GAL expression, the shorter the disease-free survival [19][87].
3.1. Galanin and Neuroendocrine Tumors
Neuroendocrine tumors (NETs) are a very heterogeneous tumor group including: (1) carcinoid gastroenteropancreatic tumors; (2) non-carcinoid gastroenteropancreatic tumors (vasoactive intestinal peptide (VIP)oma, gastrinoma, insulinoma); (3) catecholamine-secreting tumors (neuroblastoma, sympathoblastoma, ganglioneuroblastoma, ganglioneuroma, paraganglioma, phaeochromocytoma); (4) chromophobe pituitary tumors; (5) medullary carcinoma of the thyroid; (6) Merkel cell tumors; and (7) small-cell lung cancer. NETs originate from neuroendocrine cells, which release peptides (e.g., GAL, somatostatin, pancreatic polypeptide, chromogranins) and express their corresponding receptors [105][106][107]. Thus, a high expression of peptidergic receptors has been reported in NETs for neurotensin, gastrin-releasing peptide, cholecystokinin, somatostatin and vasoactive intestinal peptide [106]. Importantly, the expression of the peptidergic systems in NETs has been correlated with prognosis and tumor stage [108].
Regarding the galaninergic system, many data demonstrated its involvement in NETs pathophysiology and carcinogenesis; for example, high doses of estrogens or dopamine agonists reversed rat pituitary hyperplasia and decreased the expression of GAL, suggesting that the peptide acted as a proliferative agent [109][110][111][112][113]. GAL expression is restricted to some NETs [88]: the peptide was observed in adrenal phaeochromocytoma (62%), jugulo tympanic paraganglioma (40%) and carotid body paraganglioma (18%), but it was not found in metastatic or recurrent paraganglioma, extra-adrenal phaeochromocytoma and carcinoid tumor [88][89]. Moreover, endocrine tumors from gastrointestinal tract, pancreas and lung did not show GAL [88]. This means that the utility of GAL as a diagnostic marker is limited to certain NETs. In this section, the involvement of the galaninergic system in those NETs (phaeochromocytoma, insulinoma, neuroblastic tumor, pituitary tumor, small-cell lung cancer) expressing this system will be summarized (Table 1).
Table 1. Involvement of the galaninergic system in neuroendocrine tumors.
| Cancer | Actions/Presence | References |
|---|---|---|
| Corticotroph adenoma Human |
- High GAL expression (RIA) | [83] |
| - GAL in 84% of tumors (IH) | [84] | |
| - GAL expression: smaller adenomas and better prognosis (IH) | [86] | |
| - GAL release and responded to corticotropin-releasing factor | [114] | |
| Ganglioneuroma Human |
- No correlation between prognosis/tumor markers and GAL level (RIA) | [115] |
| - GAL1R/GAL3R immunoreactivity decrease (IH) | [116] | |
| Insulinoma Rat Rin14B cell line |
- GAL1R expression (Northern blot, in situ hybridization) | [21] |
| Insulinoma Rat RINm5F cell line |
- GAL moderately suppressed insulin accumulation, but did not affect cell proliferation | [117] |
| - Pancreatic beta-cells: GAL inhibited adenylate cyclase activity and insulin secretion | [43] | |
| Insulinoma Mouse |
- Beta TC-1 cells: GAL, released from sympathetic nerve terminals, inhibited pro-insulin gene expression stimulated by glucagon-like peptide-I (Northern blot) | [118] |
| Neuroblastic tumors Human |
- GAL mRNA, GAL immunoreactivity and GAL binding sites expression (IH, in situ hybridization) | [116] |
| - Low level of GAL binding sites correlated with survival; GAL/GALR expression related to tumor differentiation stage (RIA, IH, in situ hybridization) | [115][116] | |
| Neuroblastoma Human |
- No correlation between prognosis/tumor markers and GAL concentration | [115] |
| - GAL expression; GAL2R mRNA was less common than GAL1R mRNA (IH, in situ hybridization) | [85] | |
| - GAL1R/GAL3R highly expressed; GAL promoted tumor growth (IH, in situ hybridization) | [116] | |
| Neuroblastoma Human IMR32 cell line |
- Dense core secretory vesicles: coexistence of GAL and beta-amyloid (IH) | [119] |
| Neuroblastoma Human SH-SY5Y cell line |
- GAL2R mediated apoptosis. GAL antiproliferative potency: 100-fold higher in SY5Y/GAL2R cells than in SY5Y/GAL1R cells | [120] |
| - GAL2R transfection: cell proliferation was blocked and caspase-dependent apoptotic mechanisms induced | [120] | |
| Neuroblastoma Rat B104 cell line |
- GAL, GAL2R and GAL3R mRNAs were detected, but not GAL1R mRNA (reverse transcription-PCR) | [121] |
| - GAL promoted cell proliferation | ||
| Paraganglioma Human |
- GAL expression (IH) | [89][93][122] |
| Paraganglioma Human carotid body |
- GAL was detected in 18% of tumors (IH) | [89] |
| Paraganglioma Human jugulo tympanic |
- GAL was detected in 40% of tumors (IH) | [89] |
| Phaeochromocytoma Human |
- High GAL2R mRNA expression (Western blot) | [123] |
| - Higher GAL concentration than in normal adrenal glands (RIA) | [124] | |
| Phaeochromocytoma Rat PC12 cell line |
- GAL inhibited cell proliferation and GAL1R, GAL2R and GAL3R mRNA expression, but not GAL mRNA (reverse transcription-PCR) | [121] |
| Pituitary adenoma Human |
- GAL/GALR expression correlated with tumor stage (IH) | [82] |
| Pituitary adenoma Human |
- High GAL3R levels found in some patients who relapsed shortly after surgical intervention (q-PCR) | [125] |
| Pituitary adenoma Rat |
- GAL promoted pituitary cell proliferation and tumor development | [27] |
| Pituitary adenoma Rat MtTW-10 cell line |
- Estradiol increased GAL mRNA level | [126] |
| Prolactinoma Rat |
- GAL concentration increased and GAL promoted tumor development | [127][128] |
| - Levonorgestrel decreased GAL mRNA expression and GAL-expressing cells (IH, in situ hybridization) | [129] | |
| Small-cell lung cancer Human H345, H510 cell lines |
- GAL, via GAL2R, mediated cell proliferation | [130][131] |
| Small-cell lung cancer Human H69, H510 cell lines |
- GAL, via GAL2R, activated G proteins and promoted cell proliferation | [130] |
| - GAL increased the levels of inositol phosphate and intracellular Ca2+ and promoted cell growth | [132] | |
| Small-cell lung cancer Human H345, H510 cell lines |
- Ca2+-mobilizing peptides (e.g., GAL) promoted cell growth. Broad spectrum antagonists directed against multiple Ca2+-mobilizing receptors inhibited cell growth | [131][133] |
| Small-cell lung cancer Human H69, H345, H510 cell lines |
- GAL, via the p42MAPK pathway, promoted cell growth. Protein kinase C inhibitors blocked cell growth induced by GAL | [134][135] |
| Small-cell lung cancer Human SBC-3A cell line, mouse SBC-3A tumor |
- SBC-3A cells secreted the pre-pro-GAL precursor which was extracellular processed to GAL1-20 by plasmin | [136][137] |
| Somatotroph adenoma Human |
- Low GAL level (RIA) | [83] |
| - GAL increased circulating growth hormone level and growth hormone-producing tumors expressed GAL (IH) | [138] | |
| - GAL blocked growth hormone release | [139] | |
| Somatotroph adenoma Rat GH1 cell line |
- GAL inhibited growth hormone release | [140] |
| Somatotroph adenoma Mouse |
- GAL mRNA level and peptide concentration increased | [127] |
| - GAL secretion increased | [141] | |
| Thyrotroph adenoma Rat |
- GAL gene expression blocked | [127] |
| Thyrotroph adenoma Mouse |
- GAL synthesis inhibited | [141] |
IH: immunohistochemistry; q-PCR: quantitative real time PCR; RIA: radioimmunoassay.
3.2. Galanin and Gastric Cancer
In nerve cells, the galaninergic system plays an important role in tumor development. In human stomach samples, obtained from the vicinity of invasive cancer cells, neurons located in the myenteric plexus showed a high expression of both caspases 3 and 8, but a low expression of GAL [34][142] (Table 2). In carcinoma-affected regions of the human stomach, an increase of the GAL-immunoreactive fibers in the longitudinal muscle layer, lamina muscularis mucosae and in the vicinity of the neoplastic proliferation was observed; thus, carcinoma invasion affected GAL stomach wall innervation [143]. In patients suffering from gastric cancer, lower levels of GAL were observed in pre-operative samples (and in plasma) when compared with those found in post-operative samples obtained from the same patients or from samples of healthy donors [144]. Moreover, the levels of GAL/GAL1R were lower in gastric cancer tissues compared with those found in adjacent regions; however, the GAL2R/GAL3R levels did not change [144]. The low level of GAL could be used as a biomarker in gastric cancer and, importantly, in these patients (pre-operative samples), the GAL protein/mRNA levels have been related to tumor size, tumor node metastasis stage and lymph node metastasis [144].
Table 2. Involvement of the galaninergic system in gastric and colorectal cancer.
| Actions/Presence | References | |
|---|---|---|
| Gastric Cancer | ||
| Human | - Fibers containing GAL: increased in longitudinal muscle layer, lamina muscularis mucosae and neoplastic proliferation vicinity (IH) | [143] |
| - Myenteric plexus: neurons showed a high expression of caspases 3/8 and low GAL expression (IH) | [143] | |
| - GAL/GAL1R level reduced | [144] | |
| - GAL2R/GAL3R level unchanged (RT-PCR) | [144] | |
| - Lower level of GAL in pre-operative samples (and plasma) when compared with that found in post-operative samples or in healthy donors. Gastric cancer tissues: GAL/GAL1R level was lower compared with that found in adjacent regions GAL2R/GAL3R: no change (Western blot; RT-PCR; ELISA) | [144] | |
| - GAL low level: used as biomarker. GAL protein/mRNA level related to tumor size, tumor node metastasis stage and lymph node metastasis | [144] | |
| Human Gastric cancer cell lines |
- GAL expression decreased: restored with a demethylating agent. GAL hypermethylation: impaired GAL tumor suppressor action. GAL downregulation: due to epigenetic inactivation (Q-MSP, Western blot) | [145] |
| - GAL: decreased cell proliferation | [146] | |
| Rats | - GAL blocked gastric carcinogenesis by inhibiting antral epithelial cell proliferation | [147] |
| Colorectal Cancer (CRC) | ||
| Human | - GAL/GAL1R silencing: apoptosis in drug-sensitive/resistant cell lines and enhanced the effects mediated by chemotherapy. GAL mRNA: overexpressed. High GAL level: related to poor disease-free survival of early-stage CRC patients (IH, ELISA, RT-PCR, Western blot) | [68][87][98][102] |
| - Enteric nervous system: number of neurons containing GAL increased in regions located close to the tumor (IH) (IH, RT-PCR, ELISA) | [35] | |
| - CRC patients: more GAL-immunoreactive neurons in comparison to healthy samples (IH, ELISA) | [102] | |
| - GAL in the vicinity of cancer cell invasion (IH, ELISA) | [102] | |
| - Blood samples: increased GAL concentration. High GAL level: cancer cells. Lowest GAL level: muscular layer placed distant from tumors. GAL: CRC tumor biomarker (ELISA, IH) | [148] | |
| - GAL mRNA level: related to adenocarcinoma size/stage. Correlation between higher GAL expression and shorter disease-free survival (RT-PCR) | [87][98] | |
| - CRC cells showed a high GAL expression: more malignant and involved in tumor recurrence. High GAL expression: spread of cancer stem cells (metastasis) (RT-PCR) | [149] | |
| - High GAL expression: associated with poor prognosis (stage II) and tumor recurrence. GAL expression: related to CRC aggressive behavior (RT-PCR) | [149] | |
| Human (tissue and cell lines) | - CRC cells/tissues: higher GAL levels than non-tumor cells/tissues | [87][98][148][149] |
| - CRC tissue: increased GAL gene/protein expression. CRC cell lines: GAL/GAL1R silencing promoted apoptosis. GAL1R silencing promoted FLIPL down-regulation (IH, ELISA, RT-PCR) | [87][98][102] | |
| Human HCT116 cell line |
- Cells overexpressing GAL2R were more chemosensitive to bevacizumab than control cells | [150] |
| Rat | - GAL decreased the incidence of colon tumors | [151] |
IH: immunohistochemistry; Q-MSP: quantitative methylation-specific PCR; RT-PCR: real time-PCR.
3.3. Galanin and Colorectal Cancer
Colorectal cancer (CRC), the third most prevalent cancer worldwide, is an invasive tumor process due to the proliferation of epithelial cells that acquire a neoplastic phenotype [35]. This process is known as epithelial-to-mesenchymal transition, in which epithelial cells lose many morphological and functional characteristics (e.g., shape, cell polarity, intercellular junctions) [35]. Tumor cells digest the extracellular matrix of the intestine wall, activating growth factors that promote cell proliferation, the blockade of apoptotic mechanisms and also favor the spreading of cancer cells [35]. Then, the invasion of cancer cells destroys the enteric nervous system, leading to the atrophy of the submucosal/myenteric plexuses. The galaninergic system is involved in colon cancer [87][98][102] (Table 2); thus, for example, the siRNA-mediated silencing of the GAL gene reduced both invasive and proliferative potential in CRC cells [98].
3.4. Galanin and Head and Neck Squamous Cell Carcinoma
Head and neck squamous cell carcinoma arises from mucosal surfaces of the head and neck [152] (Table 3). Perineural invasion (PNI), a mechanism of tumor dissemination via nerves, predicts poor survival in some cancers including head and neck squamous cell carcinoma (HNSCC), pancreatic cancer, stomach cancer and colon cancer, and is a sign of cancer cell invasion and metastasis [153]. An interaction between nerves and tumor cells occurs in PNI. PNI, mediated by molecular signals, promoted neuritogenesis and the survival, proliferation and invasion of tumor cells [154][155][156][157]. These cells are attracted to nerves and communicate with them. GAL (released from nerves) exerted a nerve–tumor crosstalk by activating GAL2R expressed in tumor cells and by inducing NFATC2-mediated transcription of cyclooxygenase-2 and GAL; then, GAL released from tumor cells promoted neuritogenesis, favoring PNI [154].
Table 3. Involvement of the galaninergic system in head and neck squamous cell carcinoma.
| Actions/Presence | References | |
|---|---|---|
| Human | - High GAL level (RT-PCR) | [101] |
| - GAL1R gene promoter: frequently methylated (Q-MSP) | [158] | |
| - Methylation status of some peptide-encoding genes, including GAL, is related with survival and recurrence. Methylation changes: possible molecular marker for HNSCC risk/prognosis (Q-MSP) | [159] | |
| - GAL/GALR epigenetic variants: markers for prognosis prediction (Q-MSP) | [160][161] | |
| - Poor survival: associated with methylation of GAL/GAL1R genes. Hypermethylation: inactivation of GAL/GAL1R/GAL2R genes (Q-MSP) | [162] | |
| Human Cell lines |
- Apoptosis: mediated by GAL2R but not by GAL1R. GAL1R/GAL2R: tumor suppressors in a p53-independent manner | [163] |
| - GAL2R transfection into HNSCC cells: cell proliferation inhibited. GAL2R re-expression: blocked cell proliferation (showing mutant p53) | [94][164][165] | |
| - GAL1R/GAL2R negative HNSCC cells: GAL1R re-expression suppressed tumor cell proliferation via ERK1/2-mediated actions on cyclin-dependent kinase inhibitors and cyclin D1 | [94][165] | |
| - GAL/GAL1R blocked HNSCC and oral tumor cell proliferation by cell-cycle arrest (RT-PCR, ELISA, Q-MSP) | [104][145][164][166] | |
| - GAL1R blocked tumor cell proliferation through the activation of ERK1/2 | [164] | |
| - GAL2R promoted an antitumor effect by inducing cell cycle arrest and apoptotic mechanisms (caspase 3-dependent) | [165] | |
| - GAL2R suppressed HNSCC cell viability. HEp-2 cells: GAL2R mediated apoptotic mechanisms (caspase-independent) by downregulating ERK1/2 and inducing Bim | [167] | |
| Human Cell lines, tumor samples |
- GAL2R overexpression: favored survival/proliferation by activating PI3K/Akt and MAPK/ERK-dependent pathways. Ras-related protein 1 (Rap1): involved in HNSCC progression. | [103] |
| - GAL/GAL1R: tumor suppressor. GAL1R absent in some cell lines (Q-MSP, RT-PCR) | [145][146][166] | |
| - GAL1R promoter: widely hypermethylated and related to reduced GAL1R expression. GAL1R/GAL2R hypermethylation: associated with higher recurrence rate and reduced disease-free survival (RT-PCR, Q-MSP) | [158][161][166][168] | |
| - GAL1R methylation status: potential biomarker for predicting clinical outcomes. Methylation: related to carcinogenesis and decreased GAL1R expression (RT-PCR, Q-MSP) | [160][161][166] | |
| Human (cell lines) Mouse |
- GAL (released from nerves) activated GAL2R expressed in tumor cells inducing NFATC2-mediated transcription of cyclooxygenase-2 and GAL. GAL released from tumor cells promoted neuritogenesis, favoring perineural invasion | [154] |
| Mouse | - GAL2R promoted tumor angiogenesis through the p38-MAPK-mediated inhibition of tristetraprolin (TTP), leading to an enhanced secretion of cytokines. GAL2R activated Ras-related protein 1b (Rap1B) favoring a p38-mediated inactivation of TTP, which acted as a destabilize cytokine transcript | [169] |
Q-MSP: quantitative methylation-specific PCR. RT-PCR: real-time PCR.
3.5. Galanin and Glioma
The GAL/GALR system has been described in glioma [19][99] in which the most abundant receptor observed was GAL1R, followed by GAL3R; GAL2R was not found (astrocytic/oligodendroglia tumors) [19] (Table 4). A reduced level of GAL has been observed in the cerebrospinal fluid of patients with glioblastoma [170], and regarding the expressions of GAL and GAL3R, no correlation with oligodendroglial, astrocytic and mixed neural–glial tumors was reported [19]. Moreover, no correlation was observed between the proliferative activity and GAL/GAL binding levels [99]. However, the high-grade glioma (WHO grade IV) has been related to the expression of GAL3R [19]. GAL has been reported in gliosarcoma and glioblastoma multiforme [99]; in the latter, the most abundant receptor found was GAL1R, followed by GAL3R and GAL2R [99]. In glioma, endothelial and immune (e.g., macrophages, neutrophils) cells expressed GAL3R, but GAL1R/GAL2R were not observed around the blood vessels [19]. This means that tumor-associated cells are involved in tumor microenvironment homeostasis. Glioma-associated macrophages (GAMs) are involved in tumor progression; although macrophages produce/secrete GAL, GAMs do not express GAL, but express GAL3R, and this means that GAL could regulate the activity of GAMs [171][172].
Table 4. Involvement of the galaninergic system in glioma.
| Actions/Presence | References | |
|---|---|---|
| Human | - GAL/GAL3R expression: no correlation with oligodendroglial, astrocytic and mixed neural–glial tumors | [19] |
| - High-grade glioma (WHO grade IV): related to GAL3R expression | [19] | |
| - Endothelial/immune cells: GAL3R expression. Around blood vessels: GAL1R/GAL2R not observed (IH) | [19] | |
| - GAL1R, followed by GAL3R; GAL2R absent (astrocytic/oligodendroglia tumors) (IH, autoradiography, reverse transcription-PCR) | [19][99] | |
| - Glioma-associated macrophages: GAL3R expression (quantitative PCR) | [171][172] | |
| - No correlation between proliferative activity and GAL/GAL binding levels (IH, autoradiography, reverse transcription-PCR) | [99] | |
| - Cerebrospinal fluid (glioblastoma): reduced GAL level | [170] | |
| Human Mice |
- GAL blocked, via GAL1R, the proliferation of glioma cells and tumor growth. These effects were mediated through ERK1/2 signal activation. No cytotoxic/apoptotic effect was observed | [173] |
IH: immunohistochemistry.
3.6. Galanin and Other Cancers
Although the expressions of GAL and pre-pro-GAL mRNA have been reported in breast cancer, it has been suggested that the GALN gene (which encodes the pre-pro-GAL protein) is an unlikely candidate oncogene in breast tumors because an increase in pre-pro-GAL mRNA expression with GALN amplification was not observed [82][174] (Table 5). Many nerve fibers containing GAL have been reported in cardiac and esophageal carcinomas [175]; these fibers contacted closely with cancer cells, including those encircling tumor cells. GAL favored the extension of processes by dorsal root ganglion neurons, but the action of the peptide on tumor cells is currently unknown [175]. GAL1R DNA methylation is among the most epigenetic molecular alterations in endometrial cancer; this methylation indicates malignancy with a high degree of sensitivity and specificity [176]. The methylation of the GAL1R gene in bladder cancer has been involved in the prognosis of the disease, but the role played by the galaninergic system in this cancer is currently unknown [177].
Table 5. Involvement of the galaninergic system in other cancers.
| Actions/Presence | References | |
|---|---|---|
| Breast cancer Human |
- GAL/pre-pro-GAL mRNA level expression. GALN gene: unlike candidate oncogene (Northern blot) | [82][174] |
| Carcinoma (cardiac, esophageal) Human |
- Fibers containing GAL contacted closely with cancer cells (IH) | [175] |
| Endometrial cancer Human |
- GAL1R DNA methylation indicated malignancy (q-PCR) | [176] |
| Bladder cancer Human |
- GAL1R gene methylation involved in prognosis | [177] |
| Salivary duct carcinoma Human |
- GAL1R/GAL2R: therapeutic targets/prognostic factors. GAL1R/GAL2R methylation rates correlated with overall survival decrease (IH, Q-MSP) | [178] |
| Melanoma Human |
- GAL/GAL1R expression (IH) | [82][100] |
| Pancreas Human |
- GAL promoted SW1990 cell proliferation | [179] |
| Pancreas Rat |
- GAL blocked carcinogenesis and decreased norepinephrine level (IH, HPLC) | [180] |
HPLC: high-performance liquid chromatography; IH: immunohistochemistry; Q-MSP: quantitative methylation-specific PCR; q-PCR: quantitative real-time PCR.
4. The Galaninergic System and Cancer: Signaling Pathways
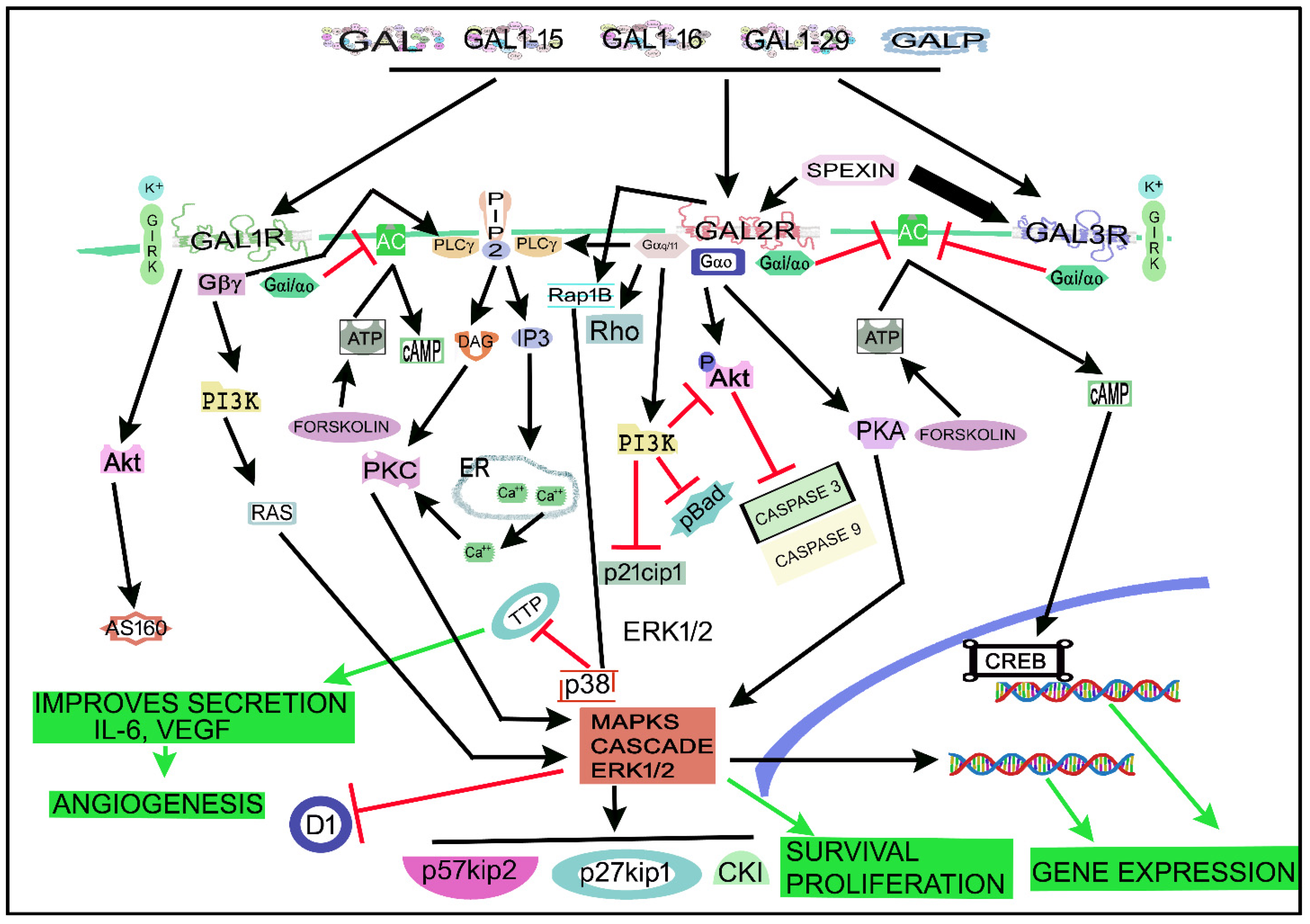
Figure 3 shows the main signaling pathways in which the galaninergic system is involved. A GAL/GALR signaling network map focused on the signaling cascades regulated by the galaninergic system has recently been published [181]. GALRs (via PKC) activate the rat sarcoma virus (Ras, a small GTPase)/MAPK/ERK pathway by increasing the intracellular Ca2+ concentration [35]. The galaninergic system activates many signal transduction pathways depending on the coupled G protein type: GAL1R/GAL3R, mainly coupled to Gi/o, decrease the cAMP level and inactivate PKA, whereas GAL2R, preferably coupled to Gq/11 mobilizing intracellular Ca2+, promotes (via PKC) the activation of cell survival (via Akt or PKB) and MAPK1/MAPK3-dependent cell proliferation pathways [6][19][181]. GAL1R can also be coupled to Gβγ- and/or Gi-signaling pathways and then the activation of MAPKs occurs in a Ras/Raf-dependent manner [6][14][181]. GAL1R activation also favors the Akt/Akt substrate of the 160 kDa (AS160) cascade [181], regulates GIRK channels [64][182] and activates the ERK1/2 signal through the Gα/i subunit and not via the PI3K pathway linked to the Gβγ subunit [164]. GAL1R induces cell-cycle control proteins (p27kip1, p57kip2) and suppresses cyclin D1 in cancer cells [9]. GAL2R, mainly coupled to Gq/11, mediated the activation of PLC and small GTPase proteins in the Rho family [181]. PLC converted phosphatidylinositol, 4, 5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphate (IP3), which mediated PKC activation and increased the intracellular concentration of Ca2+ [64]. GAL2R activated the small GTPase protein Rho A in SCLC cells, suggesting the coupling to G12/13 [9]. GAL2R inhibited the production of cAMP, meaning that the receptor was coupled to Gi protein [183]. GAL2R decreased cofilin activation and Rho and Cdc42 GTPase activity [9]. In tumor cells, GAL2R activated the MAPK/ERK pathway in a PKC manner, meaning that GAL2R was coupled to a Go protein [9]. GAL2R regulated cell-cycle control proteins (p27kip1, p57kip2) and cyclin D1 and promoted apoptosis (caspase 3-dependent) in HNSCC cells [15]. GAL2R decreased the expression of p21cip1, phosphorylated BAD forms (pBad) and phosphorylated Akt (pAkt), downstream of the Gq11/PI3K pathway [15]. The GAL-mediated Akt pathway blocked the activity of caspases 3 and 9, whereas the GAL2R-mediated apoptosis in tumor cells was induced by the activation of the pro-apoptotic Bcl-2 protein Bim, through a mechanism independent of caspase [9]. GAL3R, involved in inward potassium ion (K+) currents, is coupled to the Gi/o signaling pathway and its activation favored the inhibition of cAMP and AC altering CREB phosphorylation [6][71][181]. GAL opened adenosine triphosphate (ATP)-sensitive K+ channels and hyperpolarized cell membranes in the rat RINm5F insulinoma cell line [184], and the peptide blocked the activity of AC and the secretion of insulin via the interaction with Gαi1, Gαi2 and Gαi3 proteins [43][185]. C7 peptide (GAL1-13-spantide amide), a GAL receptor antagonist, blocked hepatocellular carcinoma metastasis by targeting the hepatocyte growth factor/c-mesenchymal–epithelial transition receptor axis signaling pathway [186]. C7 inhibited the migration and invasion of tumor cells by blocking the phosphorylation of Akt and ERK1/2 [186].

Figure 3. Main signaling pathways in which the galaninergic system is involved. Black arrows indicate activation pathways, inverted red “T” indicates blockade/suppression, green arrows mean final results. AC, adenylate cyclase; Akt, Akt serine/threonine kinase family (also called PKB); AS160, Akt substrate of 160 kDa; ATP, adenosine triphosphate; Ca2+, calcium ion; cAMP, cyclic adenosine monophosphate; CKI, cyclin-dependent kinase inhibitor 1; CREB, cAMP regulatory element-binding protein; D1, a cyclin protein; DAG, diacylglycerol; ER, endoplasmic reticulum; FORSKOLIN, enzyme that produces cyclic adenosine monophosphate; GAL, galanin; GAL1-15 fragment, galanin 1–15 fragment; GAL1-16, galanin 1–16 fragment; GAL1-29, galanin 1–29 fragment; GAL1R, galanin receptor 1; GAL2R, galanin receptor 2; GAL3R, galanin receptor 3; GALP: GAL-like peptide; GIRK, G protein-coupled inwardly-rectifying potassium; Gα/11, G protein alpha subunit (11); Gαi/αo, G protein alpha i/o subunits; Gαo, G protein alpha subunit (o); Gβγ, G protein beta-gamma subunit; IL-6, interleukin 6; IP3, inositol triphosphate; K+, potassium ion; MAPK, mitogen-activated protein kinases cascade; p21cip1, a cyclin-dependent kinase inhibitor; p27kip1, cell-cycle control protein; p38, a class of mitogen-activated protein kinase; p57kip2, cell-cycle control protein; pAkt, phosphorylated Akt; pBad, phosphorylated BAD forms (induces apoptosis by inhibiting antiapoptotic BCL-2 family members); PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol bisphosphate; PIP2, phosphatidylinositol, 4, 5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; Rap1B, Ras-related protein Rap-1b; Ras, rat sarcoma virus (a small GTPase); Rho, a family of small signaling G proteins (a subfamily of the Ras superfamily); TTP, tristetraprolin; VEGF, vascular endothelial growth factor.
5. Therapeutic Strategies
Peptides play an important role in cancer; the in-depth knowledge of the functions mediated by these substances is an emerging and promising line of research that could lead to new clinical applications in oncology. One line of research could be the use of peptides coupled to cytotoxic agents to exert an antitumor action, and another, the use of peptide receptor antagonists or agonists. In the case of GAL, GALR antagonists or agonists could be used as antitumor treatments according to the different signaling pathways and actions mediated by GALRs. GALR antagonists have been administered for the treatment of food intake disorders, anxiety, depression and Alzheimer’s disease, whereas GALR agonists have been used for the treatment of chronic pain [7][74]. It has also been reported that SNAP 37889, a non-peptidergic GAL3R antagonist, promoted apoptosis in promyelocytic leukemia cells expressing GAL2R [187].
In vitro and in vivo experiments using human gastric cancer cell lines have been performed to study the antitumor action of a triple treatment with GAL, serotonin and octreotide (an octapeptide that mimics the actions mediated by somatostatin) [146]. Treatment with one compound or with a double/triple combination decreased cell proliferation and viability in vitro, and tumor volume/weight was reduced in vivo after the triple treatment. However, this reduction was not due to apoptosis or cell proliferation inhibition; thus, other unknown mechanisms were involved [146]. In experimental animals, implanted human colon cancer cells were treated with the triple treatment (octreotide, serotonin and GAL were administered subcutaneously or intraperitoneally) [188][189][190]: tumor volume/weight, number of viable cells, proliferation index and tumor vascularization decreased, whereas the apoptotic index increased. In nude mice implanted with colonic adenocarcinoma cells and treated with the triple treatment, the tumor volume decreased and the apoptotic index and volume density of the tumor necrotic tissue increased [191]. The triple therapy did not show any apparent side effects [192].
6. Conclusions
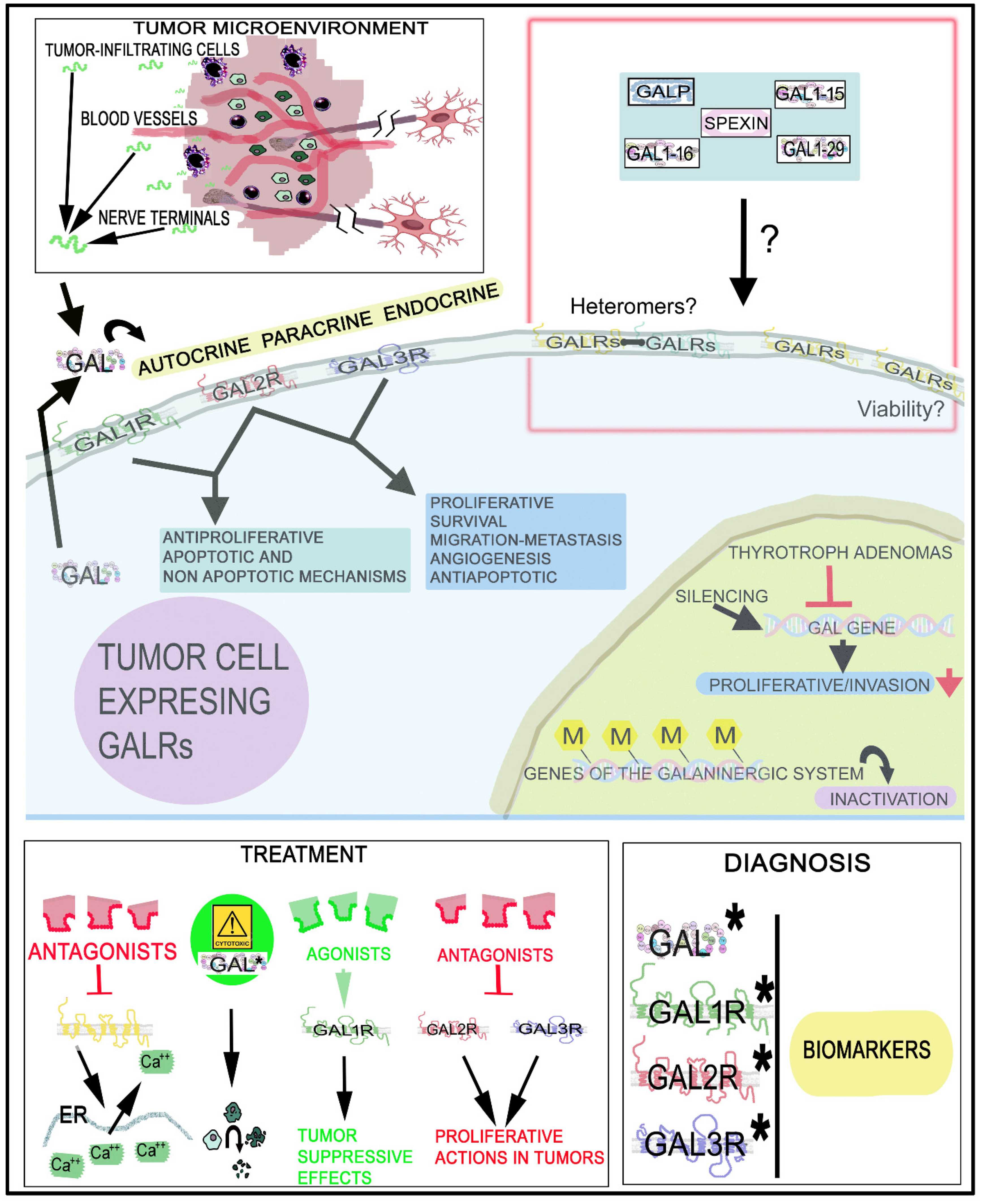
The galaninergic system is involved in tumorigenesis, invasion and migration and has been correlated with tumor stage/subtypes and metastasis and, in this system, epigenetic mechanisms have been related with carcinogenesis and recurrence rates. GALRs play a crucial role in cancer and their specific actions must be clearly understood in many tumor types because GALRs mediate different signal transduction pathways and actions depending on the tumor cell type and the particular G protein involved. GALRs could be used as a therapeutic target and diagnostic marker for the treatment, prognosis and surgical outcome in certain tumors. Different from other peptidergic systems, the galaninergic system exerts a proliferative action on tumor cells, but GAL also suppresses the development of tumor. Thus, in-depth studies using GALRs agonists or antagonists as antitumor agents must be conducted to search for therapeutic strategies (alone or in combination with chemotherapy/radiotherapy) against tumor development. The involvement of the galaninergic system in cancer is a line of research that has been abandoned, but it must be re-opened and developed in the future. Additional studies must be carried out, for example, on the use of GALR agonists/antagonists as antitumor agents, the activation of signaling transduction pathways, the involvement of heteromers, targeted radionuclide cancer therapy and the viability of GALRs. This knowledge is crucial to establish future potential clinical antitumor applications, although unfortunately, the pharmaceutical industry has generally had no interest in this line of research; however, the data reported here suggest that the galaninergic system is a promising target for the treatment of tumors (Figure 4).

Figure 4. Involvement of the GAL/GALR system in cancer, diagnosis and treatment. GAL1R/GAL2R mediate an antiproliferative effect, whereas GAL2R/GAL3R promote a proliferative action on tumor cells. GAL originates from tumor cells, tumor-infiltrating cells and nerve cells. Circulating GAL can also bind to GALRs. ↑: increase; ↓: decrease; ?: mechanisms that must be investigated (presence/functions of heteromers in tumor cells, involvement of GALRs in the viability of cancer cells and involvement of GAL fragments and other peptides belonging to the GAL family of peptides in cancer). *, biomarkers; M: methylation.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674.
- Tatemoto, K.; Rökaeus, Å.; Jörnvall, H.; McDonald, T.J.; Mutt, V. Galanin-a Novel Biologically Active Peptide from Porcine Intestine. FEBS Lett. 1983, 164, 124–128.
- Bersani, M.; Johnsen, A.H.; Højrup, P.; Dunning, B.E.; Andreasen, J.J.; Holst, J.J. Human Galanin: Primary Structure and Indentification of Two Molecular Forms. FEBS Lett. 1991, 283, 189–194.
- Schmidt, W.E.; Kratzin, H.; Eckart, K.; Drevs, D.; Mundkowski, G.; Clemens, A.; Katsoulis, S.; Schäfer, H.; Gallwitz, B.; Creutzfeldt, W. Isolation and Primary Structure of Pituitary Human Galanin, a 30-Residue Nonamidated Neuropeptide. Proc. Natl. Acad. Sci. USA 1991, 88, 11435–11439.
- Chen, D.; Kodama, Y.; Kulseng, B.; Johannessen, H.; Zhao, C. Galanin. In Handbook of BiologicallyActive Peptides; Kastin, A.J., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 1210–1218.
- Maria, E.; Vrontakis, B.S.P. Galanin: A Biologically Active Peptide. Curr. Drug Target-CNS Neurol. Disord. 2002, 1, 531–541.
- Katsoulis, S.; Clemens, A.; Morys-Wortmann, C.; Schwörer, H.; Schaube, H.; Klomp, H.-J.; Fölsch, U.R.; Schmidt, W.E. Human Galanin Modulates Human Colonic Motility in Vitro Characterization of Structural Requirements. Scand. J. Gastroenterol. 1996, 31, 446–451.
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol. Rev. 2015, 67, 118–175.
- Tran, A.; He, W.; Chen, J.T.C.; Belsham, D.D. Spexin: Its Role, Regulation, and Therapeutic Potential in the Hypothalamus. Pharmacol. Ther. 2022, 233, 108033.
- Ohtaki, T.; Kumano, S.; Ishibashi, Y.; Ogi, K.; Matsui, H.; Harada, M.; Kitada, C.; Kurokawa, T.; Onda, H.; Fujino, M. Isolation and CDNA Cloning of a Novel Galanin-like Peptide (GALP) from Porcine Hypothalamus. J. Biol. Chem. 1999, 274, 37041–37045.
- Lawrence, C.; Fraley, G.S. Galanin-like Peptide (GALP) Is a Hypothalamic Regulator of Energy Homeostasis and Reproduction. Front. Neuroendocrinol. 2011, 32, 1–9.
- Santic, R.; Fenninger, K.; Graf, K.; Schneider, R.; Hauser-Kronberger, C.; Schilling, F.H.; Kogner, P.; Ratschek, M.; Jones, N.; Sperl, W.; et al. Gangliocytes in Neuroblastic Tumors Express Alarin, a Novel Peptide Derived by Differential Splicing of the Galanin-Like Peptide Gene. J. Mol. Neurosci. 2006, 29, 145–152.
- Marcos, P.; Coveñas, R. Neuropeptidergic Control of Feeding: Focus on the Galanin Family of Peptides. Int. J. Mol. Sci. 2021, 22, 2544.
- Evans, H.; Baumgartner, M.; Shine, J.; Herzog, H. Genomic Organization and Localization of the Gene Encoding Human Preprogalanin. Genomics 1993, 18, 473–477.
- Kofler, B.; Evans, H.F.; Liu, M.L.; Falls, V.; Iismaa, T.P.; Shine, J.; Herzog, H. Characterization of the 5′-Flanking Region of the Human Preprogalanin Gene. DNA Cell Biol. 1995, 14, 321–329.
- Cunningham, M.J.; Scarlett, J.M.; Steiner, R.A. Cloning and Distribution of Galanin-Like Peptide MRNA in the Hypothalamus and Pituitary of the Macaque. Endocrinology 2002, 143, 755–763.
- Landry, M.; Åman, K.; Dostrovsky, J.; Lozano, A.M.; Carlstedt, T.; Spenger, C.; Josephson, A.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Galanin Expression in Adult Human Dorsal Root Ganglion Neurons: Initial Observations. Neuroscience 2003, 117, 795–809.
- Falkenstetter, S.; Leitner, J.; Brunner, S.M.; Rieder, T.N.; Kofler, B.; Weis, S. Galanin System in Human Glioma and Pituitary Adenoma. Front. Endocrinol. 2020, 11, 1–14.
- Anselmi, L.; Stella, S.L.; Lakhter, A.; Hirano, A.; Tonini, M. Catia Sternini Galanin Receptors in the Rat Gastrointestinal Tract. Neuropeptides 2005, 39, 349–352.
- Parker, E.M.; Izzarelli, D.G.; Nowak, H.P.; Mahle, C.D.; Iben, L.G.; Wang, J.; Goldstein, M.E. Cloning and Characterization of the Rat GALR1 Galanin Receptor from Rin14B Insulinoma Cells. Mol. Brain Res. 1995, 34, 179–189.
- Waters, S.M.; Krause, J.E. Distribution of Galanin-1, -2 and -3 Receptor Messenger RNAs in Central and Peripheral Rat Tissues. Neuroscience 1999, 95, 265–271.
- Gustafson, E.L.; Smith, K.E.; Durkin, M.M.; Gerald, C.; Branchek, T.A. Distribution of a Rat Galanin Receptor MRNA in Rat Brain. NeuroReport 1996, 7, 953–957.
- Smith, K.E.; Forray, C.; Walker, M.W.; Jones, K.A.; Tamm, J.A.; Bard, J.; Branchek, T.A.; Linemeyer, D.L.; Gerald, C. Expression Cloning of a Rat Hypothalamic Galanin Receptor Coupled to Phosphoinositide Turnover. J. Biol. Chem. 1997, 272, 24612–24616.
- Bennet, W.M.; Hill, S.F.; Ghatei, M.A.; Bloom, S.R. Galanin in the Normal Human Pituitary and Brain and in Pituitary Adenomas. J. Endocrinol. 1991, 130, 463–467.
- Zhang, X.; Verge, V.M.K.; Wiesenfeld-Hallin, Z.; Piehl, F.; Hökfelt, T. Expression of Neuropeptides and Neuropeptide MRNAs in Spinal Cord after Axotomy in the Rat, with Special Reference to Motoneurons and Galanin. Exp. Brain Res. 1993, 93, 450–461.
- Perumal, P.; Vrontakis, M. Transgenic Mice Over-Expressing Galanin Exhibit Pituitary Adenomas and Increased Secretion of Galanin, Prolactin and Growth Hormone. J. Endocrinol. 2003, 179, 145–154.
- Hacker, G.W.; Bishop, A.E.; Terenghi, G.; Varndell, I.M.; Aghahowa, J.; Pollard, K.; Thurner, J.; Polak, J.M. Multiple Peptide Production and Presence of General Neuroendocrine Markers Detected in 12 Cases of Human Phaeochromocytoma and in Mammalian Adrenal Glands. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 399–411.
- Melander, T.; Hijkfelt, T.; Riikaeus, A. Coexistence of Galanin-like Lmmunoreactivity with Catecholamines, 5=Hydroxytryptamine, GABA and Neuropeptides in the Rat CNS. J. Neurosci. 1986, 6, 15.
- Xu, Z.-Q.D.; Shi, T.-J.S.; Hökfelt, T. Galanin/GMAP- and NPY-like Immunoreactivities in Locus Coeruleus and Noradrenergic Nerve Terminals in the Hippocampal Formation and Cortex with Notes on the Galanin-R1 and -R2 Receptors. J. Comp. Neurol. 1998, 392, 227–251.
- Merchenthaler, I.; Lopez, F.J.; Negro-Vilar, A. Colocalization of Galanin and Luteinizing Hormone-Releasing Hormone in a Subset of Preoptic Hypothalamic Neurons: Anatomical and Functional Correlates. Proc. Natl. Acad. Sci. USA 1990, 87, 6326–6330.
- Zhang, X.; Nicholas, A.P.; Ho¨kfelt, T. Ultrastructural Studies on Peptides in the Dorsal Horn of the Spinal Cord—I. Co-Existence of Galanin with Other Peptides in Primary Afferents in Normal Rats. Neuroscience 1993, 57, 365–384.
- Zhang, X.; Nicholas, A.P.; Hökfelt, T. Ultrastructural Studies on Peptides in the Dorsal Horn of the Rat Spinal Cord—II. Co-Existence of Galanin with Other Peptides in Local Neurons. Neuroscience 1995, 64, 875–891.
- Gundlach, A.L.; Ryan, P.J. Galanin and GALP. In Handbook of BiologicallyActive Peptides; Kastin, A.J., Ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 766–775.
- Godlewski, J.; Kmiec, Z. Colorectal Cancer Invasion and Atrophy of the Enteric Nervous System: Potential Feedback and Impact on Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3391.
- McKnight, G.L.; Karlsen, A.E.; Kowalyk, S.; Mathewes, S.L.; Sheppard, P.O.; O’Hara, P.J.; Taborsky, G.J. Sequence of Human Galanin and Its Inhibition of Glucose-Stimulated Insulin Secretion From RIN Cells. Diabetes 1992, 41, 82–87.
- Hökfelt, T.; Tatemoto, K. Galanin—25 Years with a Multitalented Neuropeptide. Cell. Mol. Life Sci. 2008, 65, 1791–1795.
- Scott, M.K.; Ross, T.M.; Lee, D.H.S.; Wang, H.-Y.; Shank, R.P.; Wild, K.D.; Davis, C.B.; Crooke, J.J.; Potocki, A.C.; Reitz, A.B. 2,3-Dihydro-Dithiin and -Dithiepine-1,1,4,4-Tetroxides: Small Molecule Non-Peptide Antagonists of the Human Galanin HGAL-1 Receptor. Bioorg. Med. Chem. 2000, 8, 1383–1391.
- Hobson, S.-A.; Bacon, A.; Elliot-Hunt, C.R.; Holmes, F.E.; Kerr, N.C.H.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin Acts as a Trophic Factor to the Central and Peripheral Nervous Systems. Cell. Mol. Life Sci. 2010, 102, 25–38.
- Elliott-Hunt, C.R.; Marsh, B.; Bacon, A.; Pope, R.; Vanderplank, P.; Wynick, D. Galanin Acts as a Neuroprotective Factor to the Hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 5105–5110.
- Quynh, N.T.T.; Islam, S.M.; Florén, A.; Bartfai, T.; Langel, Ü.; Östenson, C.-G. Effects of Galnon, a Non-Peptide Galanin-Receptor Agonist, on Insulin Release from Rat Pancreatic Islets. Biochem. Biophys. Res. Commun. 2005, 328, 213–220.
- Giustina, A.; Schettino, M.; Bodini, C.; Doga, M.; Licini, M.; Giustina, G. Effect of Galanin on the Growth Hormone Response to Growth Hormone-Releasing Hormone in Acromegaly. Metabolism 1992, 41, 1291–1294.
- Mazancourt, P.D.; Goldsmith, P.K.; Weinstein, L.S. Inhibition of Adenylate Cyclase Activity by Galanin in Rat Insulinoma Cells Is Mediated by the G-Protein G13. Biochem. J. 1994, 303, 369–375.
- Guipponi, M.; Chentouf, A.; Webling, K.E.B.; Freimann, K.; Crespel, A.; Nobile, C.; Lemke, J.R.; Hansen, J.; Dorn, T.; Lesca, G.; et al. Galanin Pathogenic Mutations in Temporal Lobe Epilepsy. Hum. Mol. Genet. 2015, 24, 3082–3091.
- Webling, K.E.B.; Runesson, J.; Bartfai, T.; Langel, Ü. Galanin Receptors and Ligands. Front. Endocrinol. 2012, 3, 1–14.
- Chan-Palay, V. Galanin Hyperinnervates Surviving Neurons of the Human Basal Nucleus of Meynert in Dementias of Alzheimer’s and Parkinson’s Disease: A Hypothesis for the Role of Galanin in Accentuating Cholinergic Dysfunction in Dementia. J. Comp. Neurol. 1988, 273, 543–557.
- Wiesenfeld-Hallin, Z.; Villar, M.J.; Ho¨kfelt, T. The Effects of Intrathecal Galanin and C-Fiber Stimulation on the Flexor Reflex in the Rat. Brain Res. 1989, 486, 205–213.
- Wang, P.; Li, H.; Barde, S.; Zhang, M.-D.; Sun, J.; Wang, T.; Zhang, P.; Luo, H.; Wang, Y.; Yang, Y.; et al. Depression-like Behavior in Rat: Involvement of Galanin Receptor Subtype 1 in the Ventral Periaqueductal Gray. Proc. Natl. Acad. Sci. USA 2016, 113, 4726–4735.
- Jurkowski, W.; Yazdi, S.; Elofsson, A. Ligand Binding Properties of Human Galanin Receptors. Mol. Membr. Biol. 2013, 30, 206–216.
- Probst, W.C.; Snyder, L.A.; Schuster, D.I.; Brosius, J.; Sealfon, S.C. Sequence Alignment of the G-Protein Coupled Receptor Superfamily. DNA Cell Biol. 1992, 11, 1–20.
- Krishna, A.G.; Menon, S.T.; Terry, T.J.; Sakmar, T.P. Evidence That Helix 8 of Rhodopsin Acts as a Membrane-Dependent Conformational Switch. Biochemistry 2002, 41, 8298–8309.
- Kolakowski, L.F.; O’Neill, G.P.; Howard, A.D.; Broussard, S.R.; Sullivan, K.A.; Feighner, S.D.; Sawzdargo, M.; Nguyen, T.; Kargman, S.; Shiao, L.-L.; et al. Molecular Characterization and Expression of Cloned Human Galanin Receptors GALR2 and GALR3. J. Neurochem. 2002, 71, 2239–2251.
- Lang, R.; Gundlach, A.; Kofler, B. The Galanin Peptide Family: Receptor Pharmacology, Pleiotropic Biological Actions, and Implications in Health and Disease. Pharmacol. Ther. 2007, 115, 177–207.
- Habert-Ortoli, E.; Amiranoff, B.; Loquet, I.; Laburthe, M.; Mayaux, J.F. Molecular Cloning of a Functional Human Galanin Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 9780–9783.
- Wang, S.; Hashemi, T.; Fried, S.; Clemmons, A.L.; Hawes, B.E. Differential Intracellular Signaling of the GalR1 and GalR2 Galanin Receptor Subtypes. Biochemistry 1998, 37, 6711–6717.
- Wang, S.; He, C.; Maguire, M.T.; Clemmons, A.L.; Burrier, R.E.; Guzzi, M.F.; Strader, C.D.; Parker, E.M.; Bayne, M.L. Genomic Organization and Functional Characterization of the Mouse GalR1 Galanin Receptor. FEBS Lett. 1997, 411, 225–230.
- Badie-Mahdavi, H.; Lu, X.; Behrens, M.M.; Bartfai, T. Role of Galanin Receptor 1 and Galanin Receptor 2 Activation in Synaptic Plasticity Associated with 3′,5′-Cyclic AMP Response Element-Binding Protein Phosphorylation in the Dentate Gyrus: Studies with a Galanin Receptor 2 Agonist and Galanin Receptor 1 Knockout Mice. Neuroscience 2005, 133, 591–604.
- Zachariou, V.; Georgescu, D.; Kansal, L.; Merriam, P.; Picciotto, M.R. Galanin Receptor 1 Gene Expression Is Regulated by Cyclic AMP through a CREB-Dependent Mechanism: Galanin Receptor I Gene Expression. J. Neurochem. 2008, 76, 191–200.
- Hawes, J.J.; Brunzell, D.H.; Wynick, D.; Zachariou, V.; Picciotto, M.R. GalR1, but Not GalR2 or GalR3, Levels Are Regulated by Galanin Signaling in the Locus Coeruleus through a Cyclic AMP-Dependent Mechanism: GalR Regulation in the Locus Coeruleus. J. Neurochem. 2005, 93, 1168–1176.
- Jacoby, A.S.; Webb, G.C.; Liu, M.L.; Kofler, B.; Hort, Y.J.; Fathi, Z.; Bottema, C.D.K.; Shine, J.; Iismaa, T.P. Structural Organization of the Mouse and Human GALR1 Galanin Receptor Genes (GalnrandGALNR) and Chromosomal Localization of the Mouse Gene. Genomics 1997, 45, 496–508.
- Lorimer, D.D.; Benya, R.V. Cloning and Quantification of Galanin-1 Receptor Expression by Mucosal Cells Lining the Human Gastrointestinal Tract. Biochem. Biophys. Res. Commun. 1996, 222, 379–385.
- Sullivan, K.A.; Shiao, L.-L.; Cascieri, M.A. Pharmacological Characterization and Tissue Distribution of the Human and Rat GALR1 Receptors. Biochem. Biophys. Res. Commun. 1997, 233, 823–828.
- Howard, A.D.; Tan, C.; Shiao, L.-L.; Palyha, O.C.; McKee, K.K.; Weinberg, D.H.; Feighner, S.D.; Cascieri, M.A.; Smith, R.G.; Van Der Ploeg, L.H.T.; et al. Molecular Cloning and Characterization of a New Receptor for Galanin. FEBS Lett. 1997, 405, 285–290.
- Demsie, D.G.; Altaye, B.M.; Weldekidan, E.; Gebremedhin, H.; Alema, N.M.; Tefera, M.M.; Tilahun, A. Galanin Receptors as Drug Target for Novel Antidepressants: Review. Biol. Targets Ther. 2020, 14, 37–45.
- Bloomquist, B.T.; Beauchamp, M.R.; Zhelnin, L.; Brown, S.-E.; Gore-Willse, A.R.; Gregor, P.; Cornfield, L.J. Cloning and Expression of the Human Galanin Receptor GalR2. Biochem. Biophys. Res. Commun. 1998, 243, 474–479.
- Mitchell, V.; Bouret, S.; Howard, A.D.; Beauvillain, J.-C. Expression of the Galanin Receptor Subtype Gal-R2 MRNA in the Rat Hypothalamus. J. Chem. Neuroanat. 1999, 16, 265–277.
- O’Donnell, D.; Ahmad, S.; Wahlestedt, C.; Walker, P. Expression of the Novel Galanin Receptor Subtype GALR2 in the Adult Rat CNS: Distinct Distribution from GALR. J. Comp. Neurol. 1999, 409, 2.
- Colucci, R.; Blandizzi, C.; Ghisu, N.; Florio, T.; Del Tacca, M. Somatostatin Inhibits Colon Cancer Cell Growth through Cyclooxygenase-2 Downregulation: Somatostatin and Cyclooxygenase-2 in Colon Cancer. Br. J. Pharmacol. 2008, 155, 198–209.
- Mons, N.; Decorte, L.; Jaffard, R.; Cooper, D. Ca2+-Sensitive Adenylyl Cyclases, Key Integrators of Cellular Signalling. Life Sci. 1998, 62, 1647–1652.
- Wang, S.; He, C.; Hashemi, T.; Bayne, M. Cloning and Expressional Characterization of a Novel Galanin Receptor. J. Biol. Chem. 1997, 272, 31949–31952.
- Smith, K.E.; Walker, M.W.; Artymyshyn, R.; Bard, J.; Borowsky, B.; Tamm, J.A.; Yao, W.-J.; Vaysse, P.J.-J.; Branchek, T.A.; Gerald, C.; et al. Cloned Human and Rat Galanin GALR3 Receptors. J. Biol. Chem. 1998, 273, 23321–23326.
- Mennicken, F.; Hoffert, C.; Pelletier, M.; Ahmad, S.; O’Donnell, D. Restricted Distribution of Galanin Receptor 3 (GalR3) MRNA in the Adult Rat Central Nervous System. J. Chem. Neuroanat. 2002, 24, 257–268.
- Kim, D.-K.; Yun, S.; Son, G.H.; Hwang, J.-I.; Park, C.R.; Kim, J.I.; Kim, K.; Vaudry, H.; Seong, J.Y. Coevolution of the Spexin/Galanin/Kisspeptin Family: Spexin Activates Galanin Receptor Type II and III. Endocrinology 2014, 155, 1864–1873.
- Kask, K.; Berthold, M.; Bourne, J.; Andell, S.; Langel, Ü.; Bartfai, T. Binding and Agonist/Antagonist Actions of M35, Galanin(1-13)-Bradykinin(2-9) Amide Chimeric Peptide, in Rin m 5F Insulinoma Cells. Regul. Pept. 1995, 59, 341–348.
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Gago, B.; Santín, L.; Castilla-Ortega, E.; Narváez, J.Á.; Fuxe, K.; Díaz-Cabiale, Z. The Neuropeptides Galanin and Galanin (1–15) in Depression-like Behaviours. Neuropeptides 2017, 64, 39–45.
- Díaz-Cabiale, Z.; Parrado, C.; Vela, C.; Razani, H.; Coveñas, R.; Fuxe, K.; Narváez, J.A. Role of Galanin and Galanin (1–15) on Central Cardiovascular Control. Neuropeptides 2005, 39, 185–190.
- Millón, C.; Flores-Burgess, A.; Castilla-Ortega, E.; Gago, B.; García-Fernandez, M.; Serrano, A.; Rodriguez de Fonseca, F.; Narváez, J.A.; Fuxe, K.; Santín, L.; et al. Central Administration of Galanin N-Terminal Fragment 1–15 Decreases the Voluntary Alcohol Intake in Rats: Galanin (1–15) and Alcohol. Addict. Biol. 2019, 24, 76–87.
- Díaz-Cabiale, Z.; Parrado, C.; Narváez, M.; Millón, C.; Puigcerver, A.; Fuxe, K.; Narváez, J.A. The Galanin N-Terminal Fragment (1–15) Interacts with Neuropeptide Y in Central Cardiovascular Control: Involvement of the NPY Y2 Receptor Subtype. Regul. Pept. 2010, 163, 130–136.
- Borroto-Escuela, D.O.; Narvaez, M.; Di Palma, M.; Calvo, F.; Rodriguez, D.; Millon, C.; Carlsson, J.; Agnati, L.F.; Garriga, P.; Díaz-Cabiale, Z.; et al. Preferential Activation by Galanin 1–15 Fragment of the GalR1 Protomer of a GalR1–GalR2 Heteroreceptor Complex. Biochem. Biophys. Res. Commun. 2014, 452, 347–353.
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Calvo, F.; Garriga, P.; Tena, M.; Narvaez, M.; Millón, C.; Parrado, C.; et al. On the Existence and Function of Galanin Receptor Heteromers in the Central Nervous System. Front. Endocrinol. 2012, 3, 127.
- Flores-Burgess, A.; Millón, C.; Gago, B.; García-Durán, L.; Cantero-García, N.; Coveñas, R.; Narváez, J.A.; Fuxe, K.; Santín, L.; Díaz-Cabiale, Z. Galanin (1–15)-Fluoxetine Interaction in the Novel Object Recognition Test. Involvement of 5-HT1A Receptors in the Prefrontal Cortex of the Rats. Neuropharmacology 2019, 155, 104–112.
- Rauch, I.; Kofler, B. The Galanin System in Cancer. In Galanin; Experientia Supplementum; Hökfelt, T., Ed.; Springer Basel: Basel, Switzerland, 2010; Volume 102, pp. 223–241. ISBN 978-3-0346-0227-3.
- Grenbäck, E.; Bjellerup, P.; Wallerman, E.; Lundblad, L.; Änggård, A.; Ericson, K.; Åman, K.; Landry, M.; Schmidt, W.E.; Hökfelt, T.; et al. Galanin in Pituitary Adenomas. Regul. Pept. 2004, 117, 127–139.
- Hsu, D.W.; Hooi, S.C.; Tessa, E.; Strauss, R.M.; Kaplan, L.M. Coexpression of Galanin and Adrenocorticotropic Hormone in Human Pituitary and Pituitary Adenomas. Am. J. Pathol. 1991, 138, 13.
- Tuechler, C.; Hametner, R.; Jones, N.; Jones, R.; Iismaa, T.P.; Sperl, W.; Kofler, B. Galanin and Galanin Receptor Expression in Neuroblastoma a. Ann. N. Y. Acad. Sci. 1998, 863, 438–441.
- Leung, B.; Lismaa, T.P.; Leung, K.-C.; Hort, Y.J.; Turner, J.; Sheehy, J.P.; Ho, K.K.Y. Galanin in Human Pituitary Adenomas: Frequency and Clinical Significance. Clin. Endocrinol. 2002, 56, 397–403.
- Kim, K.Y.; Kee, M.K.; Chong, S.A.; Nam, M.J. Galanin Is Up-Regulated in Colon Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2373–2378.
- Sano, T.; Vrontakis, M.E.; Kovacs, K.; Asa, S.L.; Friesen, H.G. Galanin Immunoreactivity in Neuroendocrine Tumors. Arch. Pathol. Lab. Med. 1991, 1, 926–929.
- Tadros, T.S.; Strauss, R.M.; Cohen, C.; Gal, A.A. Galanin Immunoreactivity in Paragangliomas but Not in Carcinoid Tumors. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 250–252.
- Hulting, A.-L.; Meister, B.; Grimetlius, L.; Wersäll, J.; Änggård, A.; Hökfelt, T. Production of a Galanin-like Peptide by a Human Pituitary Adenoma: ImmunohistochemicaI Evidence. Acta Physiol. Scand. 1989, 137, 561–562.
- Vrontakis, M.E.; Sano, T.; Kovacs, K.; Friesen, H.G. Presence of Galanin-Like Immunoreactivity in Nontumorous Corticotrophs and Corticotroph Adenomas of the Human Pituitary. J. Clin. Endocrinol. Metab. 1990, 70, 747–751.
- Felix, I.; Bilbao, J.M.; Asa, S.L.; Tyndel, F.; Kovacs, K.; Becker, L.E. Cerebral and Cerebellar Gangliocytomas: A Morphological Study of Nine Cases. Acta Neuropathol. 1994, 88, 246–251.
- Fried, G.; Wikström, L.-M.; Höög, A.; Arver, S.; Cedermark, B.; Hamberger, B.; Grimelius, L.; Meister, B. Multiple Neuropetide Immunoreactivities in a Renin-Producing Human Paraganglioma. Cancer 1994, 74, 142–151.
- Kanazawa, T.; Kommareddi, P.K.; Iwashita, T.; Kumar, B.; Misawa, K.; Misawa, Y.; Jang, I.; Nair, T.S.; Iino, Y.; Carey, T.E. Galanin Receptor Subtype 2 Suppresses Cell Proliferation and Induces Apoptosis in P53 Mutant Head and Neck Cancer Cells. Clin. Cancer Res. 2009, 15, 2222–2230.
- Skotheim, R.I.; Lind, G.E.; Monni, O.; Nesland, J.M.; Abeler, V.M.; Fosså, S.D.; Duale, N.; Brunborg, G.; Kallioniemi, O.; Andrews, P.W.; et al. Differentiation of Human Embryonal Carcinomas In Vitro and In Vivo Reveals Expression Profiles Relevant to Normal Development. Cancer Res. 2005, 65, 5588–5598.
- Kepron, C.; Reis, P.; Bharadwaj, R.; Shaw, J.; Kamel-Reid, S.; Ghazarian, D. Identification of Genomic Predictors of Non-Melanoma Skin Cancer in Solid Organ Transplant Recipients. Eur. J. Dermatol. 2009, 19, 278–280.
- Berger, A.; Santic, R.; Hauser-Kronberger, C.; Schilling, F.H.; Kogner, P.; Ratschek, M.; Gamper, A.; Jones, N.; Sperl, W.; Kofler, B. Galanin and Galanin Receptors in Human Cancers. Neuropeptides 2005, 39, 353–359.
- Stevenson, L.; Allen, W.L.; Turkington, R.; Jithesh, P.V.; Proutski, I.; Stewart, G.; Lenz, H.-J.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Identification of Galanin and Its Receptor GalR1 as Novel Determinants of Resistance to Chemotherapy and Potential Biomarkers in Colorectal Cancer. Clin. Cancer Res. 2012, 18, 5412–5426.
- Berger, A.; Santic, R.; Almer, D.; Hauser-Kronberger, C.; Huemer, M.; Humpel, C.; Stockhammer, G.; Sperl, W.; Kofler, B. Galanin and Galanin Receptors in Human Gliomas. Acta Neuropathol. 2003, 105, 555–560.
- Gilaberte, Y.; Vera, J.; Coscojuela, C.; Roca, M.J.; Parrado, C.; González, S. Expression of Galanin in Melanocytic Tumors. Actas Dermo-Sifiliogr. Engl. Ed. 2007, 98, 24–34.
- Sugimoto, T.; Seki, N.; Shimizu, S.; Kikkawa, N.; Tsukada, J.; Shimada, H.; Sasaki, K.; Hanazawa, T.; Okamoto, Y.; Hata, A. The Galanin Signaling Cascade Is a Candidate Pathway Regulating Oncogenesis in Human Squamous Cell Carcinoma. Genes. Chromosomes Cancer 2009, 48, 132–142.
- Godlewski, J.; Pidsudko, Z. Characteristic of Galaninergic Components of the Enteric Nervous System in the Cancer Invasion of Human Large Intestine. Ann. Anat.-Anat. Anz. 2012, 194, 368–372.
- Banerjee, R.; Henson, B.S.; Russo, N.; Tsodikov, A.; D’Silva, N.J. Rap1 Mediates Galanin Receptor 2-Induced Proliferation and Survival in Squamous Cell Carcinoma. Cell. Signal. 2011, 23, 1110–1118.
- Henson, B.S.; Neubig, R.R.; Jang, I.; Ogawa, T.; Zhang, Z.; Carey, T.E.; D’Silva, N.J. Galanin Receptor 1 Has Anti-Proliferative Effects in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2005, 280, 22564–22571.
- Barakat, M.T.; Meeran, K.; Bloom, S.R. Neuroendocrine Tumours. Endocr. Relat. Cancer 2004, 11, 1–18.
- Reubi, J.C. Regulatory Peptide Receptors as Molecular Targets for Cancer Diagnosis and Therapy. Q. J. Nucl. Med. 1997, 41, 63–70.
- Wood, S.M.; Polak, J.M.; Bloom, S.R. Gut Hormone Secreting Tumours. Scand. J. Gastroenterol. 1983, 82, 165–179.
- Kogner, P. Neuropeptides in Neuroblastomas Andganglioneuromas. Prog. Brain Res. 1995, 104, 325–338.
- Gregg, D.W.; Galkin, M.; Gorski, J. Effect of Estrogen on the Expression of Galanin MRNA in Pituitary Tumor-Sensitive and Tumor-Resistant Rat Strains. Steroids 1996, 61, 468–472.
- Hsu, D.W.; El-Azouzi, M.; Black, P.M.; Chin, W.W.; Hedley-Whyte, E.T.; Kaplan, L.M. Estrogen Increases Galanin Immunoreactivity in Hyperplastic Prolactin-Secreting Cells in Fisher 344 Rats. Endocrinology 1990, 126, 3159–3167.
- Hyde, J.F.; Howard, G. Regulation of Galanin Gene Expression in the Rat Anterior Pituitary Gland by the Somatostatin Analog SMS 201-995. Endocrinology 1992, 131, 2097–2102.
- Wynick, D.; Hammond, P.J.; Akinsanya, K.O.; Bloom, S.R. Galanin Regulates Basal and Oestrogen-Stimulated Lactotroph Function. Nature 1993, 364, 529–532.
- Wynick, D.; Small, C.J.; Bacon, A.; Holmes, F.E.; Norman, M.; Ormandy, C.J.; Kilic, E.; Kerr, N.C.H.; Ghatei, M.; Talamantes, F.; et al. Galanin Regulates Prolactin Release and Lactotroph Proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 12671–12676.
- Invitti, C.; Giraldi, F.P.; Dubini, A.; Moroni, P.; Losa, M.; Piccoletti, R.; Cavagnini, F. Galanin Is Released by Adrenocorticotropin-Secreting Pituitary Adenomas In Vivo and In Vitro. J. Clin. Endocrinol. Metab. 1999, 84, 1351–1356.
- Berger, A.; Tuechler, C.; Almer, D.; Kogner, P.; Ratschek, M.; Kerbl, R.; Iismaa, T.P.; Jones, N.; Sperl, W.; Kofler, B. Elevated Expression of Galanin Receptors in Childhood Neuroblastic Tumors. Neuroendocrinology 2002, 75, 130–138.
- Perel, Y.; Amrein, L.; Dobremez, E.; Rivel, J.; Daniel, J.Y.; Landry, M. Galanin and Galanin Receptor Expression in Neuroblastic Tumours: Correlation with Their Differentiation Status. Br. J. Cancer 2002, 86, 117–122.
- Sjöholm, Å. Regulation of Insulinoma Cell Proliferation and Insulin Accumulation by Peptides and Second Messengers. Ups. J. Med. Sci. 1995, 100, 201–216.
- Fehmann, H.C.; Habener, J.F. Galanin Inhibits Proinsulin Gene Expression Stimulated by the Insulinotropic Hormone Glucagon-like Peptide-I(7-37) in Mouse Insulinoma Beta TC-1 Cells. Endocrinology 1992, 130, 2890–2896.
- Toneff, T.; Funkelstein, L.; Mosier, C.; Abagyan, A.; Ziegler, M.; Hook, V. Beta-Amyloid Peptides Undergo Regulated Co-Secretion with Neuropeptide and Catecholamine Neurotransmitters. Peptides 2013, 46, 126–135.
- Berger, A.; Lang, R.; Moritz, K.; Santic, R.; Hermann, A.; Sperl, W.; Kofler, B. Galanin Receptor Subtype GalR2 Mediates Apoptosis in SH-SY5Y Neuroblastoma Cells. Endocrinology 2004, 145, 500–507.
- Cheng, S.; Yuan, C.-G. Differential Effect of Galanin on Proliferation of PC12 and B104 Cells. NeuroReport 2007, 18, 1379–1383.
- Squillaci, S.; Gal, A.A. Galanin Immunoreactivity in a Laryngealparaganglioma: Case Report and Literature Review. Pathologica 2004, 96, 111–116.
- Tofighi, R.; Joseph, B.; Xia, S.; Xu, Z.-Q.D.; Hamberger, B.; Hökfelt, T.; Ceccatelli, S. Galanin Decreases Proliferation of PC12 Cells and Induces Apoptosis via Its Subtype 2 Receptor (GalR2). Proc. Natl. Acad. Sci. USA 2008, 105, 2717–2722.
- Bauer, F.E.; Hacker, G.W.; Terenghi, G.; Adrian, T.E.; Polak, J.M.; Bloom, S.R. Localization and Molecular Forms of Galanin in Human Adrenals: Elevated Levels in Pheochromocytomas. J. Clin. Endocrinol. Metab. 1986, 63, 1372–1378.
- Tofighi, R.; Barde, S.; Palkovits, M.; Höög, A.; Hökfelt, T.; Ceccatelli, S.; Hulting, A.-L. Galanin and Its Three Receptors in Human Pituitary Adenoma. Neuropeptides 2012, 46, 195–201.
- Hyde, J.F.; Morrison, D.G.; Moore, J.P.; Howard, G. MtTW-10 Pituitary Tumor Cells: Galanin Gene Expression and Peptide Secretion. Endocrinology 1993, 133, 2588–2593.
- Hyde, J.F.; Moore, J.P.; Drake, K.W.; Morrison, D.G. Galanin Gene Expression in Radiothyroidectomy-Induced Thyrotroph Adenomas. Am. J. Physiol.-Endocrinol. Metab. 1996, 271, E24–E30.
- Vrontakis, M.E.; Peden, L.M.; Duckworth, M.L.; Friesen, H.G. Isolation and Characterization of a Complementary DNA (Galanin) Clone from Estrogen-Induced Pituitary Tumor Messenger RNA. J. Biol. Chem. 1987, 262, 16755–16758.
- Piroli, G.G.; Cassataro, J.; Pietranera, L.; Grillo, C.A.; Ferrini, M.; Lux-Lantos, V.; De Nicola, A.F. Progestin Regulation of Galanin and Prolactin Gene Expression in Oestrogen-Induced Pituitary Tumours: Progestin Regulation of Galanin Expression in Prolactinomas. J. Neuroendocrinol. 2001, 13, 302–309.
- Wittau, N.; Grosse, R.; Kalkbrenner, F.; Gohla, A.; Schultz, G.; Gudermann, T. The Galanin Receptor Type 2 Initiates Multiple Signaling Pathways in Small Cell Lung Cancer Cells by Coupling to Gq, Gi and G12 Proteins. Oncogene 2000, 19, 4199–4209.
- Sethi, T.; Rozengurt, E. Multiple Neuropeptides Stimulate Clonal Growth of Small Cell Lung Cancer: Effects of Bradykinin, Vasopressin, Cholecystokinin, Galanin, and Neurotensin. Cancer Res. 1991, 51, 3621–3623.
- Sethi, T.; Rozengurt’, E. Galanin Stimulates Ca2+Mobilization, Inositol Phosphate Accumulation, and Clonal Growth in Small Cell Lung Cancer Cells. Cancer Res. 1991, 51, 1674–1679.
- Sethi, T.; Langdon, S.; Smyth, J.; Rozengurt, E. Growth of Small Cell Lung Cancer Cells: Stimulation by Multiple Neuropeptides and Inhibition by Broad Spectrum Antagonists In Vitro and In Vivo. Cancer Res. 1992, 25 (Suppl. 9), 2737S–2743S.
- Seufferlein, T.; Rozengurt, E. Galanin, Neurotensin, and Phorbol Esters Rapidly Stimulate Activation of Mitogen-Activated Protein Kinase in Small Cell Lung Cancer Cells. Cancer Res. 1996, 56, 5758–5764.
- Gudermann, T.; Roelle, S. Calcium-Dependent Growth Regulation of Small Cell Lung Cancer Cells by Neuropeptides. Endocr. Relat. Cancer 2006, 13, 1069–1084.
- Yamamoto, H.; Ben, S.; Saitoh, S.; Kamata, K.; Iguchi, K.; Hoshino, M. Plasmin: Its Role in the Extracellular Processing of Progalanin in Tumor Tissue. Protein Pept. Lett. 2011, 18, 1204–1211.
- Yamamoto, H.; Iguchi, K.; Ohno, S.; Yokogawa, T.; Nishikawa, K.; Hoshino, M. Activation of Large Form Galanin-LI by Extracellular Processing in Small Cell Lung Carcinoma Tissue. Protein Pept. Lett. 2011, 18, 1058–1064.
- Hulting, A.-L.; Land, T.; Berthold, M.; Langel, Ü.; Hökfelt, T.; Bartfai, T. Galanin Receptors from Human Pituitary Tumors Assayed with Human Galanin as Ligand. Brain Res. 1993, 625, 173–176.
- Giustina, A.; Ragni, G.; Bollati, A.; Cozzi, R.; Licini, M.; Poiesi, C.; Turazzi, S.; Bonfanti, C. Inhibitory Effects of Galanin on Growth Hormone (GH) Release in Cultured GH-Secreting Adenoma Cells: Comparative Study with Octreotide, GH-Releasing Hormone, and Thyrotropin-Releasing Hormone. Metabolism 1997, 46, 425–430.
- Giustina, A.; Bonfanti, C.; Licini, M.; De Rango, C.; Milani, G. Inhibitory Effect of Galanin on Growth Hormone Release from Rat Pituitary Tumor Cells (GH1) in Culture. Life Sci. 1994, 55, 1845–1851.
- Hyde, J.F.; Moore, J.P.; Cai, A. Galanin in Normal and Hyperplastic Anterior Pituitary Cells: From Pituitary Tumor Cell Lines to Transgenic Mice. Ann. N. Y. Acad. Sci. 1998, 863, 48–55.
- Kozłowska, A.; Kozera, P.; Majewski, M.; Godlewski, J. Co-Expression of Caspase-3 or Caspase-8 with Galanin in the Human Stomach Section Affected by Carcinoma. Apoptosis 2018, 23, 484–491.
- Kozłowska, A.; Godlewski, J.; Majewski, M. Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. Int. J. Mol. Sci. 2018, 19, 3357.
- Zhang, L.; Fang, P.; Chai, C.; Shao, L.; Mao, H.; Qiao, D.; Kong, G.; Dong, X.; Shi, M.; Zhang, Z.; et al. Galanin Expression Is Down-Regulated in Patients with Gastric Cancer. J. Int. Med. Res. 2019, 47, 1241–1249.
- Yoon, D.; Bae, K.; Lee, M.-K.; Kim, J.H.; Yoon, K.-A. Galanin Is an Epigenetically Silenced Tumor Suppressor Gene in Gastric Cancer Cells. PLoS ONE 2018, 13, e0193275.
- El-Salhy, M. Effects of Octreotide, Galanin and Serotonin on a Human Gastric Cancer Cell Line. Oncol. Rep. 2005.
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Nakaizumi, A. Protection by Galanin against Gastric Carcinogenesis Induced by N-Methyl-N’-Nitro-N-Nitrosoguanidine in Wistar Rats. Cancer Res. 1994, 54, 3167–3170.
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal Cancer Patients Exhibit Increased Levels of Galanin in Serum and Colon Tissues. Oncol. Lett. 2016, 12, 3323–3329.
- Nagayoshi, K.; Ueki, T.; Tashiro, K.; Mizuuchi, Y.; Manabe, T.; Araki, H.; Oda, Y.; Kuhara, S.; Tanaka, M. Galanin Plays an Important Role in Cancer Invasiveness and Is Associated with Poor Prognosis in Stage II Colorectal Cancer. Oncol. Rep. 2015, 33, 539–546.
- Kim, J.C.; Lee, H.C.; Cho, D.H.; Choi, E.Y.; Cho, Y.K.; Ha, Y.J.; Choi, P.W.; Roh, S.A.; Kim, S.Y.; Kim, Y.S. Genome-Wide Identification of Possible Methylation Markers Chemosensitive to Targeted Regimens in Colorectal Cancers. J. Cancer Res. Clin. Oncol. 2011, 137, 1571–1580.
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Yano, H.; Nakaizumi, A. Chemoprevention by Galanin against Colon Carcinogenesis Induced by Azoxymethane in Wistar Rats. Int. J. Cancer 1995, 61, 861–863.
- Jung, K.; Narwal, M.; Min, S.Y.; Keam, B.; Kang, H. Squamous Cell Carcinoma of Head and Neck: What Internists Should Know. Korean J. Intern. Med. 2020, 35, 1031–1044.
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural Invasion in Cancer: A Review of the Literature. Cancer 2009, 115, 3379–3391.
- Scanlon, C.S.; Banerjee, R.; Inglehart, R.C.; Liu, M.; Russo, N.; Hariharan, A.; van Tubergen, E.A.; Corson, S.L.; Asangani, I.A.; Mistretta, C.M.; et al. Galanin Modulates the Neural Niche to Favour Perineural Invasion in Head and Neck Cancer. Nat. Commun. 2015, 6, 6885.
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-Related Axonogenesis and Neurogenesis in Prostate Cancer. Clin. Cancer Res. 2008, 14, 7593–7603.
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular Mechanisms of Perineural Invasion, a Forgotten Pathway of Dissemination and Metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82.
- Amit, M.; Na’ara, S.; Gil, Z. Mechanisms of Cancer Dissemination along Nerves. Nat. Rev. Cancer 2016, 16, 399–408.
- Misawa, K.; Mochizuki, D.; Imai, A.; Endo, S.; Mima, M.; Misawa, Y.; Kanazawa, T.; Carey, T.E.; Mineta, H. Prognostic Value of Aberrant Promoter Hypermethylation of Tumor-Related Genes in Early-Stage Head and Neck Cancer. Oncotarget 2016, 7, 26087–26098.
- Misawa, K.; Mima, M.; Imai, A.; Mochizuki, D.; Misawa, Y.; Endo, S.; Ishikawa, R.; Kanazawa, T.; Mineta, H. The Neuropeptide Genes SST, TAC1, HCRT, NPY, and GAL Are Powerful Epigenetic Biomarkers in Head and Neck Cancer: A Site-Specific Analysis. Clin. Epigenetics 2018, 10, 1–10.
- Kanazawa, T.; Misawa, K.; Shinmura, K.; Misawa, Y.; Kusaka, G.; Maruta, M.; Sasaki, T.; Watanabe, Y.; Carey, T.E. Promoter Methylation of Galanin Receptors as Epigenetic Biomarkers for Head and Neck Squamous Cell Carcinomas. Expert Rev. Mol. Diagn. 2019, 19, 137–148.
- Misawa, K.; Mochizuki, D.; Endo, S.; Mima, M.; Misawa, Y.; Imai, A.; Shinmura, K.; Kanazawa, T.; Carey, T.E.; Mineta, H. Site-Specific Methylation Patterns of the GAL and GALR1/2 Genes in Head and Neck Cancer: Potential Utility as Biomarkers for Prognosis: Methylation Patterns of the GAL and GALR1/2 Genes. Mol. Carcinog. 2017, 56, 1107–1116.
- Misawa, K.; Misawa, Y.; Kanazawa, T.; Mochizuki, D.; Imai, A.; Endo, S.; Carey, T.E.; Mineta, H. Epigenetic Inactivation of Galanin and GALR1/2 Is Associated with Early Recurrence in Head and Neck Cancer. Clin. Exp. Metastasis 2016, 33, 187–195.
- Kanazawa, T.; Misawa, K.; Carey, T.E. Galanin Receptor Subtypes 1 and 2 as Therapeutic Targets in Head and Neck Squamous Cell Carcinoma. Expert Opin. Ther. Targets 2010, 14, 289–302.
- Kanazawa, T.; Iwashita, T.; Kommareddi, P.; Nair, T.; Misawa, K.; Misawa, Y.; Ueda, Y.; Tono, T.; Carey, T.E. Galanin and Galanin Receptor Type 1 Suppress Proliferation in Squamous Carcinoma Cells: Activation of the Extracellular Signal Regulated Kinase Pathway and Induction of Cyclin-Dependent Kinase Inhibitors. Oncogene 2007, 26, 5762–5771.
- Kanazawa, T.; Misawa, K.; Misawa, Y.; Maruta, M.; Uehara, T.; Kawada, K.; Nagatomo, T.; Ichimura, K. Galanin Receptor 2 Utilizes Distinct Signaling Pathways to Suppress Cell Proliferation and Induce Apoptosis in HNSCC. Mol. Med. Rep. 2014, 10, 1289–1294.
- Misawa, K.; Ueda, Y.; Kanazawa, T.; Misawa, Y.; Jang, I.; Brenner, J.C.; Ogawa, T.; Takebayashi, S.; Grenman, R.A.; Herman, J.G.; et al. Epigenetic Inactivation of Galanin Receptor 1 in Head and Neck Cancer. Clin. Cancer Res. 2008, 14, 7604–7613.
- Uehara, T.; Kanazawa, T.; Mizukami, H.; Uchibori, R.; Tsukahara, T.; Urabe, M.; Kume, A.; Misawa, K.; Carey, T.E.; Suzuki, M.; et al. Novel Anti-Tumor Mechanism of Galanin Receptor Type 2 in Head and Neck Squamous Cell Carcinoma Cells. Cancer Sci. 2014, 105, 72–80.
- Kanazawa, T.; Misawa, K.; Misawa, Y.; Uehara, T.; Fukushima, H.; Kusaka, G.; Maruta, M.; Carey, T. G-Protein-Coupled Receptors: Next Generation Therapeutic Targets in Head and Neck Cancer? Toxins 2015, 7, 2959–2984.
- Banerjee, R.; Van Tubergen, E.A.; Scanlon, C.S.; Vander Broek, R.; Lints, J.P.; Liu, M.; Russo, N.; Inglehart, R.C.; Wang, Y.; Polverini, P.J.; et al. The G Protein–Coupled Receptor GALR2 Promotes Angiogenesis in Head and Neck Cancer. Mol. Cancer Ther. 2014, 13, 1323–1333.
- Shen, F.; Zhang, Y.; Yao, Y.; Hua, W.; Zhang, H.; Wu, J.; Zhong, P.; Zhou, L. Proteomic Analysis of Cerebrospinal Fluid: Toward the Identification of Biomarkers for Gliomas. Neurosurg. Rev. 2014, 37, 367–380.
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Locker, F.; Schlager, S.; Kofler, B. Galanin Is a Potent Modulator of Cytokine and Chemokine Expression in Human Macrophages. Sci. Rep. 2019, 9, 7237.
- Locarno, C.V.; Simonelli, M.; Carenza, C.; Capucetti, A.; Stanzani, E.; Lorenzi, E.; Persico, P.; Della Bella, S.; Passoni, L.; Mavilio, D.; et al. Role of Myeloid Cells in the Immunosuppressive Microenvironment in Gliomas. Immunobiology 2020, 225, 151853.
- Mei, Z.; Yang, Y.; Li, Y.; Yang, F.; Li, J.; Xing, N.; Xu, Z.-Q.D. Galanin Suppresses Proliferation of Human U251 and T98G Glioma Cells via Its Subtype 1 Receptor. Biol. Chem. 2017, 398, 1127–1139.
- Ormandy, C.J.; Lee, C.S.L.; Ormandy, H.F.; Fanti, V.; Shine, J.; Peters, G.; Sutherland, R.L. Amplification, Expression, and Steroid Regulation of the Preprogalanin Gene in Human Breast Cancer. Cancer Res. 1998, 6, 1353–1357.
- Lü, S.-H. Peptidergic Innervation of Human Esophageal and Cardiac Carcinoma. World J. Gastroenterol. 2003, 9, 399.
- Doufekas, K.; Hadwin, R.; Kandimalla, R.; Jones, A.; Mould, T.; Crowe, S.; Olaitan, A.; Macdonald, N.; Fiegl, H.; Wik, E.; et al. GALR1 Methylation in Vaginal Swabs Is Highly Accurate in Identifying Women With Endometrial Cancer. Int. J. Gynecol. Cancer 2013, 23, 1050–1055.
- Ning, X.; Qi, Y.; Wang, F.; Li, S.; Jia, Z.; Yang, J. A Three Protein-Coding Gene Prognostic Model Predicts Overall Survival in Bladder Cancer Patients. BioMed Res. Int. 2020, 2020, 7272960.
- Kanazawa, T.; Misawa, K.; Fukushima, H.; Misawa, Y.; Sato, Y.; Maruta, M.; Imayoshi, S.; Kusaka, G.; Kawabata, K.; Mineta, H.; et al. Epigenetic Inactivation of Galanin Receptors in Salivary Duct Carcinoma of the Parotid Gland: Potential Utility as Biomarkers for Prognosis. Oncol. Lett. 2018, 15, 9043–9050.
- Tjomsland, V.; El-Salhy, M. Effects of Single, Double or Triple Combinations of Octreotide, Galanin and Serotonin on a Human Pancreatic Cancer Cell Line. Histol. Histopathol. 2005, 537–541.
- Iishi, H.; Tatsuta, M.; Baba, M.; Yano, H.; Iseki, K.; Uehara, H.; Nakaizumi, A. Inhibition by Galanin of Experimental Carcinogenesis Induced by Azaserine in Rat Pancreas. Int. J. Cancer 1998, 75, 396–399.
- Gopalakrishnan, L.; Chatterjee, O.; Raj, C.; Pullimamidi, D.; Advani, J.; Mahadevan, A.; Keshava Prasad, T.S. An Assembly of Galanin–Galanin Receptor Signaling Network. J. Cell Commun. Signal. 2021, 15, 269–275.
- Kastin, A.J. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013.
- Ding, X.; MacTavish, D.; Kar, S.; Jhamandas, J.H. Galanin Attenuates β-Amyloid (Aβ) Toxicity in Rat Cholinergic Basal Forebrain Neurons. Neurobiol. Dis. 2006, 21, 413–420.
- Dunne, M.J.; Bullett, M.J.; Li, G.D.; Wollheim, C.B.; Petersen, O.H. Galanin Activates Nucleotide-Dependent K+ Channels in Insulin-Secreting Cells via a Pertussis Toxin-Sensitive G-Protein. EMBO J. 1989, 8, 413–420.
- Cormont, M.; Le Marchand-Brustel, Y.; Van Obberghen, E.; Spiegel, A.M.; Sharp, G.W. Identification of G Protein Alpha-Subunits in RINm5F Cells and Their Selective Interaction with Galanin Receptor. Diabetes 1991, 40, 1170–1176.
- Zhao, M.; Wang, Y.; Liu, Y.; Zhang, W.; Liu, Y.; Yang, X.; Cao, Y.; Wang, S. C7 Peptide Inhibits Hepatocellular Carcinoma Metastasis by Targeting the HGF/c-Met Signaling Pathway. Cancer Biol. Ther. 2019, 20, 1430–1442.
- Koller, A.; Rid, R.; Beyreis, M.; Bianchini, R.; Holub, B.S.; Lang, A.; Locker, F.; Brodowicz, B.; Velickovic, O.; Jakab, M.; et al. In Vitro Toxicity of the Galanin Receptor 3 Antagonist SNAP 37889. Neuropeptides 2016, 56, 83–88.
- El-Salhy, M. Effects of Triple Therapy with Octreotide, Galanin and Serotonin on a Human Colon Cancer Cell Line Implanted in Mice: Comparison between Different Routes of Administration. Histol. Histopathol. 2005, 20, 19–25.
- El-Salhy, M. Comparison between Triple Therapy with Octreotide, Galanin and Serotonin, 5-Fluorouracil/Leucovorin, and Sequential Treatment with Both, on Human Colon Cancer. Oncol. Rep. 2004, 11, 11611161168.
- El-Salhy, M.; Dennerqvist, V. Effects of Triple Therapy with Octreotide, Galanin and Serotonin on Liver Metastasis of Human Colon Cancer in Xenografts. Oncol. Rep. 2004, 6, 1177–1182.
- Sitohy, B.; El-Salhy, M. A Comparison between Double and Triple Therapies of Octreotide, Galanin and Serotonin on a Rat Colon Carcinoma. Histol. Histopathol. 2003, 1, 103–110.
- El-Salhy, M.; Sitohy, B.; Norrgård, Ö. Triple Therapy with Octreotide, Galanin, and Serotonin Reduces the Size and Blood Vessel Density and Increases Apoptosis of a Rat Colon Carcinoma. Regul. Pept. 2003, 111, 145–152.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
920
Revisions:
2 times
(View History)
Update Date:
27 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




