You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by MANUEL LISARDO Sanchez SANCHEZ and Version 2 by Lindsay Dong.
Peptidergic systems play an important role in cancer progression. The galaninergic system (the peptide galanin and its receptors: galanin 1, 2 and 3) is involved in tumorigenesis, the invasion and migration of tumor cells and angiogenesis and it has been correlated with tumor stage/subtypes, metastasis and recurrence rate in many types of cancer. Galanin exerts a dual action in tumor cells: a proliferative or an antiproliferative effect depending on the galanin receptor involved in these mechanisms. Galanin receptors could be used in certain tumors as therapeutic targets and diagnostic markers for treatment, prognosis and surgical outcome.
- galanin
- galanin receptor
- galanin receptor antagonist
- galanin receptor agonist
1. Introduction
The GLOBOCAN 2020 database (World Health Organization (WHO)) states that of the 7,794,798,844 inhabitants of our planet, 19,292,789 of them were diagnosed with some type of cancer and 9,958,133 died, with prevalence cases at 5 years of 50,550,287. Female breast cancer is the most diagnosed cancer and the leading cause of cancer death is lung cancer (1.8 million deaths) [1]. In 2040, 28.4 million patients suffering from cancer are expected in the world [1]. These data are sufficiently representative of the health problem that cancer represents today. Cells, escaping from normal behavior, acquire distinctive characters (evading growth suppressors, maintaining proliferative signaling, allowing replicative immortality, resisting cell death, activating invasion/metastasis, inducing angiogenesis) that make them cancerous [2] (Figure 1). Moreover, the reprogramming of energy metabolism and evasion of immune destruction have also been added to the previous hallmarks of cancer [2]. These behaviors arise from the instability of the genome that produces genetic diversity, and inflammatory mechanisms that promote the multiple actions described above (Figure 1). Tumors are not currently considered as simple masses of cancer cells; they are more complex in that they contain a repertoire of apparently normal recruited cells that contribute to the acquisition of distinctive features by regulating the tumor microenvironment [2]. The full knowledge of the previously mentioned hallmarks will help to develop new therapeutic strategies against cancer.
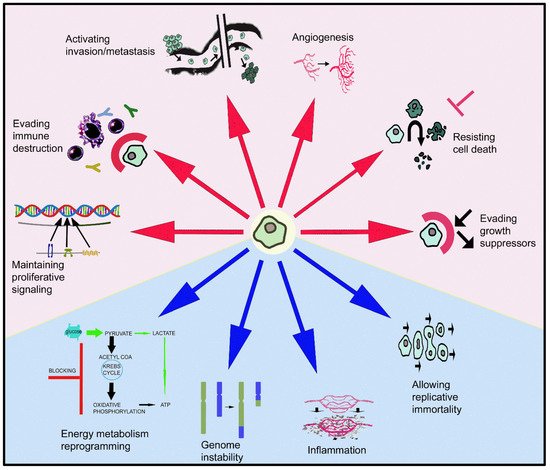
Figure 1. Ten keys of cellular/tissue behavior that make a cell a cancer cell, contrary to its normal biological destiny, leading to the formation of a primary tumor and later a secondary one. Red arrows show the involvement of the galaninergic system in these mechanisms: note that GAL is involved in six of them.
2. The Galaninergic System: Galanin and Its Receptors
GAL was discovered in porcine intestinal extracts and contains 29 amino acids [3][14]; however, in humans, the peptide contains 30 amino acid residues (Figure 2) and, unlike porcine GAL, the carboxy-terminus is not amidated [4][5][6][15,16,17]. The amino acid sequence of GAL is highly conserved among species (almost 90%) [7][18]. The C-terminus of GAL is involved in its receptor-binding affinity and the N-terminus is crucial for its biological activity [8][19]; the fifteen N-terminal residues of GAL are highly conserved throughout evolution [9][20]. GAL and other peptides (GAL message-associated peptide (GMAP), GAL-like peptide (GALP), alarin) belong to the GAL family of peptides. In addition, the peptide spexin (neuropeptide Q, 14 amino acids) is the most recently discovered member of this family; spexin has been shown to be involved in reproduction, nociception, renal function and energy homeostasis [10][21]. GALP, an endogenous ligand that activates the three known types of GALRs, was isolated from the porcine hypothalamus, contains 60 amino acids and is involved in reproduction and energy homeostasis [11][12][22,23]. Alarin (25 amino acids) is a splice variant of GALP mRNA [13][24]. The human chromosome 11q13.3-q13.5 contains the pre-pro-GAL gene-encoding GAL, which shows five introns and six exons, which in turn are translated into a pre-prohormone (123 amino precursor) containing the signal peptide, GAMP and GAL [6][14][17,25] (Figure 2). Some oncogenes have been located in the abovementioned region, which is also the breakpoint for the translocation t (11; 14) (q13; q32) in diffuse B-cell lymphoma and chronic lymphocytic leukemia [15][26]. The gene spans 6.5 kb and its first exon only encodes the 5′ untranslated sequence. In the pre-pro-GAL gene, its 5-prime flanking sequence shows a TATA box preceded by binding sites for transcription factors (e.g., NF-κB) and contains a CT-rich region that is flanked by two Alu repeats-, 2.3 kb upstream of the transcriptional start site; the region (500 bp) preceding this site contains 79% CG [16][27]. GALP and alarin are encoded by the pre-pro-GALP gene, which is located on the human chromosome 19q13.43 and comprises six exons [17][28]. The region encoding GALP is contained in exons 2–5 and alarin is formed when post-transcriptional splicing leads to the exclusion of exon 3, resulting in a frame shift and a novel precursor peptide [13][24].
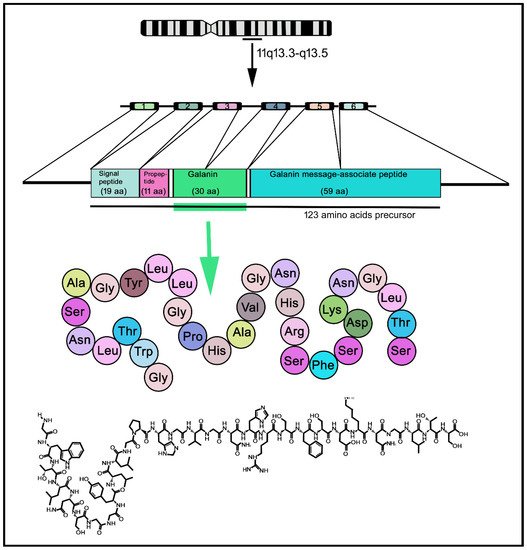
Figure 2. Transcription–maturation–translation processing of GAL, from human chromosome 11. Human GAL contains 30 amino acids residues. 1–6: exons; aa: amino acids.
The galaninergic system (GAL and GAL receptors (GALRs)) is widely distributed by the mammalian gastrointestinal tract, testis, ovary, uterus, kidney and heart, and by the immune, endocrine, peripheral and central nervous systems (e.g., endocrine pancreas, pituitary gland, paravertebral sympathetic ganglia, myenteric plexus, glial cells, dorsal root ganglion, spinal cord, brainstem, thalamus, hypothalamus, hippocampus, amygdala) [14][18][19][20][21][22][23][24][25][25,29,30,31,32,33,34,35,36]. The half-life of GAL in plasma is about five minutes and GAL coexists with many other neuroactive substances (e.g., enkephalin, vasopressin, calcitonin gene-related peptide, substance P, neuropeptide Y, cholecystokinin, growth hormone, luteinizing hormone-releasing hormone, dopamine, glutamate, noradrenalin, serotonin, acetylcholine) [18][26][27][28][29][30][31][32][33][29,37,38,39,40,41,42,43,44]. In general, GMAP in the rat central nervous system showed a similar profile of expression to GAL; however, GALP and alarin showed a more restricted expression than GAL [34][45]. Due to the widespread distribution of the galaninergic system by the whole body, GAL has been involved in many physiological actions after binding to specific G protein-coupled receptors: smooth muscle contraction, acetylcholine release inhibition, energy metabolism, food and water intake, hyperglycemia, osmotic and metabolic homeostasis, spinal reflexes, injury response, nociception, reproduction, memory, cognition, learning, arousal, sleep, neural growth, glucose-induced insulin release inhibition and respiratory, cardiovascular, neuroendocrine and gastrointestinal mechanisms [3][7][9][14][18][22][27][35][36][37][38][39][40][8,14,18,20,25,29,33,38,46,47,48,49,50]. Moreover, GAL regulates the level of growth hormone, prolactin, dopamine, pancreatic peptide, luteinizing hormone, luteinizing hormone-releasing hormone, somatostatin and insulin [7][31][41][42][43][18,42,51,52,53]. GAL acts as a neurotransmitter and neuromodulator in the central nervous system and the peptide has been involved in several diseases (e.g., anxiety, depression, stroke, alcoholism, Alzheimer’s disease, Parkinson’s disease, epilepsy); the galaninergic system also plays an important role in inflammatory bowel diseases and diabetes [7][9][14][44][45][46][47][48][18,20,25,54,55,56,57,58].
GALRs (GAL 1 receptor (GAL1R), GAL 2 receptor (GAL2R), GAL 3 receptor (GAL3R)) belong to the rhodopsin-like (class A) G protein-couple receptor family (seven transmembrane receptors or 7TM) [49][60]. They contain three extracellular loops, three intracellular loops, an extracellular N-terminus and three intercellular loops [49][50][60,61]. The helix 8 acts as a conformational switch at the C-terminus [51][62]. GALRs have sequence homologies in the transmembrane region: GAL1R-GAL3R (33%) and GAL2R-GAL3R (54%) [9][20], whereas human GAL3R and GAL2R respectively show 89% and 92% sequence homology with their receptor homologs present in the rat [52][63]. Human GAL has tens of nanomolar affinity at GAL3R, subnanomolar to nanomolar affinity at GAL2R and subnanomolar affinity at GAL1R [53][64]. Although the structure of GALRs is quite similar, different binding characteristics and intracellular signaling pathways have been reported after the activation of these receptors by ligands [49][50][60,61]. Thus, the lengths of the N-terminus (which plays an important role in the binding of ligands) and C-terminus are different in GALRs (C-terminus: GAL1R, 37 residues; GAL2R, 30; GAL3R, 13; N-terminus: GAL1R, 47 residues; GAL2R, 80; GAL3R, 62) [49][60]. The physiological actions of GAL are mediated by GAL1R, GAL2R and GAL3R; several signaling pathways are activated after the binding of GAL to these receptors: the stimulation of phospholipase C (PLC, mediated by GAL2R) or the inhibition of cyclic adenosine monophosphate (cAMP)/PKA (mediated by GAL1R/GAL3R) [15][26].
GAL1R was isolated from a human melanoma cell line [54][67]. It is coupled to Gβγ/Gαi signaling pathways and promotes, via a PKC-independent mechanism, the activation of mitogen-activated protein kinases (MAPKs) [6][55][17,68]. Moreover, the activation of GAL1R inhibited AC activity via an interaction with G-proteins (Gαi/αo), leading to G protein-coupled inwardly-rectifying potassium (GIRK) channels opening [21][54][56][32,67,69]. GAL1R activation can also inhibit the transcription factor cAMP regulatory element binding protein (CREB)-dependent signaling pathway [57][70], and the expression of GAL1R (but not that of GAL2R or GAL3R) was controlled by cAMP via CREB [58][59][71,72]. The GAL1R gene (located in chromosome 18q23) in humans shows three exons that are translated into a long protein containing 349 amino acids; GAL1R homology is high between species (e.g., in mouse, 93% of the residues are identical to those observed in humans) [60][73]. GAL1R has been located in the central (e.g., cortex, amygdala, hippocampus, thalamus, hypothalamus, locus coeruleus, medulla oblongata, spinal cord) and peripheral (e.g., dorsal root ganglion) nervous systems [22][23][33,34] and in the gastrointestinal tract [54][61][67,74].
GAL2R was first identified in the rat central nervous system [24][62][63][35,75,76] and was cloned in rat hypothalamic cells for the first time [24][35]. GAL2R contains His252/His253 (transmembrane domain 6) and Phe264/Tyr271 (extracellular loop 3) residues, which play a crucial role in the binding of ligands and in the activation of the receptor [64][77]. The sequence of human GAL2R shows a high homology with that observed in the rat (85–92%) and it was 39% identical to human GAL1R [22][52][65][33,63,78]. In the rat, GAL2R shows 38% amino acid identity with GAL1R [24][35]. In comparison with GAL1R, the distribution of GAL2R is more widespread since it has been observed in the nervous system (piriform cortex, dentate gyrus, amygdala, hypothalamus, mammillary nuclei, spinal cord), skeletal muscle, liver, testis, ovary, uterus, spleen, heart, kidney, lung, gastrointestinal tract and pituitary gland [22][24][52][66][67][33,35,63,79,80]. GAL2R mRNA expression has been reported in the neocortex, dentate gyrus, hypothalamus, cerebellar cortex, substantia nigra, vestibular complex and dorsal root ganglion [67][68][69][7,80,81].
GAL3R was first isolated from rat hypothalamic cDNA libraries [70][89]. Human GAL3R (368 amino acids long) shows 36% amino acids identity with human GAL1R, 58% with human GAL2R and 90% with rat GAL3R [52][63]. The distribution of GAL3R (olfactory cortex, hippocampus, hypothalamus, medulla oblongata) is more restricted than that reported in the brain for GAL1R or GAL2R [22][52][64][70][71][72][33,63,77,89,90,91]. GAL3R mRNA has been located in the amygdala, periaqueductal gray, locus coeruleus, brainstem reticular formation, spinal cord, pancreas, adrenal gland and testis [52][72][63,91]. GAL3R promotes the activation of Gαi/αo, blocking AC activity and opening GIRK channels [52][71][63,90]. Spexin binds to human GAL2/3Rs (not to GAL1R), exerting a higher potency toward GAL3R than GAL [10][73][21,92].
GAL agonists or antagonists (e.g., galantide, M35, M40, C7) have been used for the treatment of several disorders: GAL antagonists have been administered for the treatment of food intake disorders and Alzheimer’s disease, whereas GAL agonists have been used for the treatment of chronic pain [7][74][18,93]. Some fragments of GAL (GAL1-15; GAL1-16, GAL1-29), exerting physiological actions through GALRs (e.g., mood or cardiovascular regulation, alcohol intake), have been reported [75][76][77][78][94,95,96,97]. The conformational changes observed in GAL1R lead to a higher affinity of this receptor for GAL1-15 than for GAL, increasing the signaling (mediated by Gi/o) and decreasing AC activity and CREB level [79][98]. GALRs may form heteromers with each other and with other types of G protein-coupled receptors in the central nervous system [80][99]. Thus, the GAL1R/GAL2R heteroreceptor complex [79][98] and heteromers of GALRs with alpha2-adrenoceptors and 5-hydroxytryptamine (HT), dopamine 1, neuropeptide Y1 or Y2 receptors have been reported [9][20]. The formation of the heterotrimer GAL1R-GAL2R-5-HT1A receptor complex could explain why GAL1-15, but not GAL1-29, antagonistically moderated the serotonin receptor [80][99]. In addition, this heterotrimer has been suggested as a potential target to reverse the actions mediated by fluoxetine on memory mechanisms [75][81][94,100]. Thus, heteromers can alter the recognition of GAL ligands, and they are promising new targets for therapeutic interventions.
3R [19][30]. GAL has been reported in gliosarcoma and glioblastoma multiforme [99][118]; in the latter, the most abundant receptor found was GAL1R, followed by GAL3R and GAL2R [99][118]. In glioma, endothelial and immune (e.g., macrophages, neutrophils) cells expressed GAL3R, but GAL1R/GAL2R were not observed around the blood vessels [19][30]. This means that tumor-associated cells are involved in tumor microenvironment homeostasis. Glioma-associated macrophages (GAMs) are involved in tumor progression; although macrophages produce/secrete GAL, GAMs do not express GAL, but express GAL3R, and this means that GAL could regulate the activity of GAMs [171][172][59,204].
3. The Galaninergic System and Cancer
Peptides and their receptors are one of the molecular bases for the therapeutic targeting of tumors [82][101]. The galaninergic system is expressed in normal tissues and, in cancer cells, is involved in tumorigenesis, invasion and migration (metastasis) [19][25][28][82][83][84][85][86][87][88][89][90][91][92][93][30,36,39,101,102,103,104,105,106,107,108,109,110,111,112], although in some tumors, GAL and GALRs are silenced [94][113]. This system has been observed in neuroendocrine (e.g., phaeochromocytoma, pituitary adenoma, gangliocytoma, paraganglioma, neuroblastoma) and non-neuroendocrine (e.g., glioblastoma and other brain tumors, melanoma, basal cell carcinoma, head and neck squamous cell carcinoma, embryonic carcinoma, colon cancer, breast cancer, gastrointestinal cancer, prostate cancer) tumors [19][25][28][62][82][83][84][85][86][87][88][89][90][91][92][93][95][96][97][98][99][100][101][102][30,36,39,75,101,102,103,104,105,106,107,108,109,110,111,112,114,115,116,117,118,119,120,121]. For example, in squamous cell carcinoma, GAL1R was involved in tumor suppression and GAL2R favored tumor development and decreased survival [103][104][122,123]. GAL exerted a tumor-reducing effect in experimental murine models (gastrointestinal cancer), but in other models (adenoma formation), GAL promoted cell proliferation and tumor formation [82][101]. Thus, GAL can promote or inhibit the development of tumors; this is an important characteristic of the galaninergic system: to exert both proliferative and antiproliferative actions on tumor cells. Importantly, GAL/GALR expression has been correlated with tumor subtypes (colon carcinoma, squamous cell carcinoma, neuroblastic tumors, pituitary adenoma) or with tumor stage [82][101] and the activation of GAL1R was generally antiproliferative, whereas the activation of GAL2R showed antiproliferative or proliferative effects [82][101]. The stage and tumor size in colon cancer have been related to the GAL mRNA level: the higher the GAL expression, the shorter the disease-free survival [19][87][30,106].
3.1. Galanin and Neuroendocrine Tumors
Neuroendocrine tumors (NETs) are a very heterogeneous tumor group including: (1) carcinoid gastroenteropancreatic tumors; (2) non-carcinoid gastroenteropancreatic tumors (vasoactive intestinal peptide (VIP)oma, gastrinoma, insulinoma); (3) catecholamine-secreting tumors (neuroblastoma, sympathoblastoma, ganglioneuroblastoma, ganglioneuroma, paraganglioma, phaeochromocytoma); (4) chromophobe pituitary tumors; (5) medullary carcinoma of the thyroid; (6) Merkel cell tumors; and (7) small-cell lung cancer. NETs originate from neuroendocrine cells, which release peptides (e.g., GAL, somatostatin, pancreatic polypeptide, chromogranins) and express their corresponding receptors [105][106][107][124,125,126]. Thus, a high expression of peptidergic receptors has been reported in NETs for neurotensin, gastrin-releasing peptide, cholecystokinin, somatostatin and vasoactive intestinal peptide [106][125]. Importantly, the expression of the peptidergic systems in NETs has been correlated with prognosis and tumor stage [108][127]. Regarding the galaninergic system, many data demonstrated its involvement in NETs pathophysiology and carcinogenesis; for example, high doses of estrogens or dopamine agonists reversed rat pituitary hyperplasia and decreased the expression of GAL, suggesting that the peptide acted as a proliferative agent [109][110][111][112][113][128,129,130,131,132]. GAL expression is restricted to some NETs [88][107]: the peptide was observed in adrenal phaeochromocytoma (62%), jugulo tympanic paraganglioma (40%) and carotid body paraganglioma (18%), but it was not found in metastatic or recurrent paraganglioma, extra-adrenal phaeochromocytoma and carcinoid tumor [88][89][107,108]. Moreover, endocrine tumors from gastrointestinal tract, pancreas and lung did not show GAL [88][107]. This means that the utility of GAL as a diagnostic marker is limited to certain NETs. In this section, the involvement of the galaninergic system in those NETs (phaeochromocytoma, insulinoma, neuroblastic tumor, pituitary tumor, small-cell lung cancer) expressing this system will be summarizeviewed (Table 1).Table 1.
Involvement of the galaninergic system in neuroendocrine tumors.
| Cancer |
|---|
Involvement of the galaninergic system in gastric and colorectal cancer.
| Actions/Presence | References | ||||||
|---|---|---|---|---|---|---|---|
| Actions/Presence | References | ||||||
| Corticotroph adenoma Human |
- High GAL expression (RIA) | [83] | [102] | ||||
| - GAL in 84% of tumors (IH) | |||||||
| Gastric Cancer | |||||||
| [ | 84] | [103] | |||||
| Human | - Fibers containing GAL: increased in longitudinal muscle layer, lamina muscularis mucosae and neoplastic proliferation vicinity (IH) | [143] | [175] | - GAL expression: smaller adenomas and better prognosis (IH) | [86] | [105] | |
| - Myenteric plexus: neurons showed a high expression of caspases 3/8 and low GAL expression (IH) | [143] | [175] | ] | [192] | - GAL release and responded to corticotropin-releasing factor | [114] | [135] |
| - GAL/GAL1R level reduced | [ | ||||||
| - GAL/GALR epigenetic variants: markers for prognosis prediction (Q-MSP) | 144] | [176] | [160][161] | [193,194] | |||
| - Poor survival: associated with methylation of GAL/GAL1R genes. Hypermethylation: inactivation of GAL/GAL1R/GAL2R genes (Q-MSP) | [162] | [195] | |||||
| Human Cell lines |
Table 4.
Involvement of the galaninergic system in glioma.
| Actions/Presence | References | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | - GAL/GAL3R expression: no correlation with oligodendroglial, astrocytic and mixed neural–glial tumors | [19] | [30] | |||||||||||||
| - High-grade glioma (WHO grade IV): related to GAL3R expression | [19] | [30] | ||||||||||||||
| - Endothelial/immune cells: GAL3R expression. Around blood vessels: GAL1R/GAL2R not observed (IH) | [19] | [30] | ||||||||||||||
| - GAL1R, followed by GAL3R; GAL2R absent (astrocytic/oligodendroglia tumors) (IH, autoradiography, reverse transcription-PCR) | [19][99] | [30,118] | ||||||||||||||
| Ganglioneuroma Human |
- No correlation between prognosis/tumor markers and GAL level (RIA) | [115] | [136 | |||||||||||||
| - Glioma-associated macrophages: GAL3R expression (quantitative PCR) | [171][172] | [59,204]] | ||||||||||||||
| - GAL2R/GAL3R level unchanged (RT-PCR) | ||||||||||||||||
| Salivary duct carcinoma Human | [144] | [176 | - GAL | 1 | R/GAL | 2 | R: therapeutic targets/prognostic factors. GAL | 1 | R/GAL | 2 | R methylation rates correlated with overall survival decrease (IH, Q-MSP) | [178] | [210] | - GAL1R/GAL3R immunoreactivity decrease (IH) | [116] | [137] |
| ] | - Apoptosis: mediated by GAL2R but not by GAL1R. GAL1R/GAL2R: tumor suppressors in a p53-independent manner | Insulinoma Rat Rin14B cell line |
- GAL | |||||||||||||
| Melanoma | 1 | R expression (Northern blot, in situ hybridization) | [21] | [32] | ||||||||||||
| - Lower level of GAL in pre-operative samples (and plasma) when compared with that found in post-operative samples or in healthy donors. Gastric cancer tissues: GAL/GAL1R level was lower compared with that found in adjacent regions GAL2R/GAL3R: no change (Western blot; RT-PCR; ELISA) | [[ | |||||||||||||||
| - No correlation between proliferative activity and GAL/GAL binding levels (IH, autoradiography, reverse transcription-PCR) | Human | 144[99] | [118] | - GAL/GAL | 1 | Insulinoma Rat RINm5F cell line |
- GAL moderately suppressed insulin accumulation, but did not affect cell proliferation | [117] | [138] | |||||||
| ] | [176] | |||||||||||||||
| R expression (IH) | 163 | ] | [11] | |||||||||||||
| [ | 82 | ] | - GAL low level: used as biomarker. GAL protein/mRNA level related to tumor size, tumor node metastasis stage and lymph node metastasis | [144] | [176] | - GAL2R transfection into HNSCC cells: cell proliferation inhibited. GAL2R re-expression: blocked cell proliferation (showing mutant p53) | [94][164] | [113 | [165] | , | - Pancreatic beta-cells: GAL inhibited adenylate cyclase activity and insulin secretion | [ | ||||
| 196 | ,197] | Human | ||||||||||||||
| - Cerebrospinal fluid (glioblastoma): reduced GAL level | [ | Gastric cancer cell lines |
- GAL1R/GAL2R negative HNSCC cells: GAL1R re-expression suppressed tumor cell proliferation via ERK1/2-mediated actions on cyclin-dependent kinase inhibitors and cyclin D1- GAL expression decreased: restored with a demethylating agent. GAL hypermethylation: impaired GAL tumor suppressor action. GAL downregulation: due to epigenetic inactivation (Q-MSP, Western blot) | [94][165] | [113,197] | [145] | [177] | 43] | ||||||||
| - GAL: decreased cell proliferation | [53] | |||||||||||||||
| [ | 146] | [178] | Insulinoma Mouse |
- Beta TC-1 cells: GAL, released from sympathetic nerve terminals, inhibited pro-insulin gene expression stimulated by glucagon-like peptide-I (Northern blot) | [118] | [139] | ||||||||||
| Rats | - GAL blocked gastric carcinogenesis by inhibiting antral epithelial cell proliferation | [147] | [13] | Neuroblastic tumors Human |
- GAL mRNA, GAL immunoreactivity and GAL binding sites expression (IH, in situ hybridization) | [116] | [137] | |||||||||
| Colorectal Cancer (CRC) | - Low level of GAL binding sites correlated with survival; GAL/GALR expression related to tumor differentiation stage (RIA, IH, in situ hybridization) | [115][116] | [136,137] | |||||||||||||
| Human | - GAL/GAL1R silencing: apoptosis in drug-sensitive/resistant cell lines and enhanced the effects mediated by chemotherapy. GAL mRNA: overexpressed. High GAL level: related to poor disease-free survival of early-stage CRC patients (IH, ELISA, RT-PCR, Western blot) | [68][87][98][102] | [7,106,117,121] | Neuroblastoma Human |
- No correlation between prognosis/tumor markers and GAL concentration | [115] | [136] | |||||||||
| - Enteric nervous system: number of neurons containing GAL increased in regions located close to the tumor (IH) (IH, RT-PCR, ELISA) | [35] | [8] | - GAL expression; GAL2R mRNA was less common than GAL1R mRNA (IH, in situ hybridization) | [85] | [104 | |||||||||||
| - CRC patients: more GAL-immunoreactive neurons in comparison to healthy samples (IH, ELISA) | ] | |||||||||||||||
| [ | 102] | [121] | - GAL1R/GAL3R highly expressed; GAL promoted tumor growth (IH, in situ hybridization) | [116] | [137] | |||||||||||
| Neuroblastoma Human IMR32 cell line |
- Dense core secretory vesicles: coexistence of GAL and beta-amyloid (IH) | [119] | [140] | |||||||||||||
| Neuroblastoma Human SH-SY5Y cell line |
- GAL2R mediated apoptosis. GAL antiproliferative potency: 100-fold higher in SY5Y/GAL2R cells than in SY5Y/GAL1R cells | [120] | [12] | |||||||||||||
| - GAL2R transfection: cell proliferation was blocked and caspase-dependent apoptotic mechanisms induced | ||||||||||||||||
| - GAL in the vicinity of cancer cell invasion (IH, ELISA) | [102] | [121] | ||||||||||||||
| - Blood samples: increased GAL concentration. High GAL level: cancer cells. Lowest GAL level: muscular layer placed distant from tumors. GAL: CRC tumor biomarker (ELISA, IH) | [148] | [179] | ||||||||||||||
| - GAL mRNA level: related to adenocarcinoma size/stage. Correlation between higher GAL expression and shorter disease-free survival (RT-PCR) | [87][98] | [106,117] | [120] | [12] | ||||||||||||
| - CRC cells showed a high GAL expression: more malignant and involved in tumor recurrence. High GAL expression: spread of cancer stem cells (metastasis) (RT-PCR) | [149] | [180] | Neuroblastoma Rat B104 cell line |
- GAL, GAL2R and GAL3R mRNAs were detected, but not GAL1R mRNA (reverse transcription-PCR) | [121] | [141] | ||||||||||
| - High GAL expression: associated with poor prognosis (stage II) and tumor recurrence. GAL expression: related to CRC aggressive behavior (RT-PCR) | [149] | [180] | - GAL promoted cell proliferation | |||||||||||||
| Human (tissue and cell lines) | - CRC cells/tissues: higher GAL levels than non-tumor cells/tissues | [87][98][148][149] | [106,117,179,180] | Paraganglioma Human |
||||||||||||
| - CRC tissue: increased GAL gene/protein expression. CRC cell lines: GAL/GAL1R silencing promoted apoptosis. GAL1R silencing promoted FLIPL down-regulation (IH, ELISA, RT-PCR) | - GAL expression (IH) | [87][98][102] | [106,117, | [89][93][122] | [108,112,142] | |||||||||||
| 121 | ] | Paraganglioma Human carotid body |
- GAL was detected in 18% of tumors (IH) | |||||||||||||
| Human HCT116 cell line |
- Cells overexpressing GAL | 2 | R were more chemosensitive to bevacizumab than control cells | [89] | [108] | |||||||||||
| [ | 150 | ] | [181] | Paraganglioma Human jugulo tympanic |
- GAL was detected in 40% of tumors (IH) | [89] | [108] | |||||||||
| Phaeochromocytoma Human |
- High GAL2R mRNA expression (Western blot) | [123] | [143] | |||||||||||||
| - Higher GAL concentration than in normal adrenal glands (RIA) | [124] | [144] | ||||||||||||||
| Phaeochromocytoma Rat PC12 cell line |
- GAL inhibited cell proliferation and GAL | 1 | R, GAL | 2 | R and GAL | 3 | R mRNA expression, but not GAL mRNA (reverse transcription-PCR) | [121] | [141] | |||||||
| Pituitary adenoma Human |
- GAL/GALR expression correlated with tumor stage (IH) | [82] | [101] | |||||||||||||
| Pituitary adenoma Human |
- High GAL | 3 | R levels found in some patients who relapsed shortly after surgical intervention (q-PCR) | [125] | [145] | |||||||||||
| Pituitary adenoma Rat |
- GAL promoted pituitary cell proliferation and tumor development | [27] | [38] | |||||||||||||
| Pituitary adenoma Rat MtTW-10 cell line |
- Estradiol increased GAL mRNA level | [126] | [146] | |||||||||||||
| Rat | - GAL decreased the incidence of colon tumors | [151 | Prolactinoma Rat |
- GAL concentration increased and GAL promoted tumor development | [127] | [147 | [128] | ,148] | ||||||||
| - Levonorgestrel decreased GAL mRNA expression and GAL-expressing cells (IH, in situ hybridization) | [129] | [149] | ||||||||||||||
| Small-cell lung cancer Human H345, H510 cell lines |
- GAL, via GAL | 2 | R, mediated cell proliferation | [130][131] | [88,150] | |||||||||||
| Small-cell lung cancer Human H69, H510 cell lines |
- GAL, via GAL2R, activated G proteins and promoted cell proliferation | [130] | [88] | |||||||||||||
| - GAL increased the levels of inositol phosphate and intracellular Ca | 2+ | and promoted cell growth | [132] | [151] | ||||||||||||
| Small-cell lung cancer Human H345, H510 cell lines |
- Ca | 2+ | -mobilizing peptides (e.g., GAL) promoted cell growth. Broad spectrum antagonists directed against multiple Ca | 2+ | -mobilizing receptors inhibited cell growth | [131][133] | [150,152] | |||||||||
| Small-cell lung cancer Human H69, H345, H510 cell lines |
- GAL, via the p42MAPK pathway, promoted cell growth. Protein kinase C inhibitors blocked cell growth induced by GAL | [134][135] | [153,154] | |||||||||||||
| Small-cell lung cancer Human SBC-3A cell line, mouse SBC-3A tumor |
- SBC-3A cells secreted the pre-pro-GAL precursor which was extracellular processed to GAL1-20 by plasmin | [136][137] | [155,156] | |||||||||||||
| Somatotroph adenoma Human |
- Low GAL level (RIA) | [83] | [102] | |||||||||||||
| - GAL increased circulating growth hormone level and growth hormone-producing tumors expressed GAL (IH) | [138] | [157] | ||||||||||||||
| - GAL blocked growth hormone release | [139] | [158] | ||||||||||||||
| Somatotroph adenoma Rat GH1 cell line |
- GAL inhibited growth hormone release | [140] | [159] | |||||||||||||
| Somatotroph adenoma Mouse |
- GAL mRNA level and peptide concentration increased | [127] | [147] | |||||||||||||
| - GAL secretion increased | [141] | [160] | ||||||||||||||
| Thyrotroph adenoma Rat |
- GAL gene expression blocked | [127] | [147] | |||||||||||||
| Thyrotroph adenoma Mouse |
- GAL synthesis inhibited | [141] | [160] |
IH: immunohistochemistry; q-PCR: quantitative real time PCR; RIA: radioimmunoassay.
3.2. Galanin and Gastric Cancer
In nerve cells, the galaninergic system plays an important role in tumor development. In human stomach samples, obtained from the vicinity of invasive cancer cells, neurons located in the myenteric plexus showed a high expression of both caspases 3 and 8, but a low expression of GAL [34][142][45,174] (Table 2). In carcinoma-affected regions of the human stomach, an increase of the GAL-immunoreactive fibers in the longitudinal muscle layer, lamina muscularis mucosae and in the vicinity of the neoplastic proliferation was observed; thus, carcinoma invasion affected GAL stomach wall innervation [143][175]. In patients suffering from gastric cancer, lower levels of GAL were observed in pre-operative samples (and in plasma) when compared with those found in post-operative samples obtained from the same patients or from samples of healthy donors [144][176]. Moreover, the levels of GAL/GAL1R were lower in gastric cancer tissues compared with those found in adjacent regions; however, the GAL2R/GAL3R levels did not change [144][176]. The low level of GAL could be used as a biomarker in gastric cancer and, importantly, in these patients (pre-operative samples), the GAL protein/mRNA levels have been related to tumor size, tumor node metastasis stage and lymph node metastasis [144][176].Table 2.
| ] |
| [ |
| 182 |
| ] |
IH: immunohistochemistry; Q-MSP: quantitative methylation-specific PCR; RT-PCR: real time-PCR.
3.3. Galanin and Colorectal Cancer
Colorectal cancer (CRC), the third most prevalent cancer worldwide, is an invasive tumor process due to the proliferation of epithelial cells that acquire a neoplastic phenotype [35][8]. This process is known as epithelial-to-mesenchymal transition, in which epithelial cells lose many morphological and functional characteristics (e.g., shape, cell polarity, intercellular junctions) [35][8]. Tumor cells digest the extracellular matrix of the intestine wall, activating growth factors that promote cell proliferation, the blockade of apoptotic mechanisms and also favor the spreading of cancer cells [35][8]. Then, the invasion of cancer cells destroys the enteric nervous system, leading to the atrophy of the submucosal/myenteric plexuses. The galaninergic system is involved in colon cancer [87][98][102][106,117,121] (Table 2); thus, for example, the siRNA-mediated silencing of the GAL gene reduced both invasive and proliferative potential in CRC cells [98][117].3.4. Galanin and Head and Neck Squamous Cell Carcinoma
Head and neck squamous cell carcinoma arises from mucosal surfaces of the head and neck [152][186] (Table 3). Perineural invasion (PNI), a mechanism of tumor dissemination via nerves, predicts poor survival in some cancers including head and neck squamous cell carcinoma (HNSCC), pancreatic cancer, stomach cancer and colon cancer, and is a sign of cancer cell invasion and metastasis [153][187]. An interaction between nerves and tumor cells occurs in PNI. PNI, mediated by molecular signals, promoted neuritogenesis and the survival, proliferation and invasion of tumor cells [154][155][156][157][84,188,189,190]. These cells are attracted to nerves and communicate with them. GAL (released from nerves) exerted a nerve–tumor crosstalk by activating GAL2R expressed in tumor cells and by inducing NFATC2-mediated transcription of cyclooxygenase-2 and GAL; then, GAL released from tumor cells promoted neuritogenesis, favoring PNI [154][84].Table 3.
Involvement of the galaninergic system in head and neck squamous cell carcinoma.
| Actions/Presence | References | |||||||
|---|---|---|---|---|---|---|---|---|
| Human | - High GAL level (RT-PCR) | [ | ||||||
| 170 | ||||||||
| ] | ||||||||
| [ | ||||||||
| 203 | ||||||||
| ] | ||||||||
| Human Mice |
- GAL blocked, via GAL | 1 | R, the proliferation of glioma cells and tumor growth. These effects were mediated through ERK1/2 signal activation. No cytotoxic/apoptotic effect was observed | [173] | [205 | |||
| - GAL/GAL1R blocked HNSCC and oral tumor cell proliferation by cell-cycle arrest (RT-PCR, ELISA, Q-MSP) | [104][145][164] | [123 | [ | ,177 | 166 | ,196 | ] | ,198] |
| - GAL1R blocked tumor cell proliferation through the activation of ERK1/2 | [164] | [196] | ||||||
| - GAL2R promoted an antitumor effect by inducing cell cycle arrest and apoptotic mechanisms (caspase 3-dependent) | [165] | [197] | ||||||
| - GAL2R suppressed HNSCC cell viability. HEp-2 cells: GAL2R mediated apoptotic mechanisms (caspase-independent) by downregulating ERK1/2 and inducing Bim | [167] | [199] | ||||||
| ] | Human Cell lines, tumor samples |
IH: immunohistochemistry.
3.6. Galanin and Other Cancers
Although the expressions of GAL and pre-pro-GAL mRNA have been reported in breast cancer, it has been suggested that the GALN gene (which encodes the pre-pro-GAL protein) is an unlikely candidate oncogene in breast tumors because an increase in pre-pro-GAL mRNA expression with GALN amplification was not observed [82][174][101,206] (Table 5). Many nerve fibers containing GAL have been reported in cardiac and esophageal carcinomas [175][207]; these fibers contacted closely with cancer cells, including those encircling tumor cells. In this study, GAL favored the extension of processes by dorsal root ganglion neurons, but the action of the peptide on tumor cells is currently unknown [175][207]. GAL1R DNA methylation is among the most epigenetic molecular alterations in endometrial cancer; this methylation indicates malignancy with a high degree of sensitivity and specificity [176][208]. The methylation of the GAL1R gene in bladder cancer has been involved in the prognosis of the disease, but the role played by the galaninergic system in this cancer is currently unknown [177][209].Table 5.
Involvement of the galaninergic system in other cancers.
| Actions/Presence | References | ||||||
|---|---|---|---|---|---|---|---|
| Breast cancer Human |
- GAL/pre-pro-GAL mRNA level expression. GALN gene: unlike candidate oncogene (Northern blot) | 101] | [120] | ||||
| [ | 82 | ][174] | [101,206] | - GAL1R gene promoter: frequently methylated (Q-MSP) | |||
| Carcinoma (cardiac, esophageal) Human | [158] | [191] | |||||
| - Fibers containing GAL contacted closely with cancer cells (IH) | [ | 175] | [207] | - Methylation status of some peptide-encoding genes, including GAL, is related with survival and recurrence. Methylation changes: possible molecular marker for HNSCC risk/prognosis (Q-MSP) | |||
| Endometrial cancer Human | [159 | - GAL | 1 | R DNA methylation indicated malignancy (q-PCR) | [176] | [208] | |
| Bladder cancer Human |
- GAL | 1 | R gene methylation involved in prognosis | [177] | [209][101 | [100] | ,119] |
| Pancreas Human |
- GAL promoted SW1990 cell proliferation | [179] | [211] | ||||
| Pancreas Rat |
- GAL blocked carcinogenesis and decreased norepinephrine level (IH, HPLC) | [180] | [212] | ||||
| - GAL2R overexpression: favored survival/proliferation by activating PI3K/Akt and MAPK/ERK-dependent pathways. Ras-related protein 1 (Rap1): involved in HNSCC progression. | |||||||
| [ | |||||||
| 103 | |||||||
| ] | [ | 122] | |||||
| - GAL/GAL1R: tumor suppressor. GAL1R absent in some cell lines (Q-MSP, RT-PCR) | [145][146][166] | [177,178,198] | |||||
| - GAL1R promoter: widely hypermethylated and related to reduced GAL1R expression. GAL1R/GAL2R hypermethylation: associated with higher recurrence rate and reduced disease-free survival (RT-PCR, Q-MSP) | [158][161][166][168] | [191,194,198,200] | |||||
| - GAL1R methylation status: potential biomarker for predicting clinical outcomes. Methylation: related to carcinogenesis and decreased GAL1R expression (RT-PCR, Q-MSP) | [160][161][166] | [193,194,198] | |||||
| Human (cell lines) Mouse |
- GAL (released from nerves) activated GAL | 2 | R expressed in tumor cells inducing NFATC2-mediated transcription of cyclooxygenase-2 and GAL. GAL released from tumor cells promoted neuritogenesis, favoring perineural invasion | [154] | [84] | ||
| Mouse | - GAL | 2 | R promoted tumor angiogenesis through the p38-MAPK-mediated inhibition of tristetraprolin (TTP), leading to an enhanced secretion of cytokines. GAL | 2 | R activated Ras-related protein 1b (Rap1B) favoring a p38-mediated inactivation of TTP, which acted as a destabilize cytokine transcript | [169] | [201] |
Q-MSP: quantitative methylation-specific PCR. RT-PCR: real-time PCR.
3.5. Galanin and Glioma
The GAL/GALR system has been described in glioma [19][99][30,118] in which the most abundant receptor observed was GAL1R, followed by GAL3R; GAL2R was not found (astrocytic/oligodendroglia tumors) [19][30] (Table 4). A reduced level of GAL has been observed in the cerebrospinal fluid of patients with glioblastoma [170][203], and regarding the expressions of GAL and GAL3R, no correlation with oligodendroglial, astrocytic and mixed neural–glial tumors was reported [19][30]. Moreover, no correlation was observed between the proliferative activity and GAL/GAL binding levels [99][118]. However, the high-grade glioma (WHO grade IV) has been related to the expression of GALHPLC: high-performance liquid chromatography; IH: immunohistochemistry; Q-MSP: quantitative methylation-specific PCR; q-PCR: quantitative real-time PCR.
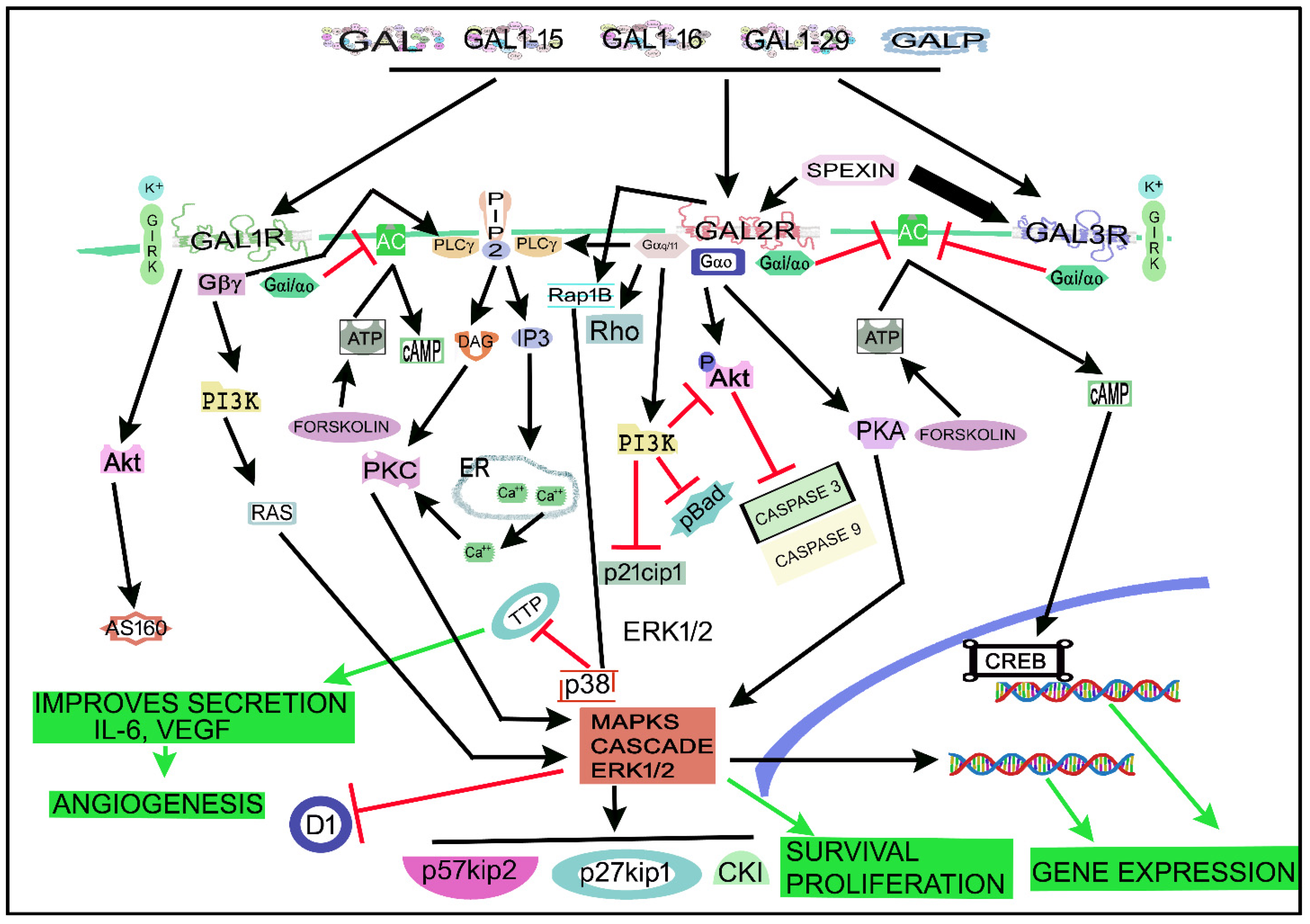
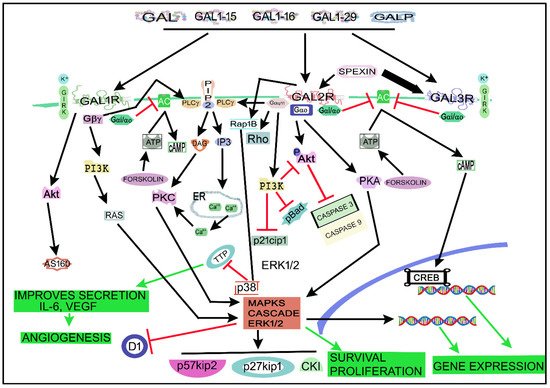
4. The Galaninergic System and Cancer: Signaling Pathways
Figure 34 shows the main signaling pathways in which the galaninergic system is involved. A GAL/GALR signaling network map focused on the signaling cascades regulated by the galaninergic system has recently been published [181][87]. GALRs (via PKC) activate the rat sarcoma virus (Ras, a small GTPase)/MAPK/ERK pathway by increasing the intracellular Ca2+ concentration [35][8]. The galaninergic system activates many signal transduction pathways depending on the coupled G protein type: GAL1R/GAL3R, mainly coupled to Gi/o, decrease the cAMP level and inactivate PKA, whereas GAL2R, preferably coupled to Gq/11 mobilizing intracellular Ca2+, promotes (via PKC) the activation of cell survival (via Akt or PKB) and MAPK1/MAPK3-dependent cell proliferation pathways [6][19][181][17,30,87]. GAL1R can also be coupled to Gβγ- and/or Gi-signaling pathways and then the activation of MAPKs occurs in a Ras/Raf-dependent manner [6][14][181][17,25,87]. GAL1R activation also favors the Akt/Akt substrate of the 160 kDa (AS160) cascade [181][87], regulates GIRK channels [64][182][4,77] and activates the ERK1/2 signal through the Gα/i subunit and not via the PI3K pathway linked to the Gβγ subunit [164][196]. GAL1R induces cell-cycle control proteins (p27kip1, p57kip2) and suppresses cyclin D1 in cancer cells [9][20]. GAL2R, mainly coupled to Gq/11, mediated the activation of PLC and small GTPase proteins in the Rho family [181][87]. PLC converted phosphatidylinositol, 4, 5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol triphosphate (IP3), which mediated PKC activation and increased the intracellular concentration of Ca2+ [64][77]. GAL2R activated the small GTPase protein Rho A in SCLC cells, suggesting the coupling to G12/13 [9][20]. GAL2R inhibited the production of cAMP, meaning that the receptor was coupled to Gi protein [183][83]. GAL2R decreased cofilin activation and Rho and Cdc42 GTPase activity [9][20]. In tumor cells, GAL2R activated the MAPK/ERK pathway in a PKC manner, meaning that GAL2R was coupled to a Go protein [9][20]. GAL2R regulated cell-cycle control proteins (p27kip1, p57kip2) and cyclin D1 and promoted apoptosis (caspase 3-dependent) in HNSCC cells [15][26]. GAL2R decreased the expression of p21cip1, phosphorylated BAD forms (pBad) and phosphorylated Akt (pAkt), downstream of the Gq11/PI3K pathway [15][26]. The GAL-mediated Akt pathway blocked the activity of caspases 3 and 9, whereas the GAL2R-mediated apoptosis in tumor cells was induced by the activation of the pro-apoptotic Bcl-2 protein Bim, through a mechanism independent of caspase [9][20]. GAL3R, involved in inward potassium ion (K+) currents, is coupled to the Gi/o signaling pathway and its activation favored the inhibition of cAMP and AC altering CREB phosphorylation [6][71][181][17,87,90]. GAL opened adenosine triphosphate (ATP)-sensitive K+ channels and hyperpolarized cell membranes in the rat RINm5F insulinoma cell line [184][214], and the peptide blocked the activity of AC and the secretion of insulin via the interaction with Gαi1, Gαi2 and Gαi3 proteins [43][185][53,215]. C7 peptide (GAL1-13-spantide amide), a GAL receptor antagonist, blocked hepatocellular carcinoma metastasis by targeting the hepatocyte growth factor/c-mesenchymal–epithelial transition receptor axis signaling pathway [186][216]. C7 inhibited the migration and invasion of tumor cells by blocking the phosphorylation of Akt and ERK1/2 [186][216].

Figure 34. Main signaling pathways in which the galaninergic system is involved. Black arrows indicate activation pathways, inverted red “T” indicates blockade/suppression, green arrows mean final results. AC, adenylate cyclase; Akt, Akt serine/threonine kinase family (also called PKB); AS160, Akt substrate of 160 kDa; ATP, adenosine triphosphate; Ca2+, calcium ion; cAMP, cyclic adenosine monophosphate; CKI, cyclin-dependent kinase inhibitor 1; CREB, cAMP regulatory element-binding protein; D1, a cyclin protein; DAG, diacylglycerol; ER, endoplasmic reticulum; FORSKOLIN, enzyme that produces cyclic adenosine monophosphate; GAL, galanin; GAL1-15 fragment, galanin 1–15 fragment; GAL1-16, galanin 1–16 fragment; GAL1-29, galanin 1–29 fragment; GAL1R, galanin receptor 1; GAL2R, galanin receptor 2; GAL3R, galanin receptor 3; GALP: GAL-like peptide; GIRK, G protein-coupled inwardly-rectifying potassium; Gα/11, G protein alpha subunit (11); Gαi/αo, G protein alpha i/o subunits; Gαo, G protein alpha subunit (o); Gβγ, G protein beta-gamma subunit; IL-6, interleukin 6; IP3, inositol triphosphate; K+, potassium ion; MAPK, mitogen-activated protein kinases cascade; p21cip1, a cyclin-dependent kinase inhibitor; p27kip1, cell-cycle control protein; p38, a class of mitogen-activated protein kinase; p57kip2, cell-cycle control protein; pAkt, phosphorylated Akt; pBad, phosphorylated BAD forms (induces apoptosis by inhibiting antiapoptotic BCL-2 family members); PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol bisphosphate; PIP2, phosphatidylinositol, 4, 5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; Rap1B, Ras-related protein Rap-1b; Ras, rat sarcoma virus (a small GTPase); Rho, a family of small signaling G proteins (a subfamily of the Ras superfamily); TTP, tristetraprolin; VEGF, vascular endothelial growth factor.
5. Therapeutic Strategies
Peptides play an important role in cancer; the in-depth knowledge of the functions mediated by these substances is an emerging and promising line of research that could lead to new clinical applications in oncology. One line of research could be the use of peptides coupled to cytotoxic agents to exert an antitumor action, and another, the use of peptide receptor antagonists or agonists. In the case of GAL, GALR antagonists or agonists could be used as antitumor treatments according to the different signaling pathways and actions mediated by GALRs. GALR antagonists have been administered for the treatment of food intake disorders, anxiety, depression and Alzheimer’s disease, whereas GALR agonists have been used for the treatment of chronic pain [7][74][18,93]. It has also been reported that SNAP 37889, a non-peptidergic GAL3R antagonist, promoted apoptosis in promyelocytic leukemia cells expressing GAL2R [187][217].
In vitro and in vivo experiments using human gastric cancer cell lines have been performed to study the antitumor action of a triple treatment with GAL, serotonin and octreotide (an octapeptide that mimics the actions mediated by somatostatin) [146][178]. Treatment with one compound or with a double/triple combination decreased cell proliferation and viability in vitro, and tumor volume/weight was reduced in vivo after the triple treatment. However, this reduction was not due to apoptosis or cell proliferation inhibition; thus, other unknown mechanisms were involved [146][178]. In experimental animals, implanted human colon cancer cells were treated with the triple treatment (octreotide, serotonin and GAL were administered subcutaneously or intraperitoneally) [188][189][190][218,219,220]: tumor volume/weight, number of viable cells, proliferation index and tumor vascularization decreased, whereas the apoptotic index increased. In nude mice implanted with colonic adenocarcinoma cells and treated with the triple treatment, the tumor volume decreased and the apoptotic index and volume density of the tumor necrotic tissue increased [191][221]. The triple therapy did not show any apparent side effects [192][222].
6. Conclusions
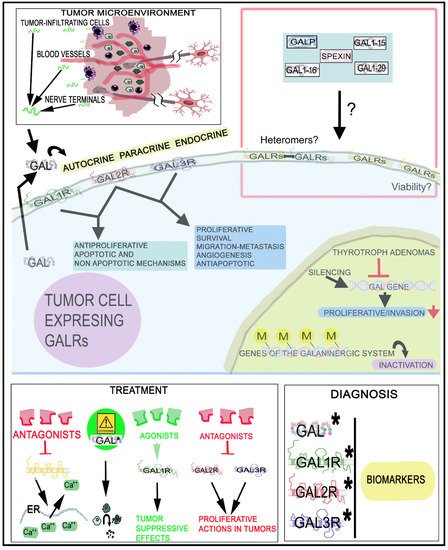
The galaninergic system is involved in tumorigenesis, invasion and migration and has been correlated with tumor stage/subtypes and metastasis and, in this system, epigenetic mechanisms have been related with carcinogenesis and recurrence rates. GALRs play a crucial role in cancer and their specific actions must be clearly understood in many tumor types because GALRs mediate different signal transduction pathways and actions depending on the tumor cell type and the particular G protein involved. GALRs could be used as a therapeutic target and diagnostic marker for the treatment, prognosis and surgical outcome in certain tumors. Different from other peptidergic systems, the galaninergic system exerts a proliferative action on tumor cells, but GAL also suppresses the development of tumor. Thus, in-depth studies using GALRs agonists or antagonists as antitumor agents must be conducted to search for therapeutic strategies (alone or in combination with chemotherapy/radiotherapy) against tumor development. The involvement of the galaninergic system in cancer is a line of research that has been abandoned, but it must be re-opened and developed in the future. Additional studies must be carried out, for example, on the use of GALR agonists/antagonists as antitumor agents, the activation of signaling transduction pathways, the involvement of heteromers, targeted radionuclide cancer therapy and the viability of GALRs. This knowledge is crucial to establish future potential clinical antitumor applications, although unfortunately, the pharmaceutical industry has generally had no interest in this line of research; however, the data reported here suggest that the galaninergic system is a promising target for the treatment of tumors (Figure 47).
Figure 47. Involvement of the GAL/GALR system in cancer, diagnosis and treatment. GAL1R/GAL2R mediate an antiproliferative effect, whereas GAL2R/GAL3R promote a proliferative action on tumor cells. GAL originates from tumor cells, tumor-infiltrating cells and nerve cells. Circulating GAL can also bind to GALRs. ↑: increase; ↓: decrease; ?: mechanisms that must be investigated (presence/functions of heteromers in tumor cells, involvement of GALRs in the viability of cancer cells and involvement of GAL fragments and other peptides belonging to the GAL family of peptides in cancer). *, biomarkers; M: methylation.
