Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashley A Stegelmeier | -- | 5200 | 2022-10-26 04:06:42 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. Use of PGPB to Promote Plant Hydroponic Growth. Encyclopedia. Available online: https://encyclopedia.pub/entry/31269 (accessed on 07 February 2026).
Stegelmeier AA, Rose DM, Joris BR, Glick BR. Use of PGPB to Promote Plant Hydroponic Growth. Encyclopedia. Available at: https://encyclopedia.pub/entry/31269. Accessed February 07, 2026.
Stegelmeier, Ashley A., Danielle M. Rose, Benjamin R. Joris, Bernard R. Glick. "Use of PGPB to Promote Plant Hydroponic Growth" Encyclopedia, https://encyclopedia.pub/entry/31269 (accessed February 07, 2026).
Stegelmeier, A.A., Rose, D.M., Joris, B.R., & Glick, B.R. (2022, October 26). Use of PGPB to Promote Plant Hydroponic Growth. In Encyclopedia. https://encyclopedia.pub/entry/31269
Stegelmeier, Ashley A., et al. "Use of PGPB to Promote Plant Hydroponic Growth." Encyclopedia. Web. 26 October, 2022.
Copy Citation
Improvements to the world’s food supply chain are needed to ensure sufficient food is produced to meet increasing population demands. Growing food in soilless hydroponic systems constitutes a promising strategy, as this method utilizes significantly less water than conventional agriculture, can be situated in urban areas, and can be stacked vertically to increase yields per acre. One method to increase hydroponic plant yields involves adding plant growth-promoting bacteria (PGPB) into these systems. PGPB are organisms that can significantly increase crop yields via a wide range of mechanisms, including stress reduction, increases in nutrient uptake, plant hormone modulation, and biocontrol.
hydroponics
plant growth-promoting bacteria
plant stress
plant growth
ACC deaminase
siderophores
food production
cannabis
space
1. Introduction
Between 1900 and 2022, the human population has quadrupled to approximately 8 billion people, with current projections expecting 10 billion inhabitants by 2050. Our existing food production systems are insufficient to meet this population increase, as it is estimated that over 800 million people already suffer from hunger worldwide, while 3.5 billion suffer from deficiencies of at least one essential nutrient [1]. As income growth continues in low- and middle-income countries, there will be increasing demand on the food production system, and this will be in addition to the food required to meet the needs of 2 billion additional people [2]. Thus, it is estimated that to meet global food requirements, agricultural production needs to increase by at least 0.5% each year by 2050 [3].
The first major problem in respect to increasing food production is that ~50% of all the world’s habitable land is already used for agriculture [4]. Of the remaining portion, 37% is forests, 11% is shrubs and grasslands, 1% is freshwater coverage, and 1% is urban areas. Historically, one of the major ways that humans have increased food production is by expanding the amount of agricultural land. One thousand years ago agricultural land made up only 4% of all habitable land, in comparison to 50% today [5]. Without an increase in productivity this would mean that 50–100% of the remaining habitable land would need to be converted into agricultural land to feed the human population, an idea that is completely unsustainable from a climate change or ecological standpoint.
The second major problem is that the quality of current agricultural land quality is on the decline. Over 50% of current agricultural land is moderately or severely impacted by soil degradation processes including erosion, acidification, compaction, salinization, and contamination [1]. Soil erosion is considered the largest contributing factor to soil degradation, accounting for a loss of 75 billion tons of fertile soil each year [6]. This means that each year, without agricultural expansion and its associated environmental costs, there is less available or productive land to produce the food our population requires.
The third major problem is that, due to climate change, the productivity of our current crops is on the decline. It is predicted that rising global temperatures and erratic weather conditions will lead to major decreases in staple crop production including maize (20–45%), wheat (5–50%), and rice (20–30%) [1]. Additionally, up to 30% of annual agricultural production could be lost due to increasing phytopathogen and pest pressure as temperatures continue to rise [7].
The fourth major problem is freshwater availability. Globally over 50% of the world relies on groundwater for their daily needs, and over 35% of agricultural irrigation uses groundwater as its source [8]. Unsustainable agricultural use combined with droughts has led to a decline in groundwater in many geographic areas [8]. In 2010 it was estimated that over 80% of people live in areas that have a high likelihood of water security threats [9]. As such, reducing agricultural usage is essential to ensure adequate fresh water supplies to meet the demands of the growing human population.
While overcoming these food production challenges may seem like an impossible task, controlled environment agriculture (CEA) systems are proving a promising solution. CEA is the process of growing crops in a manner that gives the farmer partial to full control over the environmental variables that affect plant growth. Low tech systems such as row covers, low tunnels, high tunnels, and net covers are often referred to as “protected agriculture” [10]. These systems provide less control over environmental systems in comparison to their “high tech” counterparts. High tech-controlled environment systems include greenhouses and indoor farms [11].
While the earliest recorded cases of greenhouse use stretch back to 14 CE during the Roman period, commercial-scale use did not begin until the 20th century in the Netherlands [10]. During World War II the Netherlands’ greenhouses sustained extensive damage, and during reconstruction after the war engineers in the Venlo region developed the tall, glass, multi-span greenhouse that is often seen today. Significant efforts were made post war to ensure food security in the Netherlands, including the construction of many Venlo style greenhouses for vegetable production [12]. Since the initial introduction of commercial CEA production in the Netherlands, greenhouse use has expanded across the globe [13]. Significant improvements in glazing materials, lighting sources, and growing systems have greatly increased the yield potential of greenhouse production in comparison to field agriculture. One significant advancement was the introduction of hydroponic crop production.
Hydroponics is the method of growing plants in soilless systems where the nutrients for growth are provided via a water based nutrient solution. In comparison to soil-based systems hydroponic systems offer significantly higher yields (~14× higher kg/fresh weight/m2 in vertically farmed lettuce) [14] and faster growth times as nutrients are more readily available to the plant and root growth is not hindered by mechanical interference from the soil [15][16]. Hydroponic and aquaponic systems can also be installed in areas where the soil or climate is unsuitable for traditional agricultural and aquaculture production [17]. This can help increase food production in areas where the soil has become contaminated, acidified, and/or salinized. Additionally, when combined with a greenhouse structure, hydroponic systems offer year-round local production of fresh fruits and vegetables in colder climates [18]. Furthermore, hydroponic systems have been shown to use less fertilizer than soil-based production and up to 90% less water [10][19].
While the advantages of hydroponics offer significant promise with regard to feeding the growing global population, there are still hurdles that must be overcome for this technology to overtake conventional soil-based farming. First, greenhouses have a high initial setup cost due to the materials required (namely steel and glass or plastic depending on the glazing selected) [20]. Second, hydroponic systems are far more complicated to operate than conventional growing equipment given the number of environmental variables under the growers’ control. Third, the margin for error is much lower and system failures or electrical outages can cause serious impacts on plant health [21]. Finally, pathogens can easily spread throughout an entire crop due to the proximity of plants and recirculated nutrient solution [22].
While hydroponic greenhouse growers have been able to optimize most environmental factors such as lighting, CO2, heating, and fertilizers, to date the plant microbiome has largely been ignored. Plant growth promoting bacteria (PGPB) offer significant benefits to plants in soil-based systems [23]. This includes helping the plant uptake nutrients, promoting growth, stress regulation, and pathogen prevention. However, many plant growth-promoting microbes found in soil cannot make the transition to hydroponic environments [24]. Nevertheless, PGPB offers a unique solution to several of the largest problems in hydroponic production by preventing pathogen outbreaks, improving the plants’ response to environmental stress, and increasing crop yield per m2 all of which reduces the payback period of the initial capital investment.
2. Hydroponic Systems
The earliest known records of plants grown in a hydroponic like system come from descriptions of the Hanging Gardens of Babylon built along the Euphrates River circa 600 BCE, in what is now Iraq. However, widespread commercial use of these systems did not begin until the late 1900’s when hydroponic growing systems gained popularity with greenhouse vegetable growers in Europe and North America [10]. In the 1700’s researchers began experimenting with growing plants in what would become known throughout the research community as “water culture”. In water culture studies researchers had the ability to control specifically what nutrients were available to the plants through the addition of chemicals to the water in the system. As such, this technique was used in many of the studies that determined the nutritional requirements of plants [25].
Throughout the 19th and early 20th century water culture was used solely for the purposes of academic research. However, this changed in 1929 when Dr. William Frederick Gericke from the University of California published a paper outlining the potential for growing commercial crops in water culture [26]. In Gericke’s study, a water reservoir was created using bituminous roofing paper, topped with wire mesh, burlap, and sand (which was used as a growing media for the plants; Figure 1). The reservoir was then filled with water supplemented with “the elements required for growth of plants in water” [26]. Gericke used this system to grow numerous types of plants, including those intended for commercial food production. Gericke states in his 1929 paper that the “results obtained warrant serious consideration of this method for production of certain crops grown on an intensive scale”, and the field of hydroponic crop production was born.
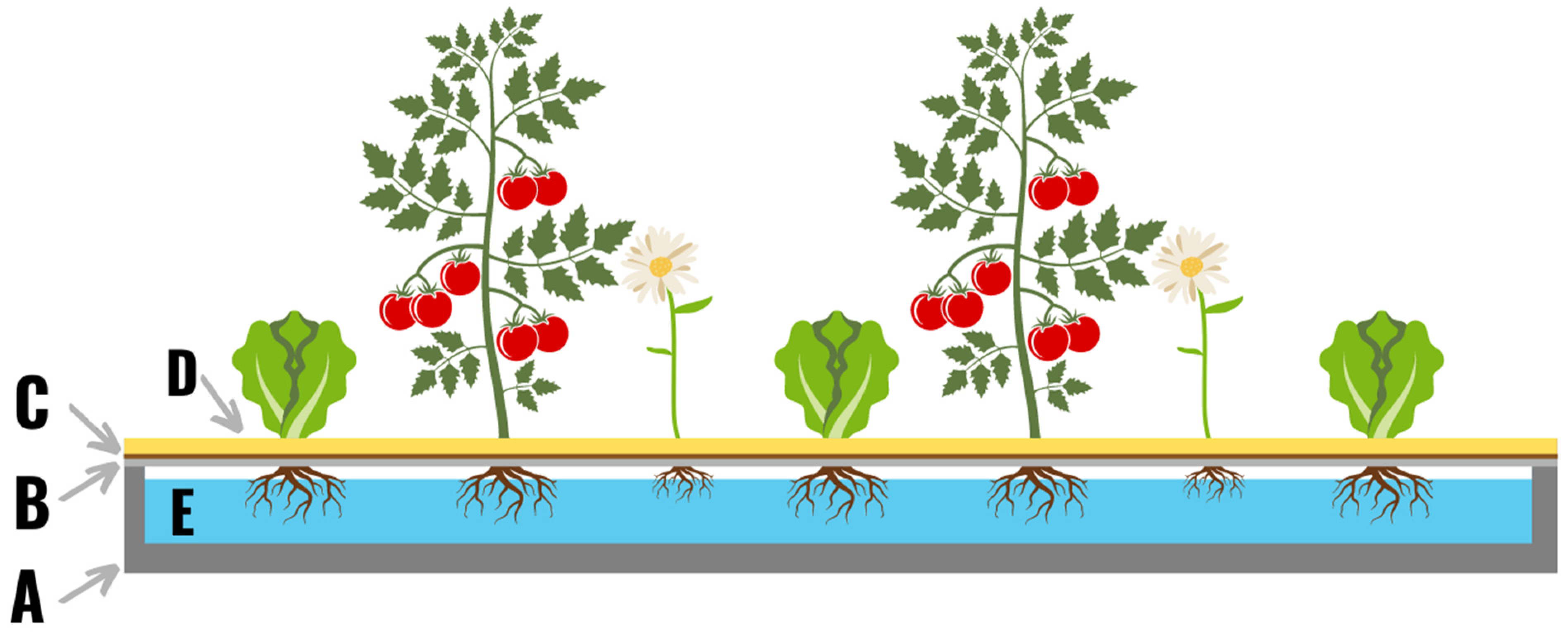
Figure 1. The original hydroponic system built by Gericke in 1929. A roll of bituminous roofing paper (36 ft × 3 ft) was folded up on every edge 6 inches to form a trough (A). On top of the trough a layer of wire netting was attached (B), and a layer of burlap (C) was added on top of the wire. A one-inch layer of sand (D) was added on top as a substrate for the plants to grow in. The trough was filled with nutrient solution (E), and a variety of crops were planted in the system.
While most modern hydroponic systems have come a long way from that reservoir made with roofing paper, the core principles of the technique remain the same. In 1938 Gericke defined hydroponics as the “art and science of crop production in liquid culture media” [25]. The term hydroponics was suggested by Dr. William Setchell from the University of California to separate the previous research focused water culture technique and the new commercial focused growing technique [27]. The term itself is a combination of the words, “hydro” (meaning water) and “ponos” (meaning labor) [25][27].
Most modern hydroponic systems can be categorized into seven main types. These systems are primarily differentiated by how the nutrient solution is applied to the root system of the plant.
3. Plant Growth-Promoting Bacteria
Although there are a wealth of hydroponic system options available for growers to select from, further advancements are needed to improve profitability via increases to crop yields. Hydroponic greenhouses need large capital investment to build, and require significant electricity and labour inputs to operate [28]. Fertilizers have significantly increased in cost in part due to increased global trade instability and supply disruptions. Indeed, prices increased 78.6% year-over-year in 2021 [29]. Moreover, there are several types of crops that are rarely profitable to grow hydroponically, including corn, potatoes, and large root vegetables (e.g., onion, carrots, and rutabaga). Solutions are needed that strengthen local food production and expand the range of viable hydroponic crops for growers to select from. Decades of research in soil suggest that microbial inoculants comprised of plant growth promoting bacteria constitute an exciting solution.
The interaction between soil bacteria and plants may be beneficial, harmful, or neutral for the plant. Beneficial plant growth-promoting bacteria (PGPB) facilitate plant growth by several different mechanisms. They are typically found in the soil along with bacteria that are deleterious to plant growth (phytopathogens) and bacteria that do not have any discernible effect on plant growth and development (commensal bacteria) (Figure 2A).
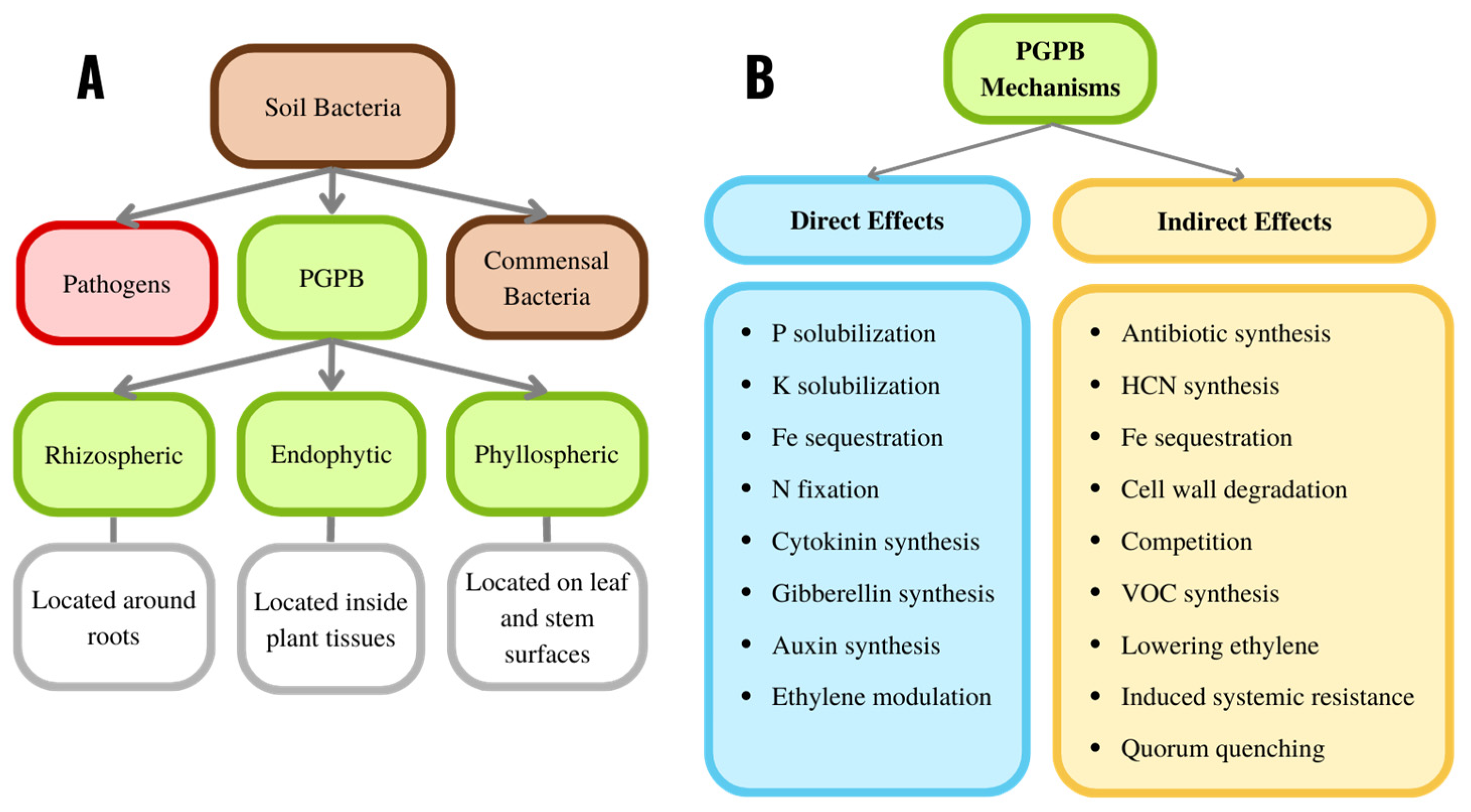
Figure 2. (A) Overview of some of the components of soil bacteria and their localization. (B) Overview of the major mechanisms used by PGPB. VOC refers to volatile organic compounds.
The soil contains a wide range of living entities including various microorganisms (bacteria, fungi, algae, and protozoa), nematodes, and earthworms with ~95% of these organisms being bacteria [30]. The number of bacteria that typically exist around the roots of plants is about 10- to 1000-fold greater than the number that are found in the bulk soil, with the highest concentration of bacteria being found immediately around the roots of plants in the rhizosphere [31]. The preponderance of microorganisms in the immediate vicinity of plant roots is a direct consequence of the fact that plants commonly exude a significant fraction of their photosynthetically fixed carbon through their roots [32][33]. Different plants produce varying amounts and compositions of root exudates; root exudates are also affected by plant age and nutrition as well as the presence of environmental stressors. Root exudates provide bacteria with carbon substrates that drive microbial metabolic processes. Each plant species (and subspecies) exudes specific small molecules so that plants “attract those microorganisms that are beneficial to plants and exclude those that are potentially pathogenic” [34]. Thus, the chemical composition of the root exudates of a particular plant species shapes the microbial community within the rhizosphere of that plant.
Many of the PGPB from the plant rhizosphere bind directly to the surface of the plant roots (i.e., they are found in the rhizoplane) (Figure 2A). Some PGPB can colonize the interior surfaces of the plant (i.e., they are found in the plant’s endosphere) without harming the plant. Other PGPB bind to the surface of plant leaves and stems (i.e., they are in the phyllosphere). Except for the determinants used by PGPB to localize rhizospherically, endophytically, or phyllospherically, all these bacteria utilize similar, if not identical, mechanisms for facilitating plant growth and development [35][36].
4. Plant Growth Promoting Bacteria Research in Hydroponics
4.1. The Hydroponic Microbiome
The Dutch botanist and microbiologist Lourens Bass-Becking once said, “Everything is everywhere, the environment selects”. Given that a hydroponic system is distinct from a soil environment surrounding the rhizosphere, it stands to reason that the same plant variety may have different rhizospheric microbial communities depending on whether it is growing in soil or a soilless environment. Hydroponic systems have different levels of moisture, oxygen [37], and nutrients [38] than soil. Oxygen exchange is highly dependent on the matrix being used: soil oxygen and moisture levels affect biogeochemical cycles and the nitrogen cycle [39]. These systems can result in changes to the crop quality. For example, hydroponic lettuce had a stronger root system and more moisture content than a soil-grown crop, although antioxidant content was reduced [40]. Barley was more susceptible to salt stress in hydroponic systems compared to soil-based plants [41]. Several research groups have conducted thorough studies to obtain empirical data on how differences between soil and soilless environments affects not just crops but the hydroponic microbiome.
An important question is whether hydroponic systems have elevated levels of human pathogens [42][43]. Every year large recalls occur due to contaminated produce, thus a growing environment that is low in pathogens is critical if the amount of produce grown in hydroponics continues to increase as projected. Human pathogens are uncommon native organisms in hydroponic systems, further supporting the safety of this agricultural method for consumers. For example, Clostridium botulinum, Escherichia coli, Salmonella, and Staphylococcus aureus were not found within lettuce hydroponic systems [42]. Water and leaves from hydroponically grown lettuce in Puerto Rico were analyzed to characterize potential human pathogens [43]. Entercoccus faecalis was the most predominant pathogen, found in 11% of leaf samples, but not in the water. A range of 4–70 CFU/mL total bacteria were quantified in leaves, which was lower than >300 total CFU/mL found in the water system. Detectable levels of E. coli O157:H7 and Salmonella were not observed within the samples. However, ~78% of their samples contained bacterial isolates, including Aeromonas, Bacillus, Corynebacterium, Mycobacterium, Pediococcus, Pseudomonas, and Serratia [43]; many of these bacteria are plant growth promoters in hydroponic systems.
High throughput sequencing has enabled scientists to ascertain which organisms are prevalent in hydroponic systems. A study of the influence of urine-derived fertilizers conveyed the range of OTUs that are native to the hydroponic lettuce rhizosphere [44]. Pseudomonas was the only genus that was a true indicator organism, present in over 90% of samples. Burkholderia and Sphinogomonas were also highly prevalent. Out of 185 identified OTUs, other highly indicative families were Rhizobiaceae, Chitinophagaceae, and Flavobacteriaceae. Hydroponic derived organisms found surviving in the plant rhizosphere are more likely to persist in a hydroponic system than soil-derived organisms. This study did not conclude that Bacillus was an indicator organism in hydroponics, which is a notable difference from soil studies of the same crops. Anzalone et al. [24] conducted a tomato rhizosphere metagenomics study comparing the communities between soil and soilless coconut fiber environments. They concluded that the tomato microbiome was controlled by the environment in which the plant grew. Significant differences in microbial communities were observed in soil vs. hydroponic systems. The hydroponic tomato rhizosphere had significantly reduced bacterial and fungal diversity, despite coming from identical nursery stock [24]. PCoA plots visualizing community similarities clearly showed that samples grouped by substrate type. These findings suggest that organisms isolated from soil may not always be able to survive on the same plant in hydroponic greenhouses.
A taxonomic survey was conducted in a lettuce hydroponic facility to determine the microbial communities present in the water, nutrient solution sump, biofilter effluent sump, and tilapia aquaculture tanks [45]. The plants had a strong influence on the microbial community present, which remained relatively constant despite various treatments. They concluded that the impact of microbial inoculants on the community structure was lower than expected and suggested that growers and scientists need to carefully balance sterilization vs. the need to maintain a healthy microbiome in the systems. However, it must be noted that the organism used to formulate these conclusions was Bacillus amyloliquefaciens, supporting evidence that Bacillus species do not always perform as well as other organisms, such as Pseudomonas, that thrive in hydroponic systems.
A study by Sheridan and colleagues analyzed microbial community changes of potato, soybean, durum wheat, and bread wheat crops after receiving a commercial microbial inoculant containing 48 strains of different organisms [46]. The authors determined that the most abundant organisms in the mix did not correlate with the most effective colonizer in a hydroponic system. They observed that microbial communities were specific to the crop type, indicating the same mixture does not interact to the same extent with all crops that were tested. Interestingly, an unexpected ten-hour 50 °C heat event in the durum wheat hydroponic system caused a shift in the crop’s microbiome, resulting in the thermophile Chlorobi OPB56 significantly increasing in abundance. As the planet warms and heat events become more frequent, the knowledge that heat shifts can change the hydroponic rhizosphere is important. A separate study that focused on which traits created the best hydroponic colonizers concluded that better PGPB colonizers of Duckweed roots contained relatively more genes for bacterial chemotaxis, flagellar assembly, and two-component systems [47]. This suggests that the ability for a bacterium to travel and move towards plant exudates increases its ability to colonize hydroponic roots.
4.2. PGPB That Increase Nutrient Uptake
Plants need a range of macro and micronutrients to thrive. The six macronutrients are carbon, hydrogen, nitrogen, oxygen, phosphorus, and potassium while the eight plant micronutrients are boron, chlorine, copper, iron, manganese, molybdenum, nickel, and zinc. PGPB that increase nutrient uptake for crops improve the availability of one or more of these nutrients to facilitate plant growth. Increasing nitrogen, phosphorus, and iron uptake are the most commonly tested strategies in the hydroponic PGPB literature.
Nitrogen is an essential plant nutrient that is critical for amino acid synthesis, chlorophyll, and nucleic acid development. Plants cannot obtain nitrogen directly from the atmosphere, thus relying on alternative forms such as ammonia and nitrate. Nitrogen is one of the main components of chemical fertilizers. Globally, approximately 115 million tons of nitrogen is applied annually to fields [48], although only a third of this is taken up by the plants. Bacteria participate in the nitrogen cycle converting gaseous nitrogen into more available forms. Nitrogen fixation converts atmospheric N2 into ammonia; ammonia oxidation then converts ammonia into nitrite, which can then be converted into nitrate via nitrite oxidation. Nitrogen fixing bacteria include Rhizobia, Azospirillum, Azotobacter, Bacillus, and Beijerinckia. When added to a nitrogen-free hydroponic system, Azospirillum and Bacillus increased nitrogen yield in bananas by up to 144%, shoot growth by ~200%, and biomass by ~140% [49]. In one study, Azotobacter was immobilized onto beads. The author determined that adding 5g of beads per plant optimized growth of Choy sum (a Chinese flowering cabbage) [50]. The presence of Acinetobacter increased the amount of nitrogen that Duckweed could obtain from pondwater [51]. A commercial mixture of Bacillus spp. influenced the levels of ammonia, nitrite, and nitrate in an NFT system containing Red Cherokee lettuce [52]. A second lettuce study using Tiberius romaine lettuce developed a consortium containing multiple nitrogen fixers, including Azotobacter chroococcum, Azospirillum brasilense, Pseudomonas fluorescens, and Bacillus subtilis [53]. The authors observed that the amount of nitrogen uptake almost doubled, especially when (non-nitrogen-fixing) arbuscular mycorrhizal fungi were also added in with the bacteria. Lastly, a mixed culture of unspecified nitrifiers and ammonifiers was able to utilize organic nitrogen in a tomato crop [54], creating the potential for hydroponic growers to switch to organic fertilizers instead of the typical inorganic fertilizers.
Phosphorus is another macronutrient that is essential for plant health. It is a building block of nucleic acids, and contributes to photosynthesis, root growth, and maturation. Phosphorus solubilizing bacteria convert phosphorus from the nutrient solution into a more bioavailable form, as plants can only absorb phosphorus in monobasic and dibasic forms. Similar to its involvement with the nitrogen cycle, Acinetobacter increased the amount of phosphorus that duckweed could obtain from pondwater [47]. A mixture of Bacillus spp. increased phosphorus solubilization for lettuce and increased yields [52] PGPB identified in a sorghum trial included a range of the phosphorus solubilizing bacteria Pseudomonas spp., Burkholderia spp., Phylobacterium spp., and Chitinophaga japonensis [55]. Phosphate solubilization is a common trait of Pseudomonas spp. in switchgrass [56][57], and tomatoes [58][59]. Several beneficial organisms were found to have both nitrogen fixation and phosphorus solubility, including Pantoea agglomerans in rice experiments [60]. An experiment with soybeans evaluated the increase in photosynthesis capabilities after applying a commercial microbial consortium [61]. The complex mixture of bacteria, yeasts, and fungi was hypothesized to be providing multiple benefits to the soybeans, including improved nitrogen and phosphorus uptake.
The micronutrient iron is a key component of chlorophyll, and as such iron deficient plants undergo chlorosis, which is characterized by yellow leaves from a lack of chlorophyll. Iron also improves plant enzymatic functions and respiration. Iron uptake is increased by bacteria that secrete siderophores, which are high-affinity iron chelators that effectively bind iron and increase iron sequestration for both the plants and bacteria. A study in canola focused on four PGPB that all possessed siderophores amongst other beneficial traits [62]. Arthrobacter, Bacillus altitudinis, Bacillus megaterium, and Sphingomonas increased biomass production; however, the authors concluded that Sphingomonas was the best candidate for future studies. A cucumber trial that tested the beneficial fungus Trichoderma harzianum resulted in inoculated plants possessing an increase in multiple nutrients including phosphorus, iron, copper, manganese, and zinc [63]. Although the authors did not test for siderophores, they observed a 90% increase in phosphorus and 30% increase in iron within the plants. More recent advances have shown that T. harzianium produces a novel siderophore called harzianic acid [64]. Interestingly, a strawberry study demonstrated that not all siderophore structures behave equally [65]. They concluded that bacteria with hydroxamate siderophores produced by the PGPB Gluconacetobacter diazotrophicus were more beneficial for iron uptake to the crop than catechol siderophores produced by Azospirillum brasilense. Catechols are less stable than hydroxymates and are susceptible to oxidation.
4.3. PGPB That Regulate Hormones
Control of the phytohormone ethylene via ACC deaminase is an effective strategy to increase crop yields in hydroponic systems. PGPB with this gene have been isolated from the international space station [66]. When twenty bacterial species were analyzed for a range of PGPB traits it was determined that Pseudomonas agglomerans and Bacillus pyrocinnia both possessed multiple PGPB beneficial traits, including ACC deaminase, phosphate solubilization, and siderophore production. A study that observed over 20% increases in canola yields also used bacteria that possessed functional ACC deaminase, IAA, phosphate solubilization, and siderophores [62]. Likewise, the best strain in terms of promoting plant growth out of 305 isolates in rice experiments possessed ACC deaminase, IAA, and siderophores [67]. Additionally, bacteria with a range of beneficial traits including ACC deaminase, IAA, phosphorus solubilization, and N cycling increased wheat yields [68]. These studies highlight that single strains that possess at least three functional plant growth promoting traits including ACC deaminase are highly successful in increasing yields in a wide range of crops.
Bacterial IAA is an auxin involved in L-tryptophan metabolism that is responsible for increases in plant growth. Within cucumbers, the two most successful PGPB tested, Serratia marcescens and Pseudomonas putida, were also the two strains that produced IAA [69]. Both organisms performed better than Bacillus amyloliquefaciens and an unspecified Bacillus spp. strain 70. Consortium trials of five IAA producers increased wheat yields from 36–80% [70]. A consortium of auxin producers including Bacillus cereus, Bacillus thuringiensis, and Buttiaxella agrestis remarkably reduced the time required for banana seedling acclimatization from 90 to 25 days [71]. Many PGPB have multiple beneficial traits, as evidenced by this consortium’s ability to also produce the hormone cytokinin, hydrocyanic acid, siderophores, and solubilize phosphorus. A lettuce trial using Gluconacetobacter diazotrophicus observed up to 16% increases in yield using an organism that produces both the hormones IAA and gibberellin [72]. Similarly, bacteria possessing IAA, nitrogen fixation, and phosphorus solubilization increased rice yields up to 20% [60][73]. Pseudomonas fluorescens increased tomato crop yields by up to 18% [74]. Although the authors were unsure of the exact mechanism, they suspected growth regulating substances were involved. Many strains of Pseudomonas fluorescens have been documented as having both IAA and ACC deaminase in more recent research [75]. Indeed, IAA and ACC deaminase producing Pseudomonas outperformed other isolates in sorghum [55], switchgrass [56][57], and tomato trials [58]. Single organisms with the ability to produce both hormones and increase nutrient uptake frequently perform the best in both soil and hydroponic systems [76].
4.4. Biocontrol Agents
Crop diseases significantly harm global food production and can have a devastating effect in greenhouses that become contaminated. Implementing biocontrol efforts to reduce the severity of infections are an effective strategy to increase yields in hydroponic farms. A wide range of PGPB have been tested against hydroponic phytopathogens.
Pythium is a parasitic oomycete that causes root rot and damping off in many crops including ornamental flowers, arugula, cucumber, lettuce, spinach, sweet pepper, and tomato [77]. A study of hydroponically grown Chrysanthemums concluded that Pseudomonas chlororaphis and Bacillus cereus were the best PGPB for Pythium biocontrol after they reduced pathogen root colonization by 72–91% [78]. Pseudomonas chlororaphis also effectively prevented Pythium infection in Romaine lettuce [16] and Cubico sweet peppers [79]. In Cortina lettuce, the commercial product “Boost” containing Bacillus subtilis was more effective than other products with Enterobacter, Trichoderma, or Gliocladium [80]. The mechanism of action was unknown, but the authors suspected that the organisms were inducing plant resistance or preventing Pythium colonization. Their conclusions were further supported by a separate study that observed that Bacillus also reduced Pythium root colonization in a trial with Red Coral and Green Oak lettuce [81]. A hydroponic tomato study observed >50% decrease in disease incidence when either Pseudomonas fluorescens, Gliocladium, Trichoderma, or Streptomyces were applied [82]. Applying Lysobacter enzymogenes in combination with chitosan reduced disease in cucumber plants by 50–100% in four independent trials [83]. Another cucumber trial tested four commercial inoculants to determine their efficacy against Pythium [84]. Mixtures containing either Gliocladium catenulatum or Streptomyces griseoviridis were more effective than Trichoderma treatments. Together, these studies suggest that Pseudomonas chlororaphis, Bacillus spp., and Gliocladium work effectively in a range of crops to prevent root rot and damping off. Indeed, the body of literature suggests that Bacillus is more effective at preventing Pythium infections than it was at colonizing hydroponic roots and increasing growth in healthy non-infected plants.
Several other plant diseases are relevant to hydroponic systems and may be alleviated by inoculating crops with PGPB and fungi as a means of biocontrol. Fusarium is a filamentous fungus that causes wilt disease in a wide range of crops. Several researchers have studied PGPB in an attempt to find suitable biocontrol candidates. Like biocontrol strategies for Pythium, the bacteria Gliocladium catenulatum and Pseudomonas chlororaphis significantly reduced Fusarium seedling mortality [85]. Commercial mixtures containing Trichoderma or Streptomyces reduced disease incidence by >50% [82]. The pathogen Ralstonia is the causative agent of bacterial wilt disease. In one study, a combination of Trichoderma viride, Bacillus thuringiensis, and Pseudomonas fluorescens inhibited ~70% of bacterial wilt in Linda lettuce [86]. Another bacterial phytopathogen is Pectobacterium (formerly Erwinia), which causes vegetable soft rot; the bacterium Rhodococcus reduced maceration in potatoes by degrading the quorum sensing capabilities of the pathogen [87]. Lastly, Colletotrichum graminicola is a fungus that causes stalk rot in grains and maize. Trichoderma virens protected maize against C. graminicola infection and reduced disease severity. The T. virens inoculant produced Sm1, a compound that induced plant defense mechanisms [88].
4.5. Bioremediation and Osmotic Stress
Bacteria isolated from hydroponic crops have promising capabilities for bioremediation. Carbendazim is a fungicide that negatively impacts aquatic organisms. A biofilm consortium comprised of Flavobacterium, Flectobacillus, Klebsiella, and Stenotrophomonas was able to degrade ~35 mg/L carbendazim to ~8 mg/L in 20 h [89]. Two studies on switchgrass demonstrated that Pseudomonas species could reduce cadmium stress and increase plant growth in the presence of 20 µmol/L cadmium. The organisms also had a range of beneficial plant growth promoting traits including ACC deaminase, IAA production, and phosphorus solubilization [56][57]. Inoculated plants had elevated expression of the heat shock proteins HSP70 and HMA3, which improves cadmium tolerance in plants. A strain of Pseudomonas fluorescens also promotes cadmium uptake in the perennial plant Sedum alfredii [75], while Pantoea agglomerans reduced cadmium concentrations and increased yields by ~20% in rice [60]. Another contaminant that is toxic to plants and harms humans upon ingestion is arsenic [90]. Research in rice concluded that a combination of Pseudomonas stutzeri and Cupriavidus taiwanensis reduced arsenic toxicity in rice [73] by converting arsenic to a harmless arsenic sulfide form. The bacteria also had a range of beneficial plant growth promotion mechanisms including ACC deaminase, IAA, phosphorus solubilization, and nitrogen fixation.
Salinity stress can occur in hydroponic systems and reduce crop yields. A wide range of negative effects including reduced photosynthesis, reduced root elongation, stem diameter and plant height are a consequence of salinity stress [91]. In one study, each EC unit increase in salinity resulted in a 7.2% decrease in tomato yield [92]. Thus, adding PGPB into hydroponics to reduce these negative effects would significantly benefit growers. Some Pseudomonas strains increased osmotically stressed plant crop yields in canola by 10% [93]. The commercial inoculant TNC Bactorr consisting of Bacillus spp. and Paenibacillus polymyxa alleviated 20mM salt stress in Crispa variety lettuce [94]. Four treatments with this inoculant prevented the 15% yield decrease caused by the osmotic stress. The authors noted that the autumn harvest tolerated salinity stress better than in the spring. Multiple PGPB have reduced salt stress in rice [67][95]. Bacillus amyloliquefaciens increased yields by 15% in the presence of 200 mM salt, while upregulating 14 plant genes [95]. The authors noted that salt-stressed rice underwent a shift in the microbiome that enriched for organisms that produced osmoprotectants including trehalose. Trehalose biosynthesis was also a key mechanism in a stressed hydroponic tomato trial [96]. A study of 305 strains concluded that strain TY0307 (taxonomy undeclared) improved yields by 30% by reducing ROS stress, increasing proline concentrations, and producing ACC deaminase [67]. Lastly, a comprehensive study in wheat tested 18 bacterial strains in four salt concentrations to determine the best PGPB for salt stress [68]. The ~58% crop reduction under salt stress was decreased to ~15% when PGPB were added due to a range of beneficial traits including ACC deaminase, phosphorus solubilization, IAA, and N fixation. The most effective organisms tested were Thalassobacillus, Bacillus, Halomonas, Oceanobacillus, Zhihengliuella, and Staphylococcus succinus.
References
- Díaz-Rodríguez, A.M.; Salcedo Gastelum, L.A.; Félix Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; de los Santos-Villalobos, S. The current and future role of microbial culture collections in food security worldwide. Front. Sustain. Food Syst. 2021, 4, 614739.
- Barrett, C.B. Overcoming global food security challenges through science and solidarity. Amer. J. Agr. Econ. 2021, 103, 422–447.
- Grafton, R.Q.; Williams, J.; Jiang, Q. Food and water gaps to 2050: Preliminary results from the global food and water system (GFWS) platform. Food Secur. 2015, 7, 209–220.
- Ritchie, H.; Roser, M. Land Use. Published Online at OurWorldInData. Available online: https://ourworldindata.org/land-use (accessed on 25 August 2022).
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of plant growth-promoting bacteria in sustainable agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842.
- Pimentel, D.; Burgess, M. Soil erosion threatens food production. Agriculture 2013, 3, 443–463.
- Haider, J.; Rai, A.B. Emergence of new insect pests on vegetables during the last decade: A case study. Curr. Hortic. 2021, 9, 20–26.
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659.
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561.
- Nemali, K. History of controlled environment horticulture: Greenhouses. HortScience 2022, 57, 239–246.
- Engler, N.; Krarti, M. Review of energy efficiency in controlled environment agriculture. Renew Sustain. Energy Rev. 2021, 141, 110786.
- Berkers, E.; Geels, F.W. System innovation through stepwise reconfiguration: The case of technological transitions in Dutch greenhouse horticulture (1930–1980). Technol. Anal. Strateg. Manag. 2011, 23, 227–247.
- Muñoz-Liesa, J.; Toboso-Chavero, S.; Mendoza Beltran, A.; Cuerva, E.; Gallo, E.; Gassó-Domingo, S.; Josa, A. Building-integrated agriculture: Are we shifting environmental impacts? An environmental assessment and structural improvement of urban greenhouses. Res. Conserv. Recycl. 2021, 169, 105526.
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191.
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371.
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215.
- Vasdravanidis, C.; Alvanou, M.V.; Lattos, A.; Papadopoulos, D.K.; Chatzigeorgiou, J.; Ravani, M.; Liantas, G.; Georgoulis, I.; Feidantsis, K.; Ntinas, G.K.; et al. Aquaponics as a promising strategy to mitigate impacts of climate change on rainbow trout culture. Animals 2022, 12, 2523.
- Farhadian, M.; Razzaghi Asl, S.; Ghamari, H. Thermal performance simulation of hydroponic green wall in a cold climate. Int. J. Srchitect. Eng. Urban Plan 2019, 29, 233–246.
- Rodríguez-Delfína, A. Advances of hydroponics in Latin America. Acta Hortic. 2012, 947, 23–32.
- Peterson, A.K.; Solberg, B. Greenhouse gas emissions, life-cycle inventory and cost-efficiency of using laminated wood instead of steel construction.: Case: Beams at Gardermoen airport. Environ. Sci. Pol. 2002, 5, 169–182.
- Sumalan, R.L.; Stroia, N.; Moga, D.; Muresan, V.; Lodin, A.; Vintila, T.; Popescu, C.A. A Cost-effective embedded platform for greenhouse environment control and remote monitoring. Agronomy 2020, 10, 936.
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188.
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Cientifica 2012, 2012, 963401.
- Anzalone, A.; Mosca, A.; Dimaria, G.; Nicotra, D.; Tessitori, M.; Privitera, G.F.; Pulvirenti, A.; Leonardi, C.; Catara, V. Soil and soilless tomato cultivation promote different microbial communities that provide new models for future crop interventions. Int. J. Mol. Sci. 2022, 23, 8820.
- Gericke, W.F. Crop production without soil. Nature 1938, 141, 536–540.
- Gericke, W.F. Aquaculture: A means of crop-production. Am. J. Bot. 1929, 16, 862.
- Gericke, W.F. Hydroponics—Crop production in liquid culture media. Science 1937, 85, 177–178.
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the U.S. midwest. J. Appl. Aquac. 2018, 30, 1–14.
- Oxford Analytica. Fertiliser and Food Prices Could Be High for Years. Published Online at Oxford Analytica Expert Briefings. Available online: https://dailybrief.oxan.com/Analysis/DB268415/Fertiliser-and-food-prices-could-be-high-for-years (accessed on 1 September 2022).
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; p. 383.
- Lynch, J.M. The Rhizosphere; Wiley-Interscience: Chichester, UK, 1990; p. 458.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in the rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 32, 44–51.
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533.
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433.
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiolog. Res. 2016, 183, 92–99.
- Bhattarai, S.P.; Salvaudon, C.; Midmore, D.J. Oxygenation of the rookwool substrate for hydroponics. Aquaponics J. 2008, 49, 29–33.
- Meselmani, M.A. Nutrient solution for hydroponics. In Soiless Culture; IntechOpen: London, UK, 2022; p. 101604.
- Rubol, S.; Manzoni, S.; Bellin, A.; Porporato, A. Modeling soil moisture and oxygen effexts on soil biogeochemical cycles including dissimilatory nitrate reduction to ammononium (DNRA). Adv. Water Res. 2013, 62, 106–124.
- Lei, C.; Engeseth, N.J. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT 2021, 150, 111931.
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 2010, 37, 621–633.
- Riser, E.C.; Grabowski, J.; Glenn, E.P. Microbiology of hydroponically grown lettuce. J. Food Prot. 1984, 47, 765–769.
- Rivera, M.E.D.; Vélez, C.; Zayas, B.; Llamas, K.M. Bacterial assessment on leaves of green vegetable grown on hydroponics and its possible health risks. J. Agric. Environ. Sci. 2015, 4, 1–4.
- Van Gerrewey, T.; El-Nakhel, C.; De Pascale, S.; De Paepe, J.; Clauwaert, P.; Kerckhof, F.M.; Boon, N.; Geelen, D. Root-associated bacterial community shifts in hydroponic lettuce cultured with urine-derived fertilizer. Microorganisms 2021, 9, 1326.
- Lobanov, V.; Keesman, K.J.; Joyce, A. Plants dictate root microbial composition in hydroponics and aquaponics. Front. Microbiol. 2022, 13, 848057.
- Sheridan, C.; Depuydt, P.; De Ro, M.; Petit, C.; Van Gysegem, E.; Delaere, P.; Dixon, M.; Stasiak, M.; Aciksöz, S.B.; Frossard, E.; et al. Microbial community dynamics and response to plant growth-promoting microorganisms in the rhizosphere of four common food crops cultivated in hydroponics. Microb. Ecol. 2017, 73, 378–393.
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa101.
- Ritchie, H.; Roser, M.; Rosado, P. Fertilizers. Published Online at OurWorldInData. Available online: https://ourworldindata.org/fertilizers (accessed on 30 August 2022).
- Mia, M.A.B.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured Musa plantlets under nitrogen-free hydroponics condition. Aus. J. Crop Sci. 2010, 4, 85–90.
- Ma-on, N. Immobilization of PGPR to Increase Efficiency of Plant Growth Promotion in Hydroponic System. Master’s Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2009; pp. 3–53.
- Ishizawa, H.; Ogata, Y.; Hachiya, Y.; Tokura, K.; Kuroda, M.; Inoue, D.; Toyama, T.; Tanaka, Y.; Mori, K.; Morikawa, M.; et al. Enhanced biomass production and nutrient removal capacity of duckweed via two-step cultivation process with a plant growth promoting bacterium, Acinetobacter calcoaceticus P23. Chemosphere 2020, 238, 124682.
- da Silva Cerozi, B.; Fitzsimmons, K. Use of Bacillus spp. to enhance phosphorus availability and serve as a plant growth promoter in aquaponics systems. Sci. Hortic. 2016, 211, 277–282.
- Aini, N.; Yamika, W.S.D.; Ulum, B. Effect of nutrient concentration, PGPR and AMF on plant growth, yield, and nutrient uptake of hydroponic lettuce. Int. J. Agric. Biol. 2019, 21, 175–183.
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watabame, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Scin. Plant Nutr. 2011, 57, 190–203.
- Amora-Lazcano, E.; Quiroz-González, H.; Osornio-Ortega, C.; Cruz-Maya, J.A.; Jan-Roblero, J. Plant growth-promoting bacteria belonging to the genera Pseudomonas and Bacillus improve the growth of sorghum seedlings in a low-nutrient soil. Bot. Sci. 2022, 100, 56–66.
- Begum, N.; Afzal, S.; Zhao, H.; Lou, L.; Cai, Q. Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiol. Plant 2018, 40, 170.
- Begum, N.; Hu, Z.; Cai, Q.; Lou, L. Influence of PGPB inoculation on HSP70 and HMA3 gene expression in switchgrass under cadmium stress. Plants 2019, 8, 504.
- Gül, A.; Özaktan, L.; Yolageldi, L.; Cakir, B.; Sahin, M.; Akat, S. Effect of rhizobacteria on yield of hydroponically grown tomato plants. Acta Hort. 2012, 952, 777–784.
- Aini, N.; Yamika, W.S.D.; Pahlevi, P.W. The effect of nutrient concentration and inoculation of PGPR and AMF on the yield and fruit quality of hydroponic cherry tomatoes (Lycopersicon esculentum Mill. var. cerasiforme). J. Appl. Hortic. 2019, 21, 116–122.
- Tian, W.; Li, L.; Xiao, X.; Wu, H.; Wang, Y.; Hu, Z.; Begum, N.; Zou, Y.; Lou, L.; Chang, M.; et al. Identification of a plant endophytic growth-promoting bacteria capable of inhibiting cadmium uptake in rice. J. Appl. Microbiol. 2022, 132, 520–531.
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674.
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth-promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int. J. Phytoremed 2017, 19, 281–289.
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242.
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Maraa, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129.
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. J. Plant Growth Regul. 2020, 91, 185–199.
- Handy, D.; Hummerick, M.E.; Dixit, A.R.; Ruby, A.M.; Massa, G.; Palmer, A. Identification of plant growth promoting bacteria within space crop production systems. Front. Astron. Space Sci. 2021, 8, 735834.
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-tolerant and plant growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978.
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627.
- Gül, A.; Özaktan, L.; Kidoglu, F.; Tüzel, Y. Rhizobacteria promoted yield of cucumber plants grown in perlite under Fusarium wilt stress. Sci. Hortic. 2013, 153, 22–25.
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Maté, A.P.; Sacristán, G.; Montero, O.; Debdoubi, A.; Rad, C. A consortium of cyanobacteria and plant growth promoting rhizobacteria for wheat growth improvement in a hydroponic system. S. Afr. J. Bot. 2021, 142, 247–258.
- Araújo, R.C.; Ribeiro, M.S.; Rodrigues, F.A.; Silva, B.S.; Dória, J.; Pasqual, M. Association of growth-promoting bacteria and hydroponic system aiming at reducing the time of production of banana seedlings. Arch. Agron. Soil Sci. 2022.
- Sebring, R.L.; Duiker, S.W.; Berghage, R.D.; Regan, J.M.; Lambert, J.D.; Bryant, R.B. Gluconacetobacter diazotrophicus Inoculation of Two Lettuce Cultivars Affects Leaf and Root Growth under Hydroponic Conditions. Appl. Sci. 2022, 12, 1585.
- Thongnok, S.; Siripornadulsil, W.; Siripornadulsil, S. AsIII-oxidizing and Cd-tolerant plant growth-promoting bacteria synergistically reduce arsenic translocation, toxicity and accumulation in KDML105 rice. Environ. Exp. Bot. 2021, 192, 104660.
- Gagné, S.; Dehbi, L.; Le Quéré, D.; Cayer, F.; Morin, J.L.; Lemay, R.; Fournier, N. Increase of greenhouse tomato fruit yields by plant growth-promoting rhizobacteria (PGPR) inoculated into the peat-based growing media. Soil Biol. Biochem. 1993, 25, 269–272.
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard Matr. 2020, 395, 122661.
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22.
- Sutton, J.C.; Sopher, C.R.; Owen-Going, T.N.; Liu, W.; Grodzinski, B.; Hall, J.C.; Benchimol, R.L. Etiology and epidemiology of Pythium root rot in hydroponic crops: Current knowledge and perspectives. Summa Phytopathol. 2006, 32, 307–321.
- Liu, W.; Sutton, J.C.; Grodzinski, B.; Kloepper, J.W.; Reddy, M.S. Biological control of Pythium root rot of chrysanthemum in small-scale hydroponic units. Phytoparasitica 2007, 35, 159–178.
- Sopher, C.R.; Sutton, J.C. Quantitative relationships of Pseudomonas chlororaphis 63-28 to Pythium root rot and growth in hydroponic peppers. Trop. Plant Pathol. 2011, 36, 214–224.
- Utkhede, R.S.; Lévesque, C.A.; Dinh, D. Pythium aphanidermatum root rot in hydroponically grown lettuce and the effect of chemical and biological agents on its control. Can. J. Plant Pathol. 2000, 22, 138–144.
- Kanjanamaneesathian, M.; Wiwattanapatapee, R.; Rotniam, W.; Wongpetkhiew, W. Spraying hydroponic lettuce roots with a suspension concentrate formulation of Bacillus velezensis to suppress root rot disease and promote plant growth. Biol. Control 2014, 67, 213–219.
- Khalil, S.; Alsanius, B.W. Evaluation of biocontrol agents for managing root diseases on hydroponically grown tomato. J. Plant Dis. Protect 2010, 117, 214–219.
- Postma, J.; Stevens, L.H.; Wiegers, G.L.; Davelaar, E.; Nijhuis, E.H. Biological control of Pythium aphanidermatum in cucumber with a combined application of Lysobacter enzymogenes strain 3.1T8 and chitosan. Biol. Control 2008, 48, 301–309.
- Punja, Z.K.; Yip, R. Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Can. J. Plant Pathol. 2003, 25, 411–417.
- Rose, S.; Parker, M.; Punja, Z.K. Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Dis. 2003, 87, 1462–1470.
- Khan, P.; Bora, L.C.; Borah, P.K.; Bora, P.; Talukdar, K. Efficacy of microbial consortia against bacterial wilt caused by Ralstonia solanacearum in hydroponically grown lettuce plant. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3046–3055.
- Cirou, A.; Raffouz, A.; Diallo, S.; Latour, X.; Dessaux, Y.; Faure, D. Gamma-caprolactone stimulates growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res. Microbiol. 2011, 162, 945–950.
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889.
- Alvarado-Gutiérrez, M.L.; Ruiz-Ordaz, N.; Galíndez-Mayer, J.; Santoyo-Tepole, F.; Curiel-Quesada, E.; García-Mena, J.; Ahuatzi-Chacón, D. Kinetics of carbendazim degradation in a horizontal tubular biofilm reactor. Bioprocess Biosyst. Eng. 2017, 40, 519–528.
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794.
- Zhang, P.; Senge, M.; Day, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55.
- Qaryouti, M.M.; Qawasmi, W.; Hamdan, H.; Edwan, M. Influence of NaCl salinity stress on yield, plant water uptake, and drainage water of tomato grown in soilless culture. Acta Hortic. 2007, 747, 70.
- Gharelo, R.S.; Bandehag, A.; Toorchi, M.; Farajzadeh, D. Canola 2-dimensional proteome profiles under osmotic stress and inoculation with Pseudomonas fluorescens FY32. Plant Cell Biotech. Mol. Biol. 2016, 17, 257–266.
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 2020, 10, 1523.
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9.
- Kalozoumis, P.; Savvas, D.; Aliferis, K.; Ntatsi, G.; Marakis, G.; Simou, E.; Tampakaki, A.; Karapanos, I. Impact of plant growth-promoting rhizobacteria inoculation and grafting on tolerance of tomato to combined water and nutrient stress. Front. Plant Sci. 2021, 12, 670236.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revision:
1 time
(View History)
Update Date:
26 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




