Improvements to the world’s food supply chain are needed to ensure sufficient food is produced to meet increasing population demands. Growing food in soilless hydroponic systems constitutes a promising strategy, as this method utilizes significantly less water than conventional agriculture, can be situated in urban areas, and can be stacked vertically to increase yields per acre. One method to increase hydroponic plant yields involves adding plant growth-promoting bacteria (PGPB) into these systems. PGPB are organisms that can significantly increase crop yields via a wide range of mechanisms, including stress reduction, increases in nutrient uptake, plant hormone modulation, and biocontrol.
- hydroponics
- plant growth-promoting bacteria
- plant stress
- plant growth
- ACC deaminase
- siderophores
- food production
- cannabis
- space
1. Introduction
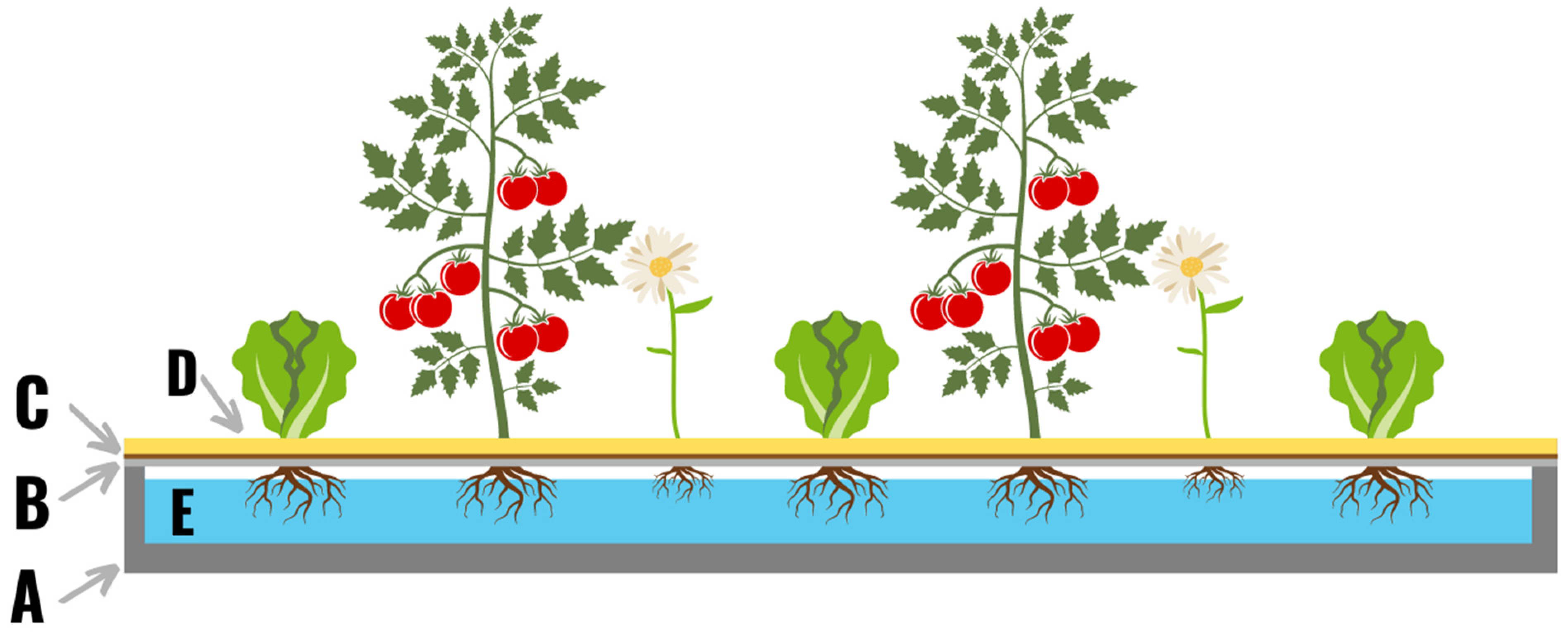
2. Hydroponic Systems
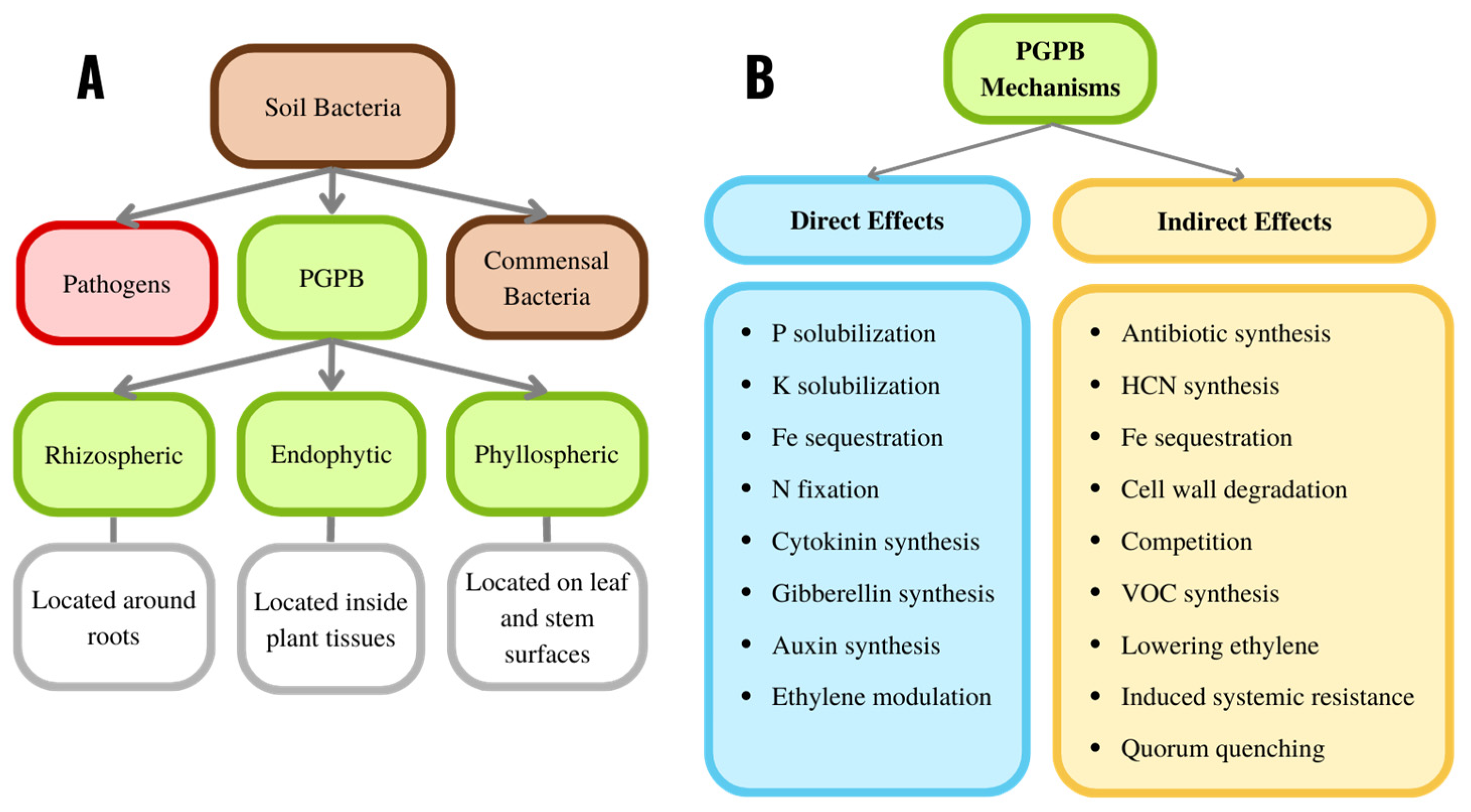
3. Plant Growth-Promoting Bacteria
4. Plant Growth Promoting Bacteria Research in Hydroponics
4.1. The Hydroponic Microbiome
4.2. PGPB That Increase Nutrient Uptake
4.3. PGPB That Regulate Hormones
4.4. Biocontrol Agents
4.5. Bioremediation and Osmotic Stress
This entry is adapted from the peer-reviewed paper 10.3390/plants11202783
References
- Díaz-Rodríguez, A.M.; Salcedo Gastelum, L.A.; Félix Pablos, C.M.; Parra-Cota, F.I.; Santoyo, G.; Puente, M.L.; Bhattacharya, D.; Mukherjee, J.; de los Santos-Villalobos, S. The current and future role of microbial culture collections in food security worldwide. Front. Sustain. Food Syst. 2021, 4, 614739.
- Barrett, C.B. Overcoming global food security challenges through science and solidarity. Amer. J. Agr. Econ. 2021, 103, 422–447.
- Grafton, R.Q.; Williams, J.; Jiang, Q. Food and water gaps to 2050: Preliminary results from the global food and water system (GFWS) platform. Food Secur. 2015, 7, 209–220.
- Ritchie, H.; Roser, M. Land Use. Published Online at OurWorldInData. Available online: https://ourworldindata.org/land-use (accessed on 25 August 2022).
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of plant growth-promoting bacteria in sustainable agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842.
- Pimentel, D.; Burgess, M. Soil erosion threatens food production. Agriculture 2013, 3, 443–463.
- Haider, J.; Rai, A.B. Emergence of new insect pests on vegetables during the last decade: A case study. Curr. Hortic. 2021, 9, 20–26.
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659.
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Reidy Liermann, C.; et al. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561.
- Nemali, K. History of controlled environment horticulture: Greenhouses. HortScience 2022, 57, 239–246.
- Engler, N.; Krarti, M. Review of energy efficiency in controlled environment agriculture. Renew Sustain. Energy Rev. 2021, 141, 110786.
- Berkers, E.; Geels, F.W. System innovation through stepwise reconfiguration: The case of technological transitions in Dutch greenhouse horticulture (1930–1980). Technol. Anal. Strateg. Manag. 2011, 23, 227–247.
- Muñoz-Liesa, J.; Toboso-Chavero, S.; Mendoza Beltran, A.; Cuerva, E.; Gallo, E.; Gassó-Domingo, S.; Josa, A. Building-integrated agriculture: Are we shifting environmental impacts? An environmental assessment and structural improvement of urban greenhouses. Res. Conserv. Recycl. 2021, 169, 105526.
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191.
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371.
- Lee, S.; Lee, J. Beneficial bacteria and fungi in hydroponic systems: Types and characteristics of hydroponic food production methods. Sci. Hortic. 2015, 195, 206–215.
- Vasdravanidis, C.; Alvanou, M.V.; Lattos, A.; Papadopoulos, D.K.; Chatzigeorgiou, J.; Ravani, M.; Liantas, G.; Georgoulis, I.; Feidantsis, K.; Ntinas, G.K.; et al. Aquaponics as a promising strategy to mitigate impacts of climate change on rainbow trout culture. Animals 2022, 12, 2523.
- Farhadian, M.; Razzaghi Asl, S.; Ghamari, H. Thermal performance simulation of hydroponic green wall in a cold climate. Int. J. Srchitect. Eng. Urban Plan 2019, 29, 233–246.
- Rodríguez-Delfína, A. Advances of hydroponics in Latin America. Acta Hortic. 2012, 947, 23–32.
- Peterson, A.K.; Solberg, B. Greenhouse gas emissions, life-cycle inventory and cost-efficiency of using laminated wood instead of steel construction.: Case: Beams at Gardermoen airport. Environ. Sci. Pol. 2002, 5, 169–182.
- Sumalan, R.L.; Stroia, N.; Moga, D.; Muresan, V.; Lodin, A.; Vintila, T.; Popescu, C.A. A Cost-effective embedded platform for greenhouse environment control and remote monitoring. Agronomy 2020, 10, 936.
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188.
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Cientifica 2012, 2012, 963401.
- Anzalone, A.; Mosca, A.; Dimaria, G.; Nicotra, D.; Tessitori, M.; Privitera, G.F.; Pulvirenti, A.; Leonardi, C.; Catara, V. Soil and soilless tomato cultivation promote different microbial communities that provide new models for future crop interventions. Int. J. Mol. Sci. 2022, 23, 8820.
- Gericke, W.F. Crop production without soil. Nature 1938, 141, 536–540.
- Gericke, W.F. Aquaculture: A means of crop-production. Am. J. Bot. 1929, 16, 862.
- Gericke, W.F. Hydroponics—Crop production in liquid culture media. Science 1937, 85, 177–178.
- Quagrainie, K.K.; Flores, R.M.V.; Kim, H.J.; McClain, V. Economic analysis of aquaponics and hydroponics production in the U.S. midwest. J. Appl. Aquac. 2018, 30, 1–14.
- Oxford Analytica. Fertiliser and Food Prices Could Be High for Years. Published Online at Oxford Analytica Expert Briefings. Available online: https://dailybrief.oxan.com/Analysis/DB268415/Fertiliser-and-food-prices-could-be-high-for-years (accessed on 1 September 2022).
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; p. 383.
- Lynch, J.M. The Rhizosphere; Wiley-Interscience: Chichester, UK, 1990; p. 458.
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in the rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266.
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 32, 44–51.
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533.
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433.
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiolog. Res. 2016, 183, 92–99.
- Bhattarai, S.P.; Salvaudon, C.; Midmore, D.J. Oxygenation of the rookwool substrate for hydroponics. Aquaponics J. 2008, 49, 29–33.
- Meselmani, M.A. Nutrient solution for hydroponics. In Soiless Culture; IntechOpen: London, UK, 2022; p. 101604.
- Rubol, S.; Manzoni, S.; Bellin, A.; Porporato, A. Modeling soil moisture and oxygen effexts on soil biogeochemical cycles including dissimilatory nitrate reduction to ammononium (DNRA). Adv. Water Res. 2013, 62, 106–124.
- Lei, C.; Engeseth, N.J. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT 2021, 150, 111931.
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. The response of barley to salinity stress differs between hydroponic and soil systems. Funct. Plant Biol. 2010, 37, 621–633.
- Riser, E.C.; Grabowski, J.; Glenn, E.P. Microbiology of hydroponically grown lettuce. J. Food Prot. 1984, 47, 765–769.
- Rivera, M.E.D.; Vélez, C.; Zayas, B.; Llamas, K.M. Bacterial assessment on leaves of green vegetable grown on hydroponics and its possible health risks. J. Agric. Environ. Sci. 2015, 4, 1–4.
- Van Gerrewey, T.; El-Nakhel, C.; De Pascale, S.; De Paepe, J.; Clauwaert, P.; Kerckhof, F.M.; Boon, N.; Geelen, D. Root-associated bacterial community shifts in hydroponic lettuce cultured with urine-derived fertilizer. Microorganisms 2021, 9, 1326.
- Lobanov, V.; Keesman, K.J.; Joyce, A. Plants dictate root microbial composition in hydroponics and aquaponics. Front. Microbiol. 2022, 13, 848057.
- Sheridan, C.; Depuydt, P.; De Ro, M.; Petit, C.; Van Gysegem, E.; Delaere, P.; Dixon, M.; Stasiak, M.; Aciksöz, S.B.; Frossard, E.; et al. Microbial community dynamics and response to plant growth-promoting microorganisms in the rhizosphere of four common food crops cultivated in hydroponics. Microb. Ecol. 2017, 73, 378–393.
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa101.
- Ritchie, H.; Roser, M.; Rosado, P. Fertilizers. Published Online at OurWorldInData. Available online: https://ourworldindata.org/fertilizers (accessed on 30 August 2022).
- Mia, M.A.B.; Shamsuddin, Z.H.; Wahab, Z.; Marziah, M. Effect of plant growth promoting rhizobacterial (PGPR) inoculation on growth and nitrogen incorporation of tissue-cultured Musa plantlets under nitrogen-free hydroponics condition. Aus. J. Crop Sci. 2010, 4, 85–90.
- Ma-on, N. Immobilization of PGPR to Increase Efficiency of Plant Growth Promotion in Hydroponic System. Master’s Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2009; pp. 3–53.
- Ishizawa, H.; Ogata, Y.; Hachiya, Y.; Tokura, K.; Kuroda, M.; Inoue, D.; Toyama, T.; Tanaka, Y.; Mori, K.; Morikawa, M.; et al. Enhanced biomass production and nutrient removal capacity of duckweed via two-step cultivation process with a plant growth promoting bacterium, Acinetobacter calcoaceticus P23. Chemosphere 2020, 238, 124682.
- da Silva Cerozi, B.; Fitzsimmons, K. Use of Bacillus spp. to enhance phosphorus availability and serve as a plant growth promoter in aquaponics systems. Sci. Hortic. 2016, 211, 277–282.
- Aini, N.; Yamika, W.S.D.; Ulum, B. Effect of nutrient concentration, PGPR and AMF on plant growth, yield, and nutrient uptake of hydroponic lettuce. Int. J. Agric. Biol. 2019, 21, 175–183.
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watabame, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Scin. Plant Nutr. 2011, 57, 190–203.
- Amora-Lazcano, E.; Quiroz-González, H.; Osornio-Ortega, C.; Cruz-Maya, J.A.; Jan-Roblero, J. Plant growth-promoting bacteria belonging to the genera Pseudomonas and Bacillus improve the growth of sorghum seedlings in a low-nutrient soil. Bot. Sci. 2022, 100, 56–66.
- Begum, N.; Afzal, S.; Zhao, H.; Lou, L.; Cai, Q. Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiol. Plant 2018, 40, 170.
- Begum, N.; Hu, Z.; Cai, Q.; Lou, L. Influence of PGPB inoculation on HSP70 and HMA3 gene expression in switchgrass under cadmium stress. Plants 2019, 8, 504.
- Gül, A.; Özaktan, L.; Yolageldi, L.; Cakir, B.; Sahin, M.; Akat, S. Effect of rhizobacteria on yield of hydroponically grown tomato plants. Acta Hort. 2012, 952, 777–784.
- Aini, N.; Yamika, W.S.D.; Pahlevi, P.W. The effect of nutrient concentration and inoculation of PGPR and AMF on the yield and fruit quality of hydroponic cherry tomatoes (Lycopersicon esculentum Mill. var. cerasiforme). J. Appl. Hortic. 2019, 21, 116–122.
- Tian, W.; Li, L.; Xiao, X.; Wu, H.; Wang, Y.; Hu, Z.; Begum, N.; Zou, Y.; Lou, L.; Chang, M.; et al. Identification of a plant endophytic growth-promoting bacteria capable of inhibiting cadmium uptake in rice. J. Appl. Microbiol. 2022, 132, 520–531.
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674.
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth-promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int. J. Phytoremed 2017, 19, 281–289.
- Yedidia, I.; Srivastva, A.K.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242.
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Maraa, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129.
- Delaporte-Quintana, P.; Lovaisa, N.C.; Rapisarda, V.A.; Pedraza, R.O. The plant growth promoting bacteria Gluconacetobacter diazotrophicus and Azospirillum brasilense contribute to the iron nutrition of strawberry plants through siderophores production. J. Plant Growth Regul. 2020, 91, 185–199.
- Handy, D.; Hummerick, M.E.; Dixit, A.R.; Ruby, A.M.; Massa, G.; Palmer, A. Identification of plant growth promoting bacteria within space crop production systems. Front. Astron. Space Sci. 2021, 8, 735834.
- Zhang, S.; Fan, C.; Wang, Y.; Xia, Y.; Xiao, W.; Cui, X. Salt-tolerant and plant growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018, 64, 968–978.
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627.
- Gül, A.; Özaktan, L.; Kidoglu, F.; Tüzel, Y. Rhizobacteria promoted yield of cucumber plants grown in perlite under Fusarium wilt stress. Sci. Hortic. 2013, 153, 22–25.
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Maté, A.P.; Sacristán, G.; Montero, O.; Debdoubi, A.; Rad, C. A consortium of cyanobacteria and plant growth promoting rhizobacteria for wheat growth improvement in a hydroponic system. S. Afr. J. Bot. 2021, 142, 247–258.
- Araújo, R.C.; Ribeiro, M.S.; Rodrigues, F.A.; Silva, B.S.; Dória, J.; Pasqual, M. Association of growth-promoting bacteria and hydroponic system aiming at reducing the time of production of banana seedlings. Arch. Agron. Soil Sci. 2022.
- Sebring, R.L.; Duiker, S.W.; Berghage, R.D.; Regan, J.M.; Lambert, J.D.; Bryant, R.B. Gluconacetobacter diazotrophicus Inoculation of Two Lettuce Cultivars Affects Leaf and Root Growth under Hydroponic Conditions. Appl. Sci. 2022, 12, 1585.
- Thongnok, S.; Siripornadulsil, W.; Siripornadulsil, S. AsIII-oxidizing and Cd-tolerant plant growth-promoting bacteria synergistically reduce arsenic translocation, toxicity and accumulation in KDML105 rice. Environ. Exp. Bot. 2021, 192, 104660.
- Gagné, S.; Dehbi, L.; Le Quéré, D.; Cayer, F.; Morin, J.L.; Lemay, R.; Fournier, N. Increase of greenhouse tomato fruit yields by plant growth-promoting rhizobacteria (PGPR) inoculated into the peat-based growing media. Soil Biol. Biochem. 1993, 25, 269–272.
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard Matr. 2020, 395, 122661.
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22.
- Sutton, J.C.; Sopher, C.R.; Owen-Going, T.N.; Liu, W.; Grodzinski, B.; Hall, J.C.; Benchimol, R.L. Etiology and epidemiology of Pythium root rot in hydroponic crops: Current knowledge and perspectives. Summa Phytopathol. 2006, 32, 307–321.
- Liu, W.; Sutton, J.C.; Grodzinski, B.; Kloepper, J.W.; Reddy, M.S. Biological control of Pythium root rot of chrysanthemum in small-scale hydroponic units. Phytoparasitica 2007, 35, 159–178.
- Sopher, C.R.; Sutton, J.C. Quantitative relationships of Pseudomonas chlororaphis 63-28 to Pythium root rot and growth in hydroponic peppers. Trop. Plant Pathol. 2011, 36, 214–224.
- Utkhede, R.S.; Lévesque, C.A.; Dinh, D. Pythium aphanidermatum root rot in hydroponically grown lettuce and the effect of chemical and biological agents on its control. Can. J. Plant Pathol. 2000, 22, 138–144.
- Kanjanamaneesathian, M.; Wiwattanapatapee, R.; Rotniam, W.; Wongpetkhiew, W. Spraying hydroponic lettuce roots with a suspension concentrate formulation of Bacillus velezensis to suppress root rot disease and promote plant growth. Biol. Control 2014, 67, 213–219.
- Khalil, S.; Alsanius, B.W. Evaluation of biocontrol agents for managing root diseases on hydroponically grown tomato. J. Plant Dis. Protect 2010, 117, 214–219.
- Postma, J.; Stevens, L.H.; Wiegers, G.L.; Davelaar, E.; Nijhuis, E.H. Biological control of Pythium aphanidermatum in cucumber with a combined application of Lysobacter enzymogenes strain 3.1T8 and chitosan. Biol. Control 2008, 48, 301–309.
- Punja, Z.K.; Yip, R. Biological control of damping-off and root rot caused by Pythium aphanidermatum on greenhouse cucumbers. Can. J. Plant Pathol. 2003, 25, 411–417.
- Rose, S.; Parker, M.; Punja, Z.K. Efficacy of biological and chemical treatments for control of Fusarium root and stem rot on greenhouse cucumber. Plant Dis. 2003, 87, 1462–1470.
- Khan, P.; Bora, L.C.; Borah, P.K.; Bora, P.; Talukdar, K. Efficacy of microbial consortia against bacterial wilt caused by Ralstonia solanacearum in hydroponically grown lettuce plant. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3046–3055.
- Cirou, A.; Raffouz, A.; Diallo, S.; Latour, X.; Dessaux, Y.; Faure, D. Gamma-caprolactone stimulates growth of quorum-quenching Rhodococcus populations in a large-scale hydroponic system for culturing Solanum tuberosum. Res. Microbiol. 2011, 162, 945–950.
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889.
- Alvarado-Gutiérrez, M.L.; Ruiz-Ordaz, N.; Galíndez-Mayer, J.; Santoyo-Tepole, F.; Curiel-Quesada, E.; García-Mena, J.; Ahuatzi-Chacón, D. Kinetics of carbendazim degradation in a horizontal tubular biofilm reactor. Bioprocess Biosyst. Eng. 2017, 40, 519–528.
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794.
- Zhang, P.; Senge, M.; Day, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55.
- Qaryouti, M.M.; Qawasmi, W.; Hamdan, H.; Edwan, M. Influence of NaCl salinity stress on yield, plant water uptake, and drainage water of tomato grown in soilless culture. Acta Hortic. 2007, 747, 70.
- Gharelo, R.S.; Bandehag, A.; Toorchi, M.; Farajzadeh, D. Canola 2-dimensional proteome profiles under osmotic stress and inoculation with Pseudomonas fluorescens FY32. Plant Cell Biotech. Mol. Biol. 2016, 17, 257–266.
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of salt stress by plant growth-promoting bacteria in hydroponic leaf lettuce. Agronomy 2020, 10, 1523.
- Nautiyal, C.S.; Srivastava, S.; Chauhan, P.S.; Seem, K.; Mishra, A.; Sopory, S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013, 66, 1–9.
- Kalozoumis, P.; Savvas, D.; Aliferis, K.; Ntatsi, G.; Marakis, G.; Simou, E.; Tampakaki, A.; Karapanos, I. Impact of plant growth-promoting rhizobacteria inoculation and grafting on tolerance of tomato to combined water and nutrient stress. Front. Plant Sci. 2021, 12, 670236.
