
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jiancheng Yang | -- | 2429 | 2022-10-24 22:32:48 | | | |
| 2 | Rita Xu | Meta information modification | 2429 | 2022-10-25 04:05:48 | | |
Video Upload Options
Iron oxide nanoparticles (IONPs) are extensively used in bone-related studies as biomaterials due to their unique magnetic properties and good biocompatibility. Through endocytosis, IONPs enter the cell where they promote osteogenic differentiation and inhibit osteoclastogenesis in vivo. This result is further supported by in-vivo findings, showing that osteoblasts and osteoclasts internalize the IONPs, yielding superior bone regeneration and weaker bone resorption. Therefore, IONPs have a potential clinical application value to promote bone regeneration and prevent osteoporosis.
1. Introduction
2. Effects of Iron Oxide Nanoparticles on Bone Remodeling
2.1. Effects of Iron Oxide Nanoparticles on Osteoblasts
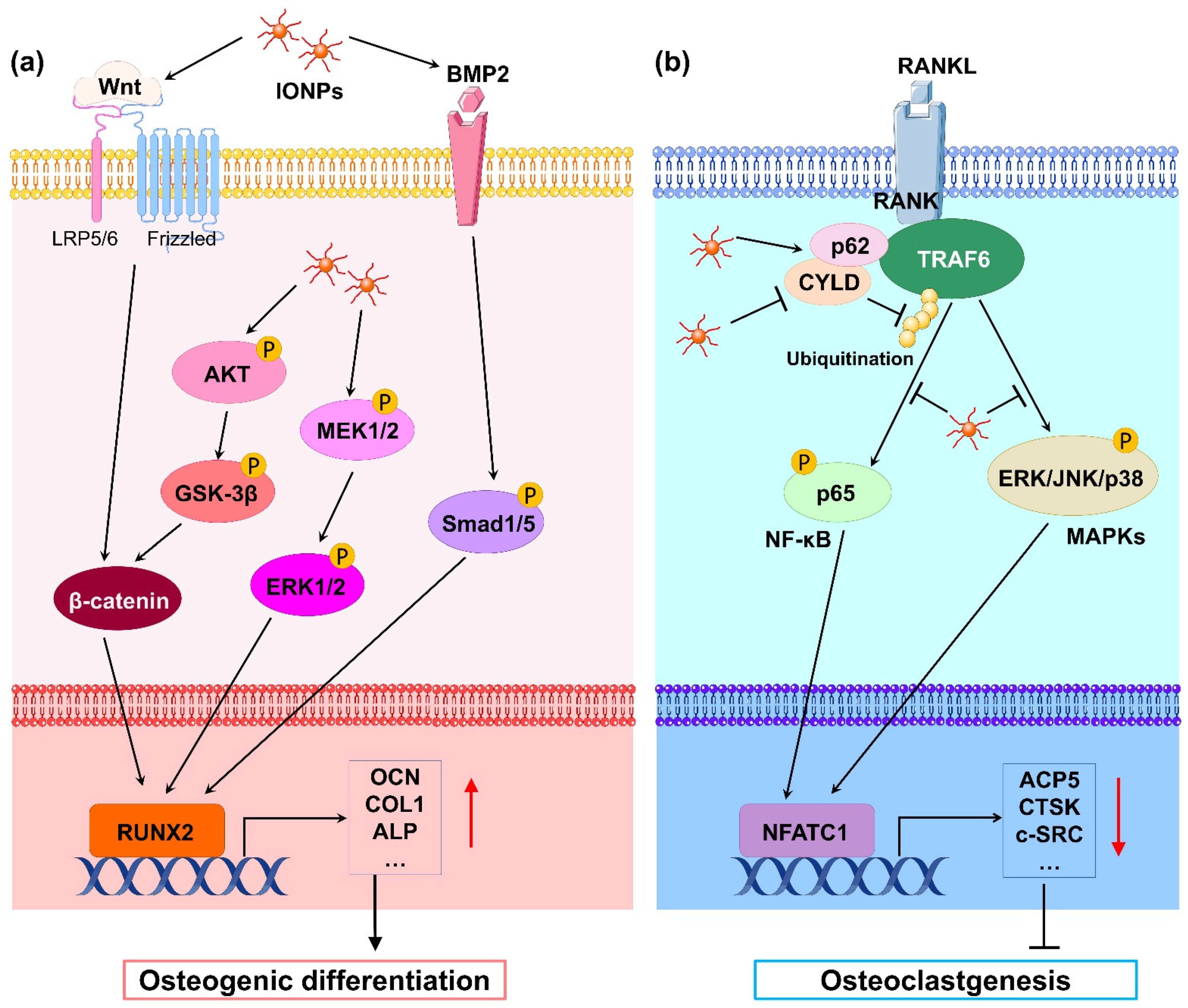
2.2. Effects of Iron Oxide Nanoparticles on Osteoclasts
3. Outlook
Previous studies on IONPs related to bone repair or osteoporosis have mainly focused on the biological effects of IONPs in animal or cell experiments. Thus, rigorous clinical trials in humans are needed before translating these findings into clinical practice. Moreover, toxicity is the most important evaluation index in clinical therapy. However, existing studies fail to evaluate the safety of IONPs in vivo. The toxicity of IONPs should be considered in a dose-, treatment-, and time-dependent manner[47]. Therefore, the absorption, distribution, metabolism, and toxicity of IONPs after implanting a composite scaffold containing IONPs in vivo should be explored in future studies.
References
- Yan Li; Dewen Ye; Mingxi Li; Ming Ma; Ning Gu; Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. ChemPhysChem 2018, 19, 1965-1979, 10.1002/cphc.201701294.
- Seyed Mohammadali Dadfar; Karolin Roemhild; Natascha I. Drude; Saskia von Stillfried; Ruth Knüchel; Fabian Kiessling; Twan Lammers; Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Advanced Drug Delivery Reviews 2019, 138, 302-325, 10.1016/j.addr.2019.01.005.
- Saeid Zanganeh; Gregor Hutter; Ryan Spitler; Olga Lenkov; Morteza Mahmoudi; Aubie Shaw; Jukka Sakari Pajarinen; Hossein Nejadnik; Jukka Sakari Pajarinen Stuart Goodman; Michael Moseley; et al.Aubie Shaw Lisa Marie CoussensHeike Daldrup-Link Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature Nanotechnology 2016, 11, 986-994, 10.1038/nnano.2016.168.
- Yue Huang; Jessica C. Hsu; Hyun Koo; David P. Cormode; Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796-816, 10.7150/thno.67375.
- Erik Fink Eriksen; Cellular mechanisms of bone remodeling. Reviews in Endocrine and Metabolic Disorders 2010, 11, 219-227, 10.1007/s11154-010-9153-1.
- Rajesh A Pareta; Eric Taylor; Thomas J Webster; Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 2008, 19, 265101, 10.1088/0957-4484/19/26/265101.
- Nhiem Tran; Thomas J. Webster; Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomaterialia 2011, 7, 1298-1306, 10.1016/j.actbio.2010.10.004.
- Nhiem Tran; Douglas Hall; Thomas J Webster; Mechanisms of enhanced osteoblast gene expression in the presence of hydroxyapatite coated iron oxide magnetic nanoparticles. Nanotechnology 2012, 23, 455104, 10.1088/0957-4484/23/45/455104.
- Nhiem Tran; Thomas J Webster; Understanding magnetic nanoparticle osteoblast receptor-mediated endocytosis using experiments and modeling. Nanotechnology 2013, 24, 185102, 10.1088/0957-4484/24/18/185102.
- Xian-Long Zhang; Si-Feng Shi; Jing-Fu Jia; Ya-Ping Zhao; Xiao-Kui Guo; De-Sheng Chen; Tao Cheng; Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. International Journal of Nanomedicine 2012, 7, 5593-5602, 10.2147/ijn.s34348.
- Guangfu Yin; Zhongbing Huang; Min Deng; Jingwen Zeng; Jianwen Gu; Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. Journal of Colloid and Interface Science 2011, 363, 393-402, 10.1016/j.jcis.2011.07.009.
- Hai-Tao Xiao; Lei Wang; Bin Yu; Superparamagnetic iron oxide promotes osteogenic differentiation of rat adipose-derived stem cells.. International journal of clinical and experimental medicine 2015, 8, 698-705.
- Yang Xia; Huimin Chen; Feimin Zhang; Lin Wang; Bo Chen; Mark A. Reynolds; Junqing Ma; Abraham Schneider; Ning Gu; Hockin H. K. Xu; et al. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 423-433, 10.1080/21691401.2018.1428813.
- Yang Xia; Huimin Chen; Yantao Zhao; Feimin Zhang; Xiaodong Li; Lin Wang; Michael D. Weir; Junqing Ma; Mark A. Reynolds; Ning Gu; et al.Hockin H.K. Xu Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Materials Science and Engineering: C 2018, 98, 30-41, 10.1016/j.msec.2018.12.120.
- Lizeng Gao; Jie Zhuang; Leng Nie; Jinbin Zhang; Yu Zhang; Ning Gu; Taihong Wang; Jing Feng; Dongling Yang; Sarah Perrett; et al.Xiyun Yan Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology 2007, 2, 577-583, 10.1038/nnano.2007.260.
- Roy H. Burdon; Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine 1995, 18, 775-794, 10.1016/0891-5849(94)00198-s.
- Dong-Ming Huang; Jong-Kai Hsiao; Ying-Chun Chen; Li-Ying Chien; Ming Yao; Yin-Kai Chen; Bor-Sheng Ko; Szu-Chun Hsu; Lin-Ai Tai; Hui-Ying Cheng; et al.Shih-Wei WangChung-Shi YangYao-Chang Chen The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645-3651, 10.1016/j.biomaterials.2009.03.032.
- Wantae Kim; Minseong Kim; Eek-Hoon Jho; Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochemical Journal 2013, 450, 9-21, 10.1042/bj20121284.
- Yang Xia; Yu Guo; Zukun Yang; Huimin Chen; Ke Ren; Michael D. Weir; Laurence C. Chow; Mark A. Reynolds; Feimin Zhang; Ning Gu; et al.Hockin H.K. Xu Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Materials Science and Engineering: C 2019, 104, 109955, 10.1016/j.msec.2019.109955.
- Buer Sen; Maya Styner; Zhihui Xie; Natasha Case; Clinton T. Rubin; Janet Rubin; Mechanical Loading Regulates NFATc1 and β-Catenin Signaling through a GSK3β Control Node. Journal of Biological Chemistry 2009, 284, 34607-34617, 10.1074/jbc.m109.039453.
- Fanglong Song; Dawei Jiang; Tianchen Wang; Yi Wang; Yi Lou; Yinquan Zhang; Hui Ma; Yifan Kang; Mechanical Stress Regulates Osteogenesis and Adipogenesis of Rat Mesenchymal Stem Cells through PI3K/Akt/GSK-3β/β-Catenin Signaling Pathway. BioMed Research International 2017, 2017, 1-10, 10.1155/2017/6027402.
- Pengjun Yu; Liming Zheng; Peng Wang; Senlin Chai; Yibo Zhang; Tianshu Shi; Lei Zhang; Rui Peng; Caoxing Huang; Baosheng Guo; et al.Qing Jiang Development of a novel polysaccharide-based iron oxide nanoparticle to prevent iron accumulation-related osteoporosis by scavenging reactive oxygen species. International Journal of Biological Macromolecules 2020, 165, 1634-1645, 10.1016/j.ijbiomac.2020.10.016.
- Eddie Rodríguez-Carballo; Beatriz Gámez; Francesc Ventura; p38 MAPK Signaling in Osteoblast Differentiation. Frontiers in Cell and Developmental Biology 2016, 4, 40-40, 10.3389/fcell.2016.00040.
- Ren Xu; Chao Zhang; Dong Yeon Shin; Jung‐Min Kim; Sarfaraz Lalani; Na Li; Yeon‐Suk Yang; Yifang Liu; Mark Eiseman; Roger J Davis; et al.Jae‐Hyuck ShimMatthew B Greenblatt c‐Jun N‐Terminal Kinases (JNKs) Are Critical Mediators of Osteoblast Activity In Vivo. Journal of Bone and Mineral Research 2017, 32, 1811-1815, 10.1002/jbmr.3184.
- Qiwei Wang; Bo Chen; Meng Cao; Jianfei Sun; Hao Wu; Peng Zhao; Jing Xing; Yan Yang; Xiquan Zhang; Min Ji; et al.Ning Gu Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11-20, 10.1016/j.biomaterials.2016.02.004.
- Mengrui Wu; Guiqian Chen; Yi-Ping Li; TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research 2016, 4, 16009, 10.1038/boneres.2016.9.
- Jia-Wei Lu; Fan Yang; Qin-Fei Ke; Xue-Tao Xie; Ya-Ping Guo; Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 811-822, 10.1016/j.nano.2017.12.025.
- Shuying Hu; Yi Zhou; Yantao Zhao; Yang Xu; Feimin Zhang; Ning Gu; Junqing Ma; Mark A. Reynolds; Yang Xia; Hockin H.K. Xu; et al. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. Journal of Tissue Engineering and Regenerative Medicine 2018, 12, e2085-e2098, 10.1002/term.2641.
- Wei Liao; Jingwei Lu; Qianjin Wang; Sen Yan; Yan Li; Yibo Zhang; Peng Wang; Qing Jiang; Ning Gu; Osteogenesis of Iron Oxide Nanoparticles-Labeled Human Precartilaginous Stem Cells in Interpenetrating Network Printable Hydrogel. Frontiers in Bioengineering and Biotechnology 2022, 10, 872149, 10.3389/fbioe.2022.872149.
- Rajendra K Singh; Kapil D. Patel; Jae Ho Lee; Eun-Jung Lee; Joong-Hyun Kim; Tae-Hyun Kim; Hae-Won Kim; Potential of Magnetic Nanofiber Scaffolds with Mechanical and Biological Properties Applicable for Bone Regeneration. PLOS ONE 2014, 9, e91584, 10.1371/journal.pone.0091584.
- S. Panseri; A. Russo; G. Giavaresi; M. Sartori; F. Veronesi; M. Fini; D. M. Salter; A. Ortolani; A. Strazzari; A. Visani; et al.C. DionigiN. BockM. SandriA. TampieriM. Marcacci Innovative magnetic scaffolds for orthopedic tissue engineering. Journal of Biomedical Materials Research Part A 2012, 100A, 2278-2286, 10.1002/jbm.a.34167.
- Cijun Shuai; Wenjing Yang; Chongxian He; Shuping Peng; Chengde Gao; Youwen Yang; Fangwei Qi; Pei Feng; A magnetic micro-environment in scaffolds for stimulating bone regeneration. Materials & Design 2019, 185, 108275, 10.1016/j.matdes.2019.108275.
- Yao Zhao; Tiantang Fan; Jingdi Chen; Jiacan Su; Xin Zhi; Panpan Pan; Lin Zou; Qiqing Zhang; Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids and Surfaces B: Biointerfaces 2018, 174, 70-79, 10.1016/j.colsurfb.2018.11.003.
- Jingjing Fan; Yanbin Tan; Liyong Jie; Xinying Wu; Risheng Yu; Minming Zhang; Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Research & Therapy 2013, 4, 44-44, 10.1186/scrt191.
- Surakshya Shrestha; Pengfei Jiang; Marcelo Henrique Sousa; Paulo Cesar Morais; Zhengwei Mao; Changyou Gao; Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. Journal of Materials Chemistry B 2015, 4, 245-256, 10.1039/c5tb02007g.
- Lizandra Ferrari Guimarães; Tatiana Kelly Da Silva Fidalgo; Gustavo Conde Menezes; Laura Guimarães Primo; Fernando Costa e Silva-Filho; Effects of citric acid on cultured human osteoblastic cells. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2010, 110, 665-669, 10.1016/j.tripleo.2010.07.003.
- Mengye Li; Shengxiang Fu; Zhongyuan Cai; Danyang Li; Li Liu; Di Deng; Rongrong Jin; Hua Ai; Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regenerative Biomaterials 2021, 8, rbab027, 10.1093/rb/rbab027.
- Dolores Shoback; Clifford J Rosen; Dennis M Black; Angela M. Cheung; Mohammad Hassan Murad; Richard Eastell; Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. The Journal of Clinical Endocrinology & Metabolism 2020, 105, 587-594, 10.1210/clinem/dgaa048.
- Li Liu; Rongrong Jin; Jimei Duan; Li Yang; Zhongyuan Cai; Wencheng Zhu; Yu Nie; Jing He; Chunchao Xia; Qiyong Gong; et al.Bin SongJames M. AndersonHua Ai Bioactive iron oxide nanoparticles suppress osteoclastogenesis and ovariectomy-induced bone loss through regulating the TRAF6-p62-CYLD signaling complex. Acta Biomaterialia 2019, 103, 281-292, 10.1016/j.actbio.2019.12.022.
- Liming Zheng; Zaikai Zhuang; Yixuan Li; Tianshu Shi; Kai Fu; Wenjin Yan; Lei Zhang; Peng Wang; Lan Li; Qing Jiang; et al. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioactive Materials 2021, 14, 250-261, 10.1016/j.bioactmat.2021.11.012.
- Jiancheng Yang; Qinghua Tang; Yuhong Zeng; Melatonin: Potential avenue for treating iron overload disorders. Ageing Res Rev 2022, 81, 101717, 10.1016/j.arr.2022.101717.
- Jaime Tsay; Zheiwei Yang; F. Patrick Ross; Susanna Cunningham-Rundles; Hong Lin; Rhima Coleman; Philipp Mayer-Kuckuk; Stephen B. Doty; Robert W. Grady; Patricia J. Giardina; et al.Adele BoskeyMaria G. Vogiatzi Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 2010, 116, 2582-2589, 10.1182/blood-2009-12-260083.
- Dulshara Sachini Amarasekara; Hyeongseok Yun; Sumi Kim; Nari Lee; Hyunjong Kim; Jaerang Rho; Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Network 2018, 18, e8, 10.4110/in.2018.18.e8.
- Wei Jin; Mikyoung Chang; Emmanuel M. Paul; Geetha Babu; Andrew J. Lee; William Reiley; Ato Wright; Minying Zhang; Jun You; Shao-Cong Sun; et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. Journal of Clinical Investigation 2008, 118, 1858-1866, 10.1172/jci34257.
- Alexander G. Robling; Lynda F. Bonewald; The Osteocyte: New Insights. Annual Review of Physiology 2020, 82, 485-506, 10.1146/annurev-physiol-021119-034332.
- Jesus Medical Delgado-Calle; Teresita Bellido; The osteocyte as a signaling cell. Physiological Reviews 2022, 102, 379-410, 10.1152/physrev.00043.2020.
- Kurtulus Gokduman; Furkan Bestepe; Lei Li; Martin L Yarmush; O. Berk Usta; Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine 2018, 13, 1267-1284, 10.2217/nnm-2017-0387.




