Iron oxide nanoparticles (IONPs) are extensively used in bone-related studies as biomaterials due to their unique magnetic properties and good biocompatibility. Through endocytosis, IONPs enter the cell where they promote osteogenic differentiation and inhibit osteoclastogenesis in vivo. This result is further supported by in-vivo findings, showing that osteoblasts and osteoclasts internalize the IONPs, yielding superior bone regeneration and weaker bone resorption. Therefore, IONPs have a potential clinical application value to promote bone regeneration and prevent osteoporosis.
- iron oxide nanoparticles
- bone remodeling
- osteoblast
- osteoclast
1. Introduction
2. Effects of Iron Oxide Nanoparticles on Bone Remodeling
2.1. Effects of Iron Oxide Nanoparticles on Osteoblasts
As early as 2008, Pareta et al.[6] used IONPs to study osteoblast proliferation and confirmed that calcium-phosphate-coated γ-Fe2O3 nanoparticles significantly increased the density of osteoblasts (i.e., promoted cell proliferation). Subsequently, Tran et al.[7] showed that hydroxyapatite (HA)-coated Fe3O4 nanoparticles significantly promoted the production of alkaline phosphatase (ALP), collagen, and calcium in osteoblasts, indicating that IONPs promote osteogenic differentiation; the authors further investigated the mechanism by which IONPs promote osteoblast differentiation and found that IONPs adsorbed a large amount of fibronectin, which can increase the function of osteoblasts, and upregulated the expression of genes related to osteoblast differentiation[8]. In addition, the authors found that osteoblasts uptake HA-coated IONPs into the cytoplasm via receptor-mediated endocytosis and increase intracellular calcium levels, which may be another reason why HA-coated IONPs promote osteoblast functions[9]. However, other IONPs coated with noncalcium materials can also promote osteoblast activity. For example, Shi et al.[10] found that chitosan-coated IONPs promoted osteoblast proliferation, reduced cell membrane damage, increased ALP activity, and enhanced extracellular calcium deposition. Yin et al.[11] treated MG-63 cells, an osteoblast cell line, with Fe3O4 nanoparticles and found that cell proliferation and ALP activity were significantly promoted. Stem cells have the ability to differentiate into a variety of cells, including osteoblasts. Xiao et al.[12] found that IONPs promoted cell proliferation, reduced apoptosis, increased ALP activity and mineralization nodule formation, and upregulated the expression of genes related to osteogenic differentiation in rat adipose-derived stem cells (ADSCs). Xia et al.[13] generated a scaffold by incorporating γFe2O3 and αFe2O3 nanoparticles into calcium phosphate cement (CPC). The authors found that human dental pulp stem cells (hDPSCs) seeded in this scaffold experienced increased osteogenic differentiation, ALP secretion, and mineral matrix synthesis compared with those seeded in scaffolds without IONPs, demonstrating that the osteogenic differentiation of hDPSCs was significantly promoted via the incorporation of IONPs into CPC. Similarly, Fe3O4-incorporated IONP–CPC scaffolds also enhanced the osteogenic differentiation of hDPSCs and promoted mandibular bone defect repair in rats[14]. In addition, studies have shown that IONPs have peroxidase activities[15]. Hydrogen peroxide (H2O2) was found to play an important role in the process of cell proliferation[16]. Huang et al.[17] treated human bone-derived mesenchymal stem cells (hBMSCs) with ferucarbotran (Resovist), an IONP approved for clinical liver MRI contrast agents, and found that ferucarbotran promoted cell proliferation by reducing intracellular H2O2 levels. These results indicate that IONPs have the ability to promote the proliferation and osteogenic differentiation of osteoblasts and stem cells in vitro. Mechanistically, numerous studies have revealed that IONPs enhance osteogenic differentiation through multiple signaling pathways. Wnt signaling is a crucial pathway that mediates osteogenesis. In the classical Wnt pathway, β-catenin acts as a key transcriptional coactivator, transmitting extracellular signals to the nucleus to activate downstream target genes such as RUNX2[18]. Xia et al.[19] revealed that γ-Fe2O3-loaded CPC scaffolds promoted the osteogenic differentiation of hDPSCs and significantly upregulated the gene expression of WNT1, RUNX2, ALP, COL1, and OCN. Moreover, β-catenin protein expression was increased, indicating that γ-Fe2O3-loaded CPC scaffolds activate Wnt/β-catenin signaling and downstream target genes. In osteoblast differentiation, increased osteoblastogenesis is dependent on the activation of β-catenin through the inhibition of GSK-3β[20], and the PI3K/Akt pathway can inhibit GSK-3β and activate β-catenin[21]. Yu et al.[22] developed a polysaccharide-based iron oxide nanoparticle (Fe2O3@PSC) and found that it has the ability to enhance osteoblast differentiation in MC3T3-E1 cells. A Western blotting assay showed that phosphorylated Akt, phosphorylated GSK-3β, and β-catenin were markedly upregulated. The authors proposed that Fe2O3@PSC promoted osteogenic differentiation by activating the Akt-GSK-3β-β-catenin signaling pathway. Mitogen-activated protein kinase (MAPK) includes three classic pathways, p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), which play a key role in skeletal development and bone homeostasis, particularly in osteoblast commitment and differentiation[23][24]. Wang et al.[25] found that polyglucose-sorbitol-carboxymethyether (PSC) coated IONPs enhanced the expression of phosphorylated MEK1/2 and ERK1/2, indicating that IONPs activate the classic ERK-MAPK signaling pathway in hBMSCs. As a result, downstream genes of this pathway such as bone morphogenic protein (BMP2) and RUNX2 were upregulated to promote osteogenic differentiation. BMP2 is a signal molecule of the transforming growth factor-beta (TGF-β) superfamily and plays a crucial role in bone formation by activating the canonical Smad-dependent pathway or noncanonical-MAPK signaling pathway[26]. Lu et al.[27] fabricated a magnetic SrFe12O19 nanoparticle-modified mesoporous bioglass (BG) and chitosan (CS) porous scaffold (MBCS) to treat hBMSCs and found that this scaffold upregulated BMP2 and phosphorylated Smad1/5 expression and promoted the expression of osteogenic-related genes including RUNX2, OCN, COL1, and ALP, suggesting that magnetic MBCS scaffolds enhance osteogenic gene expression by activating the BMP-2/Smad signaling pathway. These in vitro studies indicate that IONPs or IONP-loaded scaffolds accelerate osteogenic differentiation through the Wnt/β-catenin, Akt-GSK-3β-β-catenin, MAPK, and BMP-2/Smad signaling pathways (Figure 1a).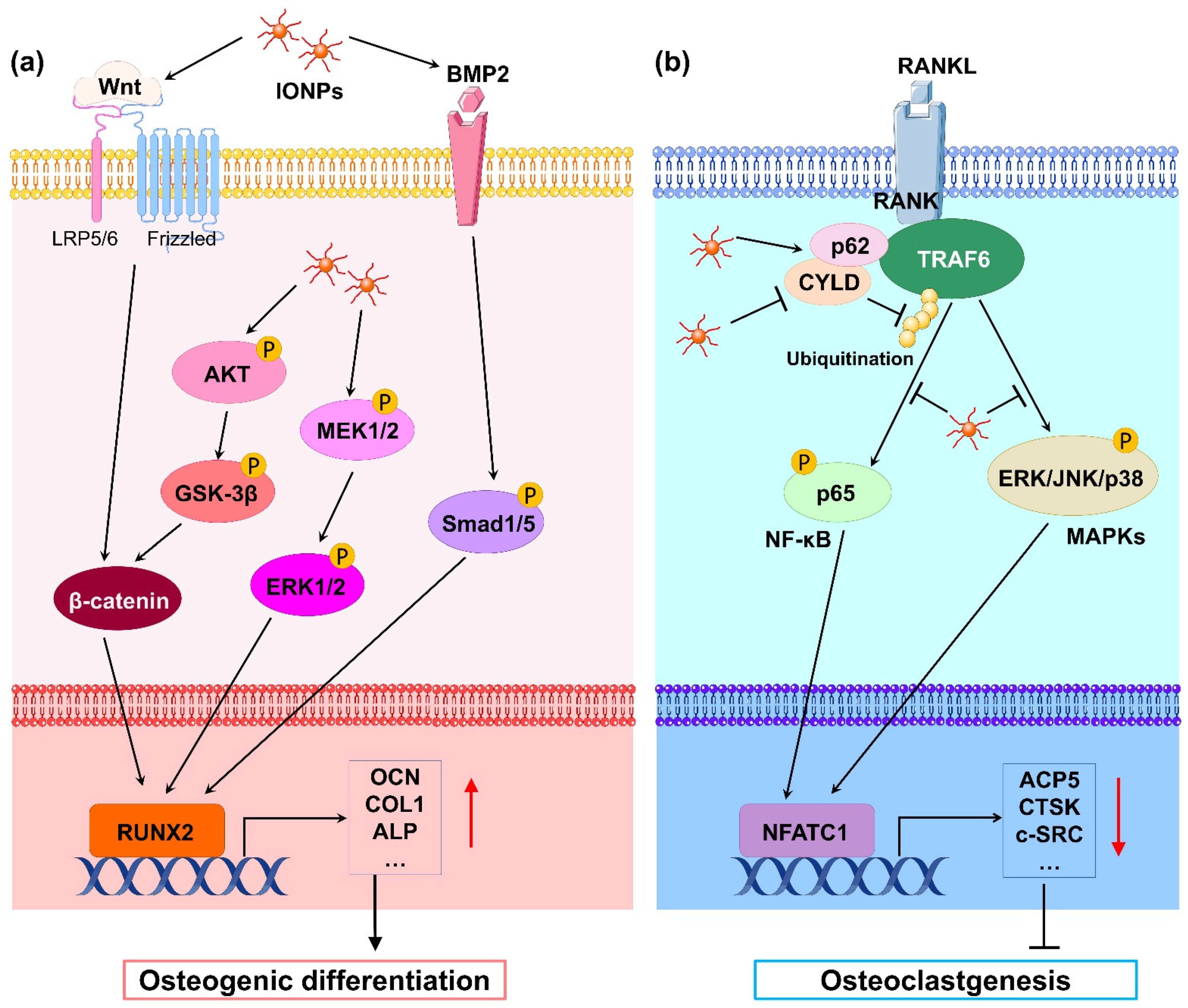
2.2. Effects of Iron Oxide Nanoparticles on Osteoclasts
Osteoclasts are differentiated from bone marrow macrophages (BMMs) under the induction of the receptor activator for nuclear factor-κ B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) and are the main cells that perform bone resorption. Compared with osteoblasts, there are fewer studies on IONPs on osteoclasts. Li et al.[37] treated mouse BMMs with PSC-coated IONPs and HA-coated IONPs, finding that both IONPs significantly inhibited osteoclast formation and downregulated osteoclast-differentiation-related gene expression. Postmenopausal osteoporosis is a disease characterized by reduced BMD, damaged bone microstructure, and increased bone fragility induced by increased osteoclast activity[38]. Bilateral ovariectomy (OVX) in animals is the most commonly used model used to mimic postmenopausal osteoporosis. Liu et al.[39] found that ferucarbotran and Feraheme inhibited the differentiation of mouse BMMs into osteoclasts, whereas the intravenous injection of two types of IONPs markedly inhibited bone resorption and OVX-induced bone loss in OVX mice. Zheng et al.[40] also revealed that PSC-coated IONPs inhibited osteoclast differentiation and prevented bone loss caused by OVX. In addition, the authors prepared IONPs loaded with alendronate, a drug used for the treatment of osteoporosis, and found that IONPs could target the bone tissue; the IONPs’ ability to inhibit bone loss was significantly better than that of alendronate alone. Iron is an essential element involved in multiple life activities of the human body, including bone metabolism[41]. However, excessive iron can induce osteoporosis by activating osteoclast activity[42]. Yu et al.[22] showed that the PSC-loaded Fe2O3 nanoparticles inhibited osteoclast differentiation of Raw 264.7 cells in vitro and prevented iron-accumulation-related osteoporosis in vivo. During osteoclast differentiation, RANKL binds to its receptor RANK on BMMs and activates many signaling pathways, including MAPKs (ERK, JNK, and p38) and nuclear factor-kB (NF-kB), by recruiting the signaling-adaptor molecule TNF receptor-associated factor 6 (TRAF6)[43]. Among them, the ubiquitination of TRAF6, which involves the important adaptor protein p62 and deubiquitinase cylindromatosis (CYLD), is a key process[44]. Liu et al.[39] revealed that IONPs enhanced the expression of p62, which resulted in the recruitment of CYLD and promoted the deubiquitination of TRAF6. Moreover, the downstream MAPK and NF-κB signaling pathway was inhibited, leading to decreased expression of osteoclastogenesis-related genes, including NFATC1, ACP5, CALCR, CTSK, and c-SRC. Similarly, Yu et al.[22] demonstrated that Fe2O3@PSC nanoparticles suppressed osteoclast differentiation by inhibiting the MAPK and NF-κB pathways in vitro. Therefore, IONPs can inhibit osteoclast differentiation through the retardation of MAPK and NF-κB signaling pathways (Figure 1b).3. Outlook
Although the effect of IONPs on bone remodeling has been well studied, there remain some interesting unresolved questions regarding the effects of IONPs on bone remodeling that deserve exploration in the future. Osteocytes descend from osteoblasts encapsulated by a mineralized bone matrix and constitute over 90% of bone cells in the adult skeleton[45]. These cells act as a coordinator in bone remodeling, modulating the differentiation and function of osteoclasts and osteoblasts through distinct signaling pathways, including the RANKL/OPG axis and SOST/Dkk1/Wnt axis[46]. Although osteocytes are very important for bone remodeling, there is no report on the effects of IONPs on osteocytes. This subject would be a rewarding direction for future studies, as osteocytes play a crucial role in bone homeostasis.Previous studies on IONPs related to bone repair or osteoporosis have mainly focused on the biological effects of IONPs in animal or cell experiments. Thus, rigorous clinical trials in humans are needed before translating these findings into clinical practice. Moreover, toxicity is the most important evaluation index in clinical therapy. However, existing studies fail to evaluate the safety of IONPs in vivo. The toxicity of IONPs should be considered in a dose-, treatment-, and time-dependent manner[47]. Therefore, the absorption, distribution, metabolism, and toxicity of IONPs after implanting a composite scaffold containing IONPs in vivo should be explored in future studies.
References
- Yan Li; Dewen Ye; Mingxi Li; Ming Ma; Ning Gu; Adaptive Materials Based on Iron Oxide Nanoparticles for Bone Regeneration. ChemPhysChem 2018, 19, 1965-1979, 10.1002/cphc.201701294.
- Seyed Mohammadali Dadfar; Karolin Roemhild; Natascha I. Drude; Saskia von Stillfried; Ruth Knüchel; Fabian Kiessling; Twan Lammers; Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Advanced Drug Delivery Reviews 2019, 138, 302-325, 10.1016/j.addr.2019.01.005.
- Saeid Zanganeh; Gregor Hutter; Ryan Spitler; Olga Lenkov; Morteza Mahmoudi; Aubie Shaw; Jukka Sakari Pajarinen; Hossein Nejadnik; Jukka Sakari Pajarinen Stuart Goodman; Michael Moseley; et al.Aubie Shaw Lisa Marie CoussensHeike Daldrup-Link Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nature Nanotechnology 2016, 11, 986-994, 10.1038/nnano.2016.168.
- Yue Huang; Jessica C. Hsu; Hyun Koo; David P. Cormode; Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796-816, 10.7150/thno.67375.
- Erik Fink Eriksen; Cellular mechanisms of bone remodeling. Reviews in Endocrine and Metabolic Disorders 2010, 11, 219-227, 10.1007/s11154-010-9153-1.
- Rajesh A Pareta; Eric Taylor; Thomas J Webster; Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 2008, 19, 265101, 10.1088/0957-4484/19/26/265101.
- Nhiem Tran; Thomas J. Webster; Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomaterialia 2011, 7, 1298-1306, 10.1016/j.actbio.2010.10.004.
- Nhiem Tran; Douglas Hall; Thomas J Webster; Mechanisms of enhanced osteoblast gene expression in the presence of hydroxyapatite coated iron oxide magnetic nanoparticles. Nanotechnology 2012, 23, 455104, 10.1088/0957-4484/23/45/455104.
- Nhiem Tran; Thomas J Webster; Understanding magnetic nanoparticle osteoblast receptor-mediated endocytosis using experiments and modeling. Nanotechnology 2013, 24, 185102, 10.1088/0957-4484/24/18/185102.
- Xian-Long Zhang; Si-Feng Shi; Jing-Fu Jia; Ya-Ping Zhao; Xiao-Kui Guo; De-Sheng Chen; Tao Cheng; Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. International Journal of Nanomedicine 2012, 7, 5593-5602, 10.2147/ijn.s34348.
- Guangfu Yin; Zhongbing Huang; Min Deng; Jingwen Zeng; Jianwen Gu; Preparation and cell response of bio-mineralized Fe3O4 nanoparticles. Journal of Colloid and Interface Science 2011, 363, 393-402, 10.1016/j.jcis.2011.07.009.
- Hai-Tao Xiao; Lei Wang; Bin Yu; Superparamagnetic iron oxide promotes osteogenic differentiation of rat adipose-derived stem cells.. International journal of clinical and experimental medicine 2015, 8, 698-705.
- Yang Xia; Huimin Chen; Feimin Zhang; Lin Wang; Bo Chen; Mark A. Reynolds; Junqing Ma; Abraham Schneider; Ning Gu; Hockin H. K. Xu; et al. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artificial Cells, Nanomedicine, and Biotechnology 2018, 46, 423-433, 10.1080/21691401.2018.1428813.
- Yang Xia; Huimin Chen; Yantao Zhao; Feimin Zhang; Xiaodong Li; Lin Wang; Michael D. Weir; Junqing Ma; Mark A. Reynolds; Ning Gu; et al.Hockin H.K. Xu Novel magnetic calcium phosphate-stem cell construct with magnetic field enhances osteogenic differentiation and bone tissue engineering. Materials Science and Engineering: C 2018, 98, 30-41, 10.1016/j.msec.2018.12.120.
- Lizeng Gao; Jie Zhuang; Leng Nie; Jinbin Zhang; Yu Zhang; Ning Gu; Taihong Wang; Jing Feng; Dongling Yang; Sarah Perrett; et al.Xiyun Yan Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology 2007, 2, 577-583, 10.1038/nnano.2007.260.
- Roy H. Burdon; Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine 1995, 18, 775-794, 10.1016/0891-5849(94)00198-s.
- Dong-Ming Huang; Jong-Kai Hsiao; Ying-Chun Chen; Li-Ying Chien; Ming Yao; Yin-Kai Chen; Bor-Sheng Ko; Szu-Chun Hsu; Lin-Ai Tai; Hui-Ying Cheng; et al.Shih-Wei WangChung-Shi YangYao-Chang Chen The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials 2009, 30, 3645-3651, 10.1016/j.biomaterials.2009.03.032.
- Wantae Kim; Minseong Kim; Eek-Hoon Jho; Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochemical Journal 2013, 450, 9-21, 10.1042/bj20121284.
- Yang Xia; Yu Guo; Zukun Yang; Huimin Chen; Ke Ren; Michael D. Weir; Laurence C. Chow; Mark A. Reynolds; Feimin Zhang; Ning Gu; et al.Hockin H.K. Xu Iron oxide nanoparticle-calcium phosphate cement enhanced the osteogenic activities of stem cells through WNT/β-catenin signaling. Materials Science and Engineering: C 2019, 104, 109955, 10.1016/j.msec.2019.109955.
- Buer Sen; Maya Styner; Zhihui Xie; Natasha Case; Clinton T. Rubin; Janet Rubin; Mechanical Loading Regulates NFATc1 and β-Catenin Signaling through a GSK3β Control Node. Journal of Biological Chemistry 2009, 284, 34607-34617, 10.1074/jbc.m109.039453.
- Fanglong Song; Dawei Jiang; Tianchen Wang; Yi Wang; Yi Lou; Yinquan Zhang; Hui Ma; Yifan Kang; Mechanical Stress Regulates Osteogenesis and Adipogenesis of Rat Mesenchymal Stem Cells through PI3K/Akt/GSK-3β/β-Catenin Signaling Pathway. BioMed Research International 2017, 2017, 1-10, 10.1155/2017/6027402.
- Pengjun Yu; Liming Zheng; Peng Wang; Senlin Chai; Yibo Zhang; Tianshu Shi; Lei Zhang; Rui Peng; Caoxing Huang; Baosheng Guo; et al.Qing Jiang Development of a novel polysaccharide-based iron oxide nanoparticle to prevent iron accumulation-related osteoporosis by scavenging reactive oxygen species. International Journal of Biological Macromolecules 2020, 165, 1634-1645, 10.1016/j.ijbiomac.2020.10.016.
- Eddie Rodríguez-Carballo; Beatriz Gámez; Francesc Ventura; p38 MAPK Signaling in Osteoblast Differentiation. Frontiers in Cell and Developmental Biology 2016, 4, 40-40, 10.3389/fcell.2016.00040.
- Ren Xu; Chao Zhang; Dong Yeon Shin; Jung‐Min Kim; Sarfaraz Lalani; Na Li; Yeon‐Suk Yang; Yifang Liu; Mark Eiseman; Roger J Davis; et al.Jae‐Hyuck ShimMatthew B Greenblatt c‐Jun N‐Terminal Kinases (JNKs) Are Critical Mediators of Osteoblast Activity In Vivo. Journal of Bone and Mineral Research 2017, 32, 1811-1815, 10.1002/jbmr.3184.
- Qiwei Wang; Bo Chen; Meng Cao; Jianfei Sun; Hao Wu; Peng Zhao; Jing Xing; Yan Yang; Xiquan Zhang; Min Ji; et al.Ning Gu Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11-20, 10.1016/j.biomaterials.2016.02.004.
- Mengrui Wu; Guiqian Chen; Yi-Ping Li; TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research 2016, 4, 16009, 10.1038/boneres.2016.9.
- Jia-Wei Lu; Fan Yang; Qin-Fei Ke; Xue-Tao Xie; Ya-Ping Guo; Magnetic nanoparticles modified-porous scaffolds for bone regeneration and photothermal therapy against tumors. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 811-822, 10.1016/j.nano.2017.12.025.
- Shuying Hu; Yi Zhou; Yantao Zhao; Yang Xu; Feimin Zhang; Ning Gu; Junqing Ma; Mark A. Reynolds; Yang Xia; Hockin H.K. Xu; et al. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. Journal of Tissue Engineering and Regenerative Medicine 2018, 12, e2085-e2098, 10.1002/term.2641.
- Wei Liao; Jingwei Lu; Qianjin Wang; Sen Yan; Yan Li; Yibo Zhang; Peng Wang; Qing Jiang; Ning Gu; Osteogenesis of Iron Oxide Nanoparticles-Labeled Human Precartilaginous Stem Cells in Interpenetrating Network Printable Hydrogel. Frontiers in Bioengineering and Biotechnology 2022, 10, 872149, 10.3389/fbioe.2022.872149.
- Rajendra K Singh; Kapil D. Patel; Jae Ho Lee; Eun-Jung Lee; Joong-Hyun Kim; Tae-Hyun Kim; Hae-Won Kim; Potential of Magnetic Nanofiber Scaffolds with Mechanical and Biological Properties Applicable for Bone Regeneration. PLOS ONE 2014, 9, e91584, 10.1371/journal.pone.0091584.
- S. Panseri; A. Russo; G. Giavaresi; M. Sartori; F. Veronesi; M. Fini; D. M. Salter; A. Ortolani; A. Strazzari; A. Visani; et al.C. DionigiN. BockM. SandriA. TampieriM. Marcacci Innovative magnetic scaffolds for orthopedic tissue engineering. Journal of Biomedical Materials Research Part A 2012, 100A, 2278-2286, 10.1002/jbm.a.34167.
- Cijun Shuai; Wenjing Yang; Chongxian He; Shuping Peng; Chengde Gao; Youwen Yang; Fangwei Qi; Pei Feng; A magnetic micro-environment in scaffolds for stimulating bone regeneration. Materials & Design 2019, 185, 108275, 10.1016/j.matdes.2019.108275.
- Yao Zhao; Tiantang Fan; Jingdi Chen; Jiacan Su; Xin Zhi; Panpan Pan; Lin Zou; Qiqing Zhang; Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids and Surfaces B: Biointerfaces 2018, 174, 70-79, 10.1016/j.colsurfb.2018.11.003.
- Jingjing Fan; Yanbin Tan; Liyong Jie; Xinying Wu; Risheng Yu; Minming Zhang; Biological activity and magnetic resonance imaging of superparamagnetic iron oxide nanoparticles-labeled adipose-derived stem cells. Stem Cell Research & Therapy 2013, 4, 44-44, 10.1186/scrt191.
- Surakshya Shrestha; Pengfei Jiang; Marcelo Henrique Sousa; Paulo Cesar Morais; Zhengwei Mao; Changyou Gao; Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. Journal of Materials Chemistry B 2015, 4, 245-256, 10.1039/c5tb02007g.
- Lizandra Ferrari Guimarães; Tatiana Kelly Da Silva Fidalgo; Gustavo Conde Menezes; Laura Guimarães Primo; Fernando Costa e Silva-Filho; Effects of citric acid on cultured human osteoblastic cells. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2010, 110, 665-669, 10.1016/j.tripleo.2010.07.003.
- Mengye Li; Shengxiang Fu; Zhongyuan Cai; Danyang Li; Li Liu; Di Deng; Rongrong Jin; Hua Ai; Dual regulation of osteoclastogenesis and osteogenesis for osteoporosis therapy by iron oxide hydroxyapatite core/shell nanocomposites. Regenerative Biomaterials 2021, 8, rbab027, 10.1093/rb/rbab027.
- Dolores Shoback; Clifford J Rosen; Dennis M Black; Angela M. Cheung; Mohammad Hassan Murad; Richard Eastell; Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. The Journal of Clinical Endocrinology & Metabolism 2020, 105, 587-594, 10.1210/clinem/dgaa048.
- Li Liu; Rongrong Jin; Jimei Duan; Li Yang; Zhongyuan Cai; Wencheng Zhu; Yu Nie; Jing He; Chunchao Xia; Qiyong Gong; et al.Bin SongJames M. AndersonHua Ai Bioactive iron oxide nanoparticles suppress osteoclastogenesis and ovariectomy-induced bone loss through regulating the TRAF6-p62-CYLD signaling complex. Acta Biomaterialia 2019, 103, 281-292, 10.1016/j.actbio.2019.12.022.
- Liming Zheng; Zaikai Zhuang; Yixuan Li; Tianshu Shi; Kai Fu; Wenjin Yan; Lei Zhang; Peng Wang; Lan Li; Qing Jiang; et al. Bone targeting antioxidative nano-iron oxide for treating postmenopausal osteoporosis. Bioactive Materials 2021, 14, 250-261, 10.1016/j.bioactmat.2021.11.012.
- Jiancheng Yang; Qinghua Tang; Yuhong Zeng; Melatonin: Potential avenue for treating iron overload disorders. Ageing Res Rev 2022, 81, 101717, 10.1016/j.arr.2022.101717.
- Jaime Tsay; Zheiwei Yang; F. Patrick Ross; Susanna Cunningham-Rundles; Hong Lin; Rhima Coleman; Philipp Mayer-Kuckuk; Stephen B. Doty; Robert W. Grady; Patricia J. Giardina; et al.Adele BoskeyMaria G. Vogiatzi Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood 2010, 116, 2582-2589, 10.1182/blood-2009-12-260083.
- Dulshara Sachini Amarasekara; Hyeongseok Yun; Sumi Kim; Nari Lee; Hyunjong Kim; Jaerang Rho; Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Network 2018, 18, e8, 10.4110/in.2018.18.e8.
- Wei Jin; Mikyoung Chang; Emmanuel M. Paul; Geetha Babu; Andrew J. Lee; William Reiley; Ato Wright; Minying Zhang; Jun You; Shao-Cong Sun; et al. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. Journal of Clinical Investigation 2008, 118, 1858-1866, 10.1172/jci34257.
- Alexander G. Robling; Lynda F. Bonewald; The Osteocyte: New Insights. Annual Review of Physiology 2020, 82, 485-506, 10.1146/annurev-physiol-021119-034332.
- Jesus Medical Delgado-Calle; Teresita Bellido; The osteocyte as a signaling cell. Physiological Reviews 2022, 102, 379-410, 10.1152/physrev.00043.2020.
- Kurtulus Gokduman; Furkan Bestepe; Lei Li; Martin L Yarmush; O. Berk Usta; Dose-, treatment- and time-dependent toxicity of superparamagnetic iron oxide nanoparticles on primary rat hepatocytes. Nanomedicine 2018, 13, 1267-1284, 10.2217/nnm-2017-0387.
