
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Naoki Asano | -- | 2003 | 2022-10-19 05:10:11 | | | |
| 2 | Catherine Yang | -3 word(s) | 2000 | 2022-10-25 03:46:45 | | |
Video Upload Options
Wnt signaling plays an essential role in aging of the gastrointestinal tract. Aberration of Wnt signaling seen in aged animals has been shown to affect regenerative capacity and differentiation of intestinal stem cells and promote aging-related deterioration. Similarly, abnormal Wnt signaling was observed in the aged stomach. Specifically, enhanced Wnt signaling in organoids established from the stomachs of aged mice induced the expression of Tbx3, a transcription factor that suppress cellular senescence, and led to augmented cellular proliferation. The enhanced Wnt signaling was due to suppressed Dkk3, a Wnt inhibitor, in aged gastric organoids. With respect to the role of TBX3 in humans, expression of TBX3 in human gastric tissues exhibited positive correlation with patients' age whereas that of DKK3 showed negative correlation with patients' age. In addition, TBX3 expression was also confirmed in gastric cancer tissues but not in normal gastric mucosae. These findings indicated that this DKK3-Wnt-TBX3 pathway may contribute to aging-related gastric carcinogenesis.
1. Wnt Signaling and Stem Cell Aging
2. Wnt Signaling and Aging of the Gastrointestinal Tract
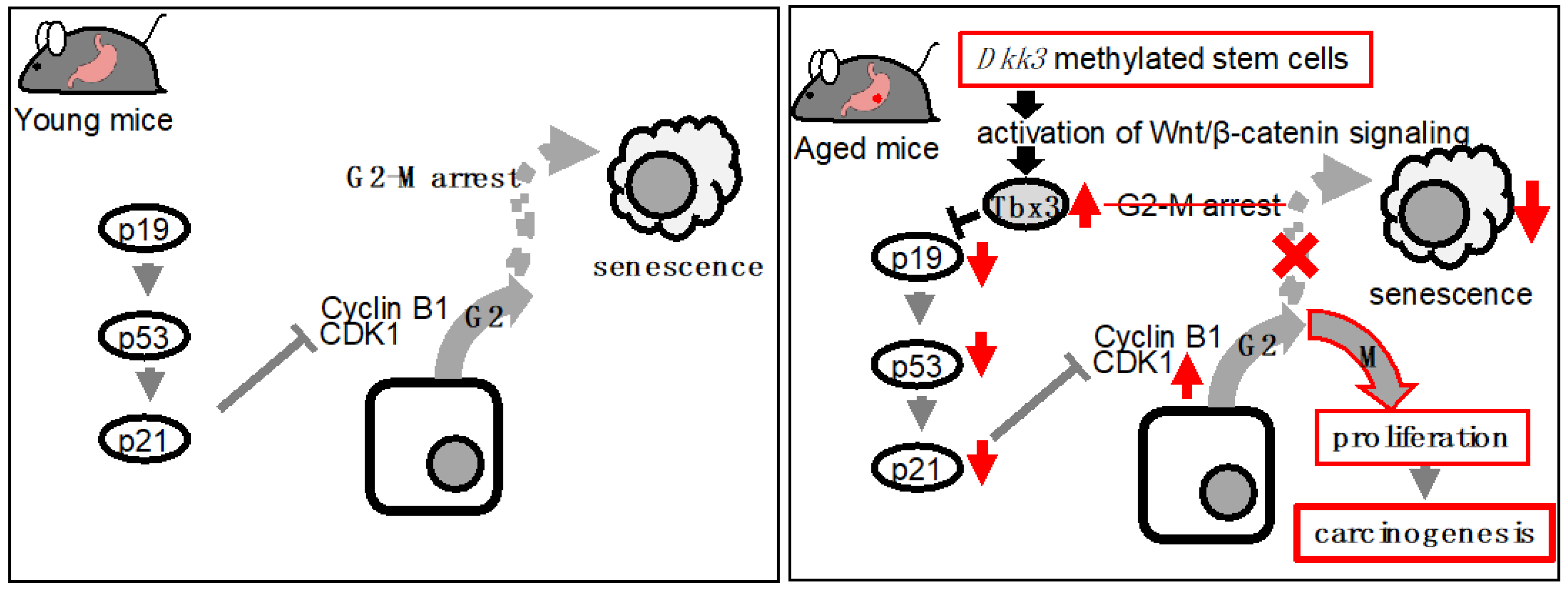
3. Wnt Signaling and Aging-Related Carcinogenesis of the Gastrointestinal Tract
References
- Juhyun Oh; Yang David Lee; Amy J Wagers; Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nature Medicine 2014, 20, 870-880, 10.1038/nm.3651.
- Mi-Hyeon Jang; Michael A. Bonaguidi; Yasuji Kitabatake; Jiaqi Sun; Juan Song; Eunchai Kang; Heechul Jun; Chun Zhong; Yijing Su; Junjie U. Guo; et al.Marie Xun WangKurt A. SailorJu-Young KimYuan GaoKimberly M. ChristianGuo-Li MingHongjun Song Secreted Frizzled-Related Protein 3 Regulates Activity-Dependent Adult Hippocampal Neurogenesis. Cell Stem Cell 2013, 12, 215-223, 10.1016/j.stem.2012.11.021.
- Chang Hoon Cho; Ki Hyun Yoo; Alfredo Oliveros; Summer Paulson; Syed Mohammed Qasim Hussaini; Jan M. Van Deursen; Mi-Hyeon Jang; sFRP3 inhibition improves age-related cellular changes in BubR1 progeroid mice. Aging Cell 2019, 18, e12899, 10.1111/acel.12899.
- Georgios Kalamakis; Daniel Brüne; Srikanth Ravichandran; Jan Bolz; Wenqiang Fan; Frederik Ziebell; Thomas Stiehl; Francisco Catalá-Martinez; Janina Kupke; Sheng Zhao; et al.Enric Llorens-BobadillaKatharina BauerStefanie LimpertBirgit BergerUrs ChristenPeter SchmezerJan Philipp MallmBenedikt BerningerSimon AndersAntonio del SolAnna Marciniak-CzochraAna Martin-Villalba Quiescence Modulates Stem Cell Maintenance and Regenerative Capacity in the Aging Brain. Cell 2019, 176, 1407-1419.e14, 10.1016/j.cell.2019.01.040.
- Désirée R.M. Seib; Nina S. Corsini; Kristina Ellwanger; Christian Plaas; Alvaro Mateos; Claudia Pitzer; Christof Niehrs; Tansu Celikel; Ana Martin-Villalba; Loss of Dickkopf-1 Restores Neurogenesis in Old Age and Counteracts Cognitive Decline. Cell Stem Cell 2013, 12, 204-214, 10.1016/j.stem.2012.11.010.
- Andrew S. Brack; Michael J. Conboy; Sudeep Roy; Mark Lee; Calvin J. Kuo; Charles Keller; Thomas A. Rando; Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807-810, 10.1126/science.1144090.
- Hellen E. Ahrens; Judith Huettemeister; Manuel Schmidt; Christoph Kaether; Julia Von Maltzahn; Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skeletal Muscle 2018, 8, 1-14, 10.1186/s13395-018-0166-x.
- Rogerio M. Castilho; Cristiane H. Squarize; Lewis A. Chodosh; Bart O. Williams; J. Silvio Gutkind; mTOR Mediates Wnt-Induced Epidermal Stem Cell Exhaustion and Aging. Cell Stem Cell 2009, 5, 279-289, 10.1016/j.stem.2009.06.017.
- Kodandaramireddy Nalapareddy; Kalpana J. Nattamai; Rupali S. Kumar; Rebekah Karns; Kathryn A. Wikenheiser-Brokamp; Leesa L. Sampson; Maxime M. Mahe; Nambirajan Sundaram; Mary-Beth Yacyshyn; Bruce Yacyshyn; et al.Michael A. HelmrathYi ZhengHartmut Geiger Canonical Wnt Signaling Ameliorates Aging of Intestinal Stem Cells. Cell Reports 2017, 18, 2608-2621, 10.1016/j.celrep.2017.02.056.
- Nalle Pentinmikko; Sharif Iqbal; Miyeko Mana; Simon Andersson; Armand B. Cognetta; Radu M. Suciu; Jatin Roper; Kalle Luopajärvi; Eino Markelin; Swetha Gopalakrishnan; et al.Olli-Pekka SmolanderSantiago NaranjoTuure SaarinenAnne JuutiKirsi PietiläinenPetri AuvinenAri RistimäkiNitin GuptaTuomas TammelaTyler JacksDavid M. SabatiniBenjamin F. CravattÖmer H. YilmazPekka Katajisto Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398-402, 10.1038/s41586-019-1383-0.
- Do-Hyung Kim; Dos D. Sarbassov; Siraj M. Ali; Jessie E. King; Robert R. Latek; Hediye Erdjument-Bromage; Paul Tempst; David M. Sabatini; mTOR Interacts with Raptor to Form a Nutrient-Sensitive Complex that Signals to the Cell Growth Machinery. Cell 2002, 110, 163-175, 10.1016/s0092-8674(02)00808-5.
- David E. Harrison; Randy Strong; Zelton Dave Sharp; James F. Nelson; Clinton M. Astle; Kevin Flurkey; Nancy L. Nadon; J. Erby Wilkinson; Krystyna Frenkel; Christy S. Carter; et al.Marco PahorMartin A. JavorsElizabeth FernandezRichard A. Miller Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392-395, 10.1038/nature08221.
- Hui Cui; Duozhuang Tang; George B. Garside; Ting Zeng; Yiting Wang; Zhendong Tao; Liu Zhang; Si Tao; Wnt Signaling Mediates the Aging-Induced Differentiation Impairment of Intestinal Stem Cells. Stem Cell Reviews and Reports 2019, 15, 448-455, 10.1007/s12015-019-09880-9.
- Yujiro Hayashi; David T. Asuzu; Michael R. Bardsley; Gabriella B. Gajdos; Sergiy M. Kvasha; David R. Linden; Rea A. Nagy; Siva Arumugam Saravanaperumal; Sabriya A. Syed; Yoshitaka Toyomasu; et al.Huihuang YanEduardo N. ChiniSimon J. GibbonsTodd A. KelloggKhashayarsha KhazaieMakoto Kuro-OJair Machado Espindola NettoMahendra Pal SinghJames G. TidballMichelle Wehling-HenricksGianrico FarrugiaTamas Ordog Wnt-induced, TRP53-mediated Cell Cycle Arrest of Precursors Underlies Interstitial Cell of Cajal Depletion During Aging. Cellular and Molecular Gastroenterology and Hepatology 2020, 11, 117-145, 10.1016/j.jcmgh.2020.07.011.
- Akio Takeuchi; Naoki Asano; Akira Imatani; Masashi Saito; Xiaoyi Jin; Masahiro Saito; Takeshi Kanno; Waku Hatta; Kaname Uno; Tomoyuki Koike; et al.Atsushi Masamune Suppressed Cellular Senescence Mediated by T-box3 in Aged Gastric Epithelial Cells may Contribute to Aging-related Carcinogenesis. Cancer Research Communications 2022, 2, 772-783, 10.1158/2767-9764.crc-22-0084.
- Thijn R. Brummelkamp; Roderik M. Kortlever; Merel Lingbeek; Flavia Trettel; Marcy E. MacDonald; Maarten van Lohuizen; René Bernards; TBX-3, the Gene Mutated in Ulnar-Mammary Syndrome, Is a Negative Regulator of p19 and Inhibits Senescence. Journal of Biological Chemistry 2002, 277, 6567-6572, 10.1074/jbc.m110492200.
- Jie Yin; Lele Yang; Yangli Xie; Yan Liu; Sheng Li; Wenjun Yang; Bo Xu; HongBin Ji; LiangHua Ding; Kun Wang; et al.Gang LiLin ChenPing Hu Dkk3 dependent transcriptional regulation controls age related skeletal muscle atrophy. Nature Communications 2018, 9, 1-13, 10.1038/s41467-018-04038-6.
- João Pedro de Magalhães; How ageing processes influence cancer. Nature Reviews Cancer 2013, 13, 357-365, 10.1038/nrc3497.
- Si Tao; Duozhuang Tang; Yohei Morita; Tobias Sperka; Omid Omrani; André Lechel; Vadim Sakk; Johann Kraus; Hans A Kestler; Michael Kühl; et al.Karl Lenhard Rudolph Wnt activity and basal niche position sensitize intestinal stem and progenitor cells toDNA damage. The EMBO Journal 2015, 34, 624-640, 10.15252/embj.201490700.
- Peter D. Adams; Heinrich Jasper; K. Lenhard Rudolph; Aging-Induced Stem Cell Mutations as Drivers for Disease and Cancer. Cell Stem Cell 2015, 16, 601-612, 10.1016/j.stem.2015.05.002.
- Emmalee R. Adelman; Hsuan-Ting Huang; Alejandro Roisman; André Olsson; Antonio Colaprico; Tingting Qin; R. Coleman Lindsley; Rafael Bejar; Nathan Salomonis; H. Leighton Grimes; et al.Maria E. Figueroa Aging Human Hematopoietic Stem Cells Manifest Profound Epigenetic Reprogramming of Enhancers That May Predispose to Leukemia. Cancer Discovery 2019, 9, 1080-1101, 10.1158/2159-8290.cd-18-1474.
- Kanji So; Gen Tamura; Teiichiro Honda; Naoyuki Homma; Takayoshi Waki; Naoyuki Togawa; Satoshi Nishizuka; Teiichi Motoyama; Multiple tumor suppressor genes are increasingly methylated with age in non-neoplastic gastric epithelia. Cancer Science 2006, 97, 1155-1158, 10.1111/j.1349-7006.2006.00302.x.
- Seung-Kyoon Kim; Hay-Ran Jang; Jeong-Hwan Kim; Mirang Kim; Seung-Moo Noh; Kyu-Sang Song; Gyeong Hoon Kang; Hee Jin Kim; Seon-Young Kim; Hyang-Sook Yoo; et al.Yong Sung Kim CpG methylation in exon 1 of transcription factor 4 increases with age in normal gastric mucosa and is associated with gene silencing in intestinal-type gastric cancers. Carcinogenesis 2008, 29, 1623-1631, 10.1093/carcin/bgn110.
- Maja Milanovic; Dorothy N. Y. Fan; Dimitri Belenki; J. Henry M. Däbritz; Zhen Zhao; Yong Yu; Jan R. Dörr; Lora Dimitrova; Dido Lenze; Ines A. Monteiro Barbosa; et al.Marco A. Mendoza-ParraTamara KanashovaMarlen MetznerKatharina PardonMaurice ReimannAndreas TrumppBernd DörkenJohannes ZuberHinrich GronemeyerMichael HummelGunnar DittmarSoyoung LeeClemens A. Schmitt Senescence-associated reprogramming promotes cancer stemness. Nature 2017, 553, 96-100, 10.1038/nature25167.




