Biodegradation is the deformation of a substance into new compounds through biochemical reactions or the actions of microorganisms such as bacteria or fungi. It is necessary for water-soluble or water-immiscible polymers because they eventually enter streams which can neither be recycled nor incinerated. It is important to consider the microbial degradation of natural and synthetic polymers in order to understand what is necessary for biodegradation and the mechanisms involved. Low/high-density polyethylene is a vital cause of environmental pollution. It occurs by choking the sewer line through mishandling, thus posing an everlasting ecological threat. Environmental pollution due to the unscrupulous consumption of synthetic polymers derived from petroleum has an adverse impact on the environment since the majority of plastics do not degrade, and the further incineration of synthetic plastics generates CO2 and dioxin. This requires understanding the interactions between materials and microorganisms and the biochemical changes involved. Widespread studies on the biodegradation of plastics have been carried out in order to overcome the environmental problems associated with synthetic plastic waste. Awareness of the waste problem and its impact on the environment has awakened new interest in the area of degradable polymers through microbes viz., bacteria, fungi, and actinomycetes. The microbial degradation of plastics is caused by certain enzymatic activities that lead to a chain cleavage of polymers into oligomers and monomers.
1. Introduction
Biodegradation is the process by which microbial organisms transform to alter (through metabolic or enzymatic action) the structure of chemicals introduced into the environment
[1][2][3][4].
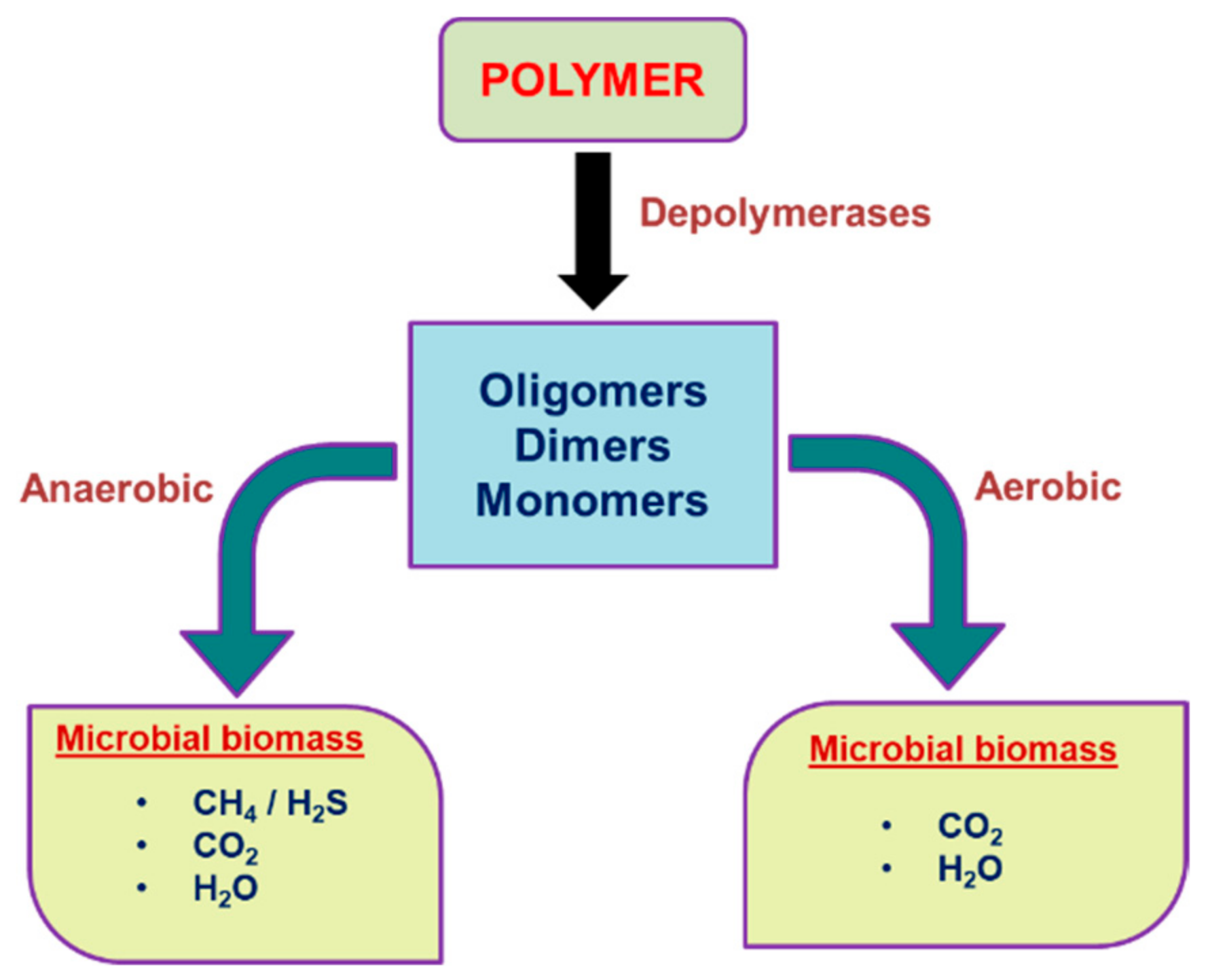
Figure 1 shows polymer degradation under aerobic and anaerobic conditions.
Figure 1. Scheme of polymer degradation under aerobic and anaerobic conditions.
2. Biodegradation of Plastic by Microbes
Biodegradation is a natural and complex process of decomposition facilitated by biochemical mechanisms and the successive mineralization of the polymer material. Biodegradable plastics open the way for new waste management strategies since these materials are designed to degrade under environmental conditions or in municipal and industrial biological waste treatment facilities
[5]. Bacteria, fungi, and actinomycetes are of particular interest in the biodegradation of natural and synthetic polymers. Many species or types of microorganisms are found broadly in nature
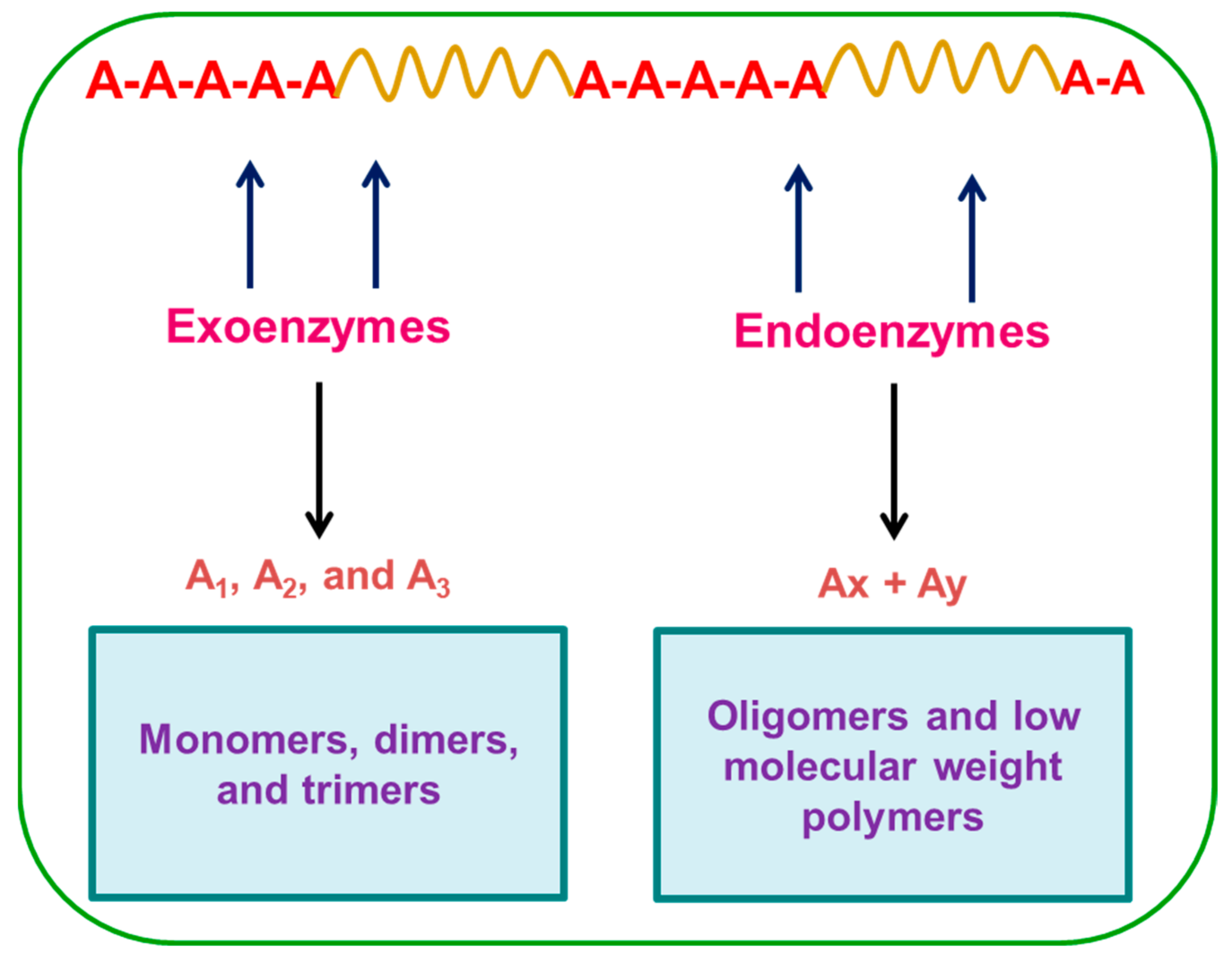
[6]. Microorganisms are highly adaptive to their environment and secrete both endoenzymes and exoenzymes that attack the substrate and cleave the molecular chains into segments
[7]. The biological degradation of these polyethylene films has been reported in pure culture studies with various microorganisms such as
Staphylococcus sp.,
Streptococcus sp.
[8],
Phanerochaete sp.
[9], and
Bacillus sp.
[10]. Mergaert et al. investigated the polymer-degrading isolates
[11], actinomycetes, and fungal isolates which were able to degrade polyhydroxy butyrate, bionolle, and polycaprolactone. Orhan et al. investigated the PE biodegradation process by using fungal isolates
[12],
Phanerochaete chrysosporium, and their extracellular polymers such as polysaccharides which can help to colonize the polymer surface
[13]. Clutario et al. showed the physical evidence of the colonization of polyethylene strips by
Xylaria sp. isolated from a termite comb.
Xylaria sp. can utilize polyethylene plastic as a co-carbon source, thereby degrading them into usable forms for self-substance
[14]. Saminathan et al. isolated a strain identified as
P. putida by performing appropriate degradation on disposable plastic items
[15]. Thilagavathy et al. reported that
Xylaria sp. from the fungal garden variety of termite could degrade 20 µm thicknesses of LDPE plastics to 25% in a period of 50 days of incubation at 25 °C
[16]. Though the fungi survived in the environments with poor nutrient supply, pH, and moisture availability, they could degrade the plastic successfully to the appreciable value. The faster growth of fungal biomass compared to bacteria, the growth extension, and the penetration into other locations in the plastic are possible through the distribution of hyphae. The distribution and penetration ability of their fungal hyphae was an added advantage
[17].
3. Mechanism of Biodegradation
In biological force, the growth of many fungi can cause small-scale swelling and bursting, as the fungi penetrate the polymer solids
[18]. Plastics are potential substrates for heterotrophic microorganisms
[19]. The microbes excrete extracellular enzymes which depolymerize the polymers outside the cells. Physical forces, such as heating, cooling, freezing, and drying can cause mechanical damage such as the cracking of polymeric materials
[20]. When exposed to soil, natural polymers such as starch, cellulose, and proteins are destroyed by a microbiological process
[21]. The microbial colonization of a polymer surface is the first requirement for its biodegradation
[22]. During degradation, exoenzymes from microorganisms break down complex polymers, yielding smaller molecules of short chains, e.g., oligomers, dimers, and monomers that are small enough to pass the semi-permeable outer bacterial membranes and then be utilized as carbon and energy sources. The process is called depolymerization. The end products are CO
2, H
2O, and CH
4, and the degradation is called mineralization
[23][24]. To facilitate the biodegradation of these polymers, a preliminary step of photo-oxidation or thermo-oxidation has routinely been employed. This oxidation of the polymer results in the formation of carbonyl residues that can be consumed by non-specific microbial populations
[25]. The results change in the bond scission, chemical transformation, and formation of new functional groups
[26]. Very small variation in the chemical structure of polymer could lead to large changes in their biodegradability. Scanning electron microscopy (SEM) is a useful imaging approach for the visualization of different polymers because it provides a consistent picture of the polymer morphology as a non-uniform structure characterized by variable thickness and variable polymer density. Akutsu et al. illustrated the surface topography of polymers with high resolution
[27]. The morphological changes in PUR during the enzyme reaction were observed by scanning electron microscopy. Ikada et al. obtained information about the degradation mechanism; the observations can be made using either scanning electron microscopy (SEM) or atomic force microscopy (AFM)
[28]. Clutario et al. observed the mycelia of fungus on polythene after sufficient adaption to the lab condition
[15]; the
Xylaria fungus penetrated into the plastic material of LDPE. After that, some chemical reaction must have taken place since evidence of bio-corrosion by the mycelium was observed through the SEM, including the tearing, pitting, and striating of the plastic strip incubated with fungus. Physico-mechanical properties have also been determined before and after the degradation of film in order to understand the rate as well as the mechanism of degradation
[29][30]. SEM images of
Xylaria over the LDPE film are shown in
Figure 2.
Figure 2. (a) SEM images of Xylaria over the LDPE, (b) cavities/cracks formed on the surface of LDPE. The LDPE surface is shown by the red color arrows. The Xylaria is depicted by the violet-colored arrows above the LDPE.
4. Enzymatic Degradation of Plastic
The biodegradation of polymers is catalyzed by extracellular, degradative enzymes that produce water-soluble, low-molecular-weight products from the macromolecular substrates. These products are water-soluble and can diffuse into the surrounding aqueous environment to be taken up by the cells of the microorganisms and used as nutrients. Mergaert et al. reported various natural polyesters, such as polyhydroxybutirate and polycaprolactone, that can also be degraded and assimilated by various microbial populations
[12]. Webb et al. have studied how the biodegradation of polythene begins with the attachment of microbes in the surface of the polymer
[32]. The microbes such as bacteria (
Streptomyces viridosporusT7A,
Streptomyces badius252, and
Streptomyces setonii75Vi2) and wood-degrading fungi produced some extracellular enzymes which led to the degradation of polythene. Polythene-cleaving enzymes belong to the group of hydrolases, which catalyze the hydrolytic cleavage of the C-O and C-N- bonds. Hydrolases include lipase and esterase enzymes. Tokiwa et al. revealed that the various esterases and lipases produced were hydrolyzing the PCL.
Particularly, the lipases of
Rhizopusdelemar and
R. arrhizus were used in the hydrolysis of polymers. Thilagavathy et al. identified that
Xylaria sp. of termite fungal comb was able to degrade 20 µm of thickness of LDPE plastics through their secretion of depolymerizers (
Figure 3)
[16]. The oxidized polymer helps in the adhesion of microorganisms (due to probable changes in the hydrophobicity of the polymer surface), which is a prerequisite for biodegradation. Johnson et al. investigated how the microorganism growing on plastic material may either utilize the plasticizer molecule of starch cellulose or the other polymer
[33]. Fungi are able to degrade a wide variety of polymers through the production of several enzymes such as cellulase and amylase
[34][35][36]. The active enzymes have been grouped as esterases, lipases, proteases, and ureases which degrade the polyurethane substrate by cleaving the ester bonds
[37]. The enzyme reacts with solid polyester PU to hydrolyze the ester bounds of PU (
Table 1). The hydrolysis of esters bound in PU is postulated to be a mechanism of PU biodegradation (
Figure 4). Prema et al. studied and purified enzyme protease that was involved in the degradation of PLA
[38]. Biological processes by both microbial and enzymatic activities are currently considered to be sustainable recycling methods in the biodegradation of plastics.
Figure 3. Enzyme-catalyzed degradation processes.
Figure 4. Hydrolysis activity of Xylaria sp in LDPE. Secretion of depolymerise in the GYP medium by Xylaria sp (xtc 1) when grown on media containing (a) cellulase; (b) caesinase; (c) lipase and (d) gelatinase respectively, as seen by the zones of clearing.