Biodegradation is the deformation of a substance into new compounds through biochemical reactions or the actions of microorganisms such as bacteria or fungi. It is necessary for water-soluble or water-immiscible polymers because they eventually enter streams which can neither be recycled nor incinerated. It is important to consider the microbial degradation of natural and synthetic polymers in order to understand what is necessary for biodegradation and the mechanisms involved. Low/high-density polyethylene is a vital cause of environmental pollution. It occurs by choking the sewer line through mishandling, thus posing an everlasting ecological threat. Environmental pollution due to the unscrupulous consumption of synthetic polymers derived from petroleum has an adverse impact on the environment since the majority of plastics do not degrade, and the further incineration of synthetic plastics generates CO2 and dioxin. This requires understanding the interactions between materials and microorganisms and the biochemical changes involved. Widespread studies on the biodegradation of plastics have been carried out in order to overcome the environmental problems associated with synthetic plastic waste. Awareness of the waste problem and its impact on the environment has awakened new interest in the area of degradable polymers through microbes viz., bacteria, fungi, and actinomycetes. The microbial degradation of plastics is caused by certain enzymatic activities that lead to a chain cleavage of polymers into oligomers and monomers.
- plastics
- biodegradation
- enzymatic activities
1. Introduction
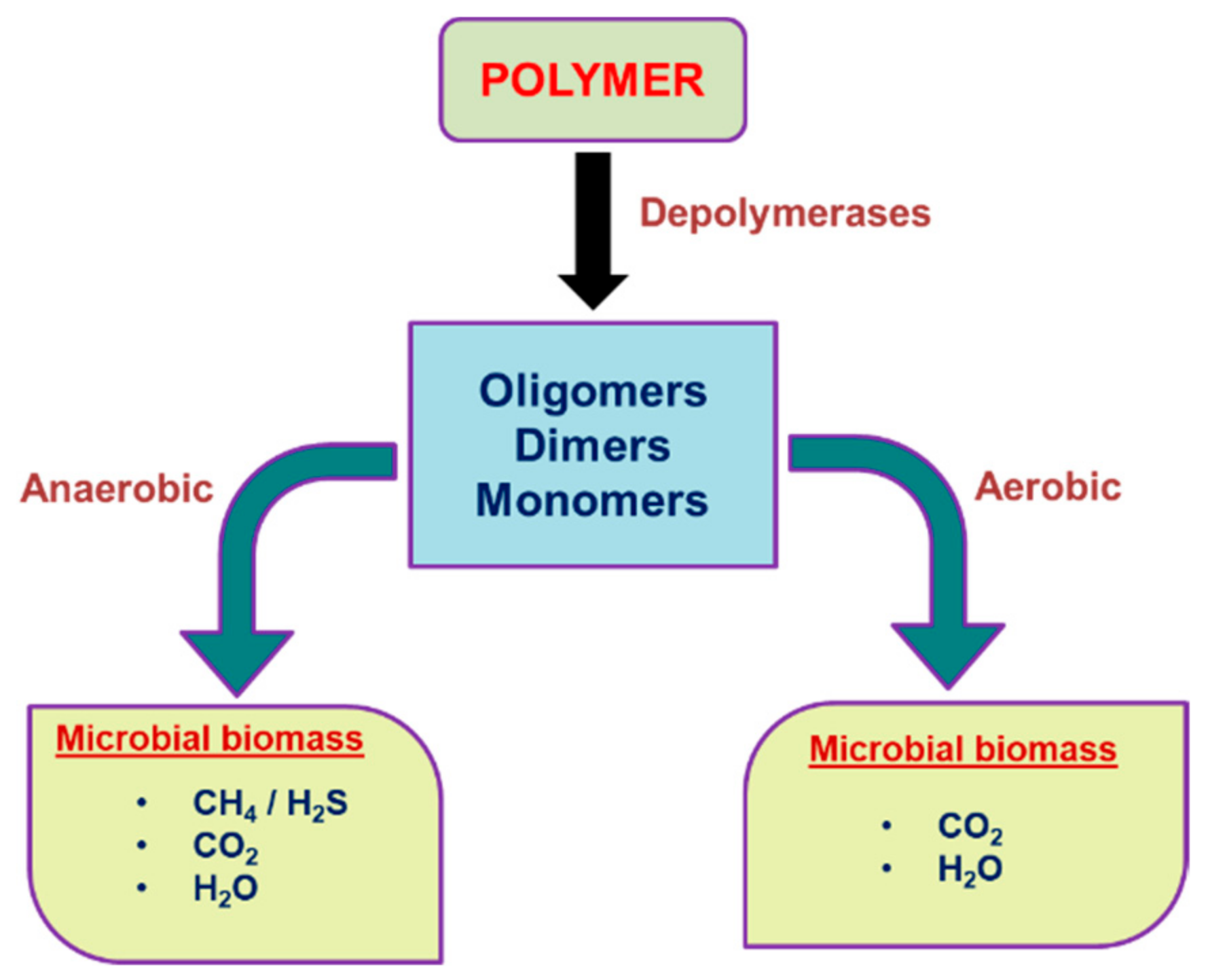
2. Biodegradation of Plastic by Microbes
3. Mechanism of Biodegradation
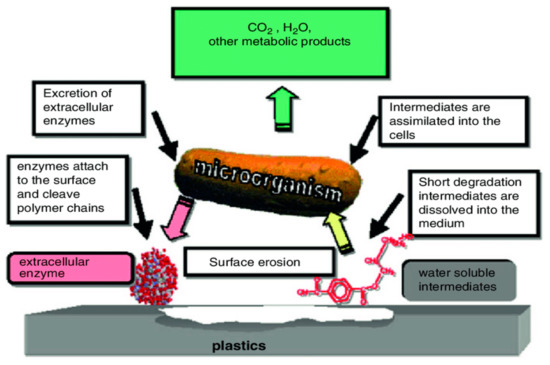

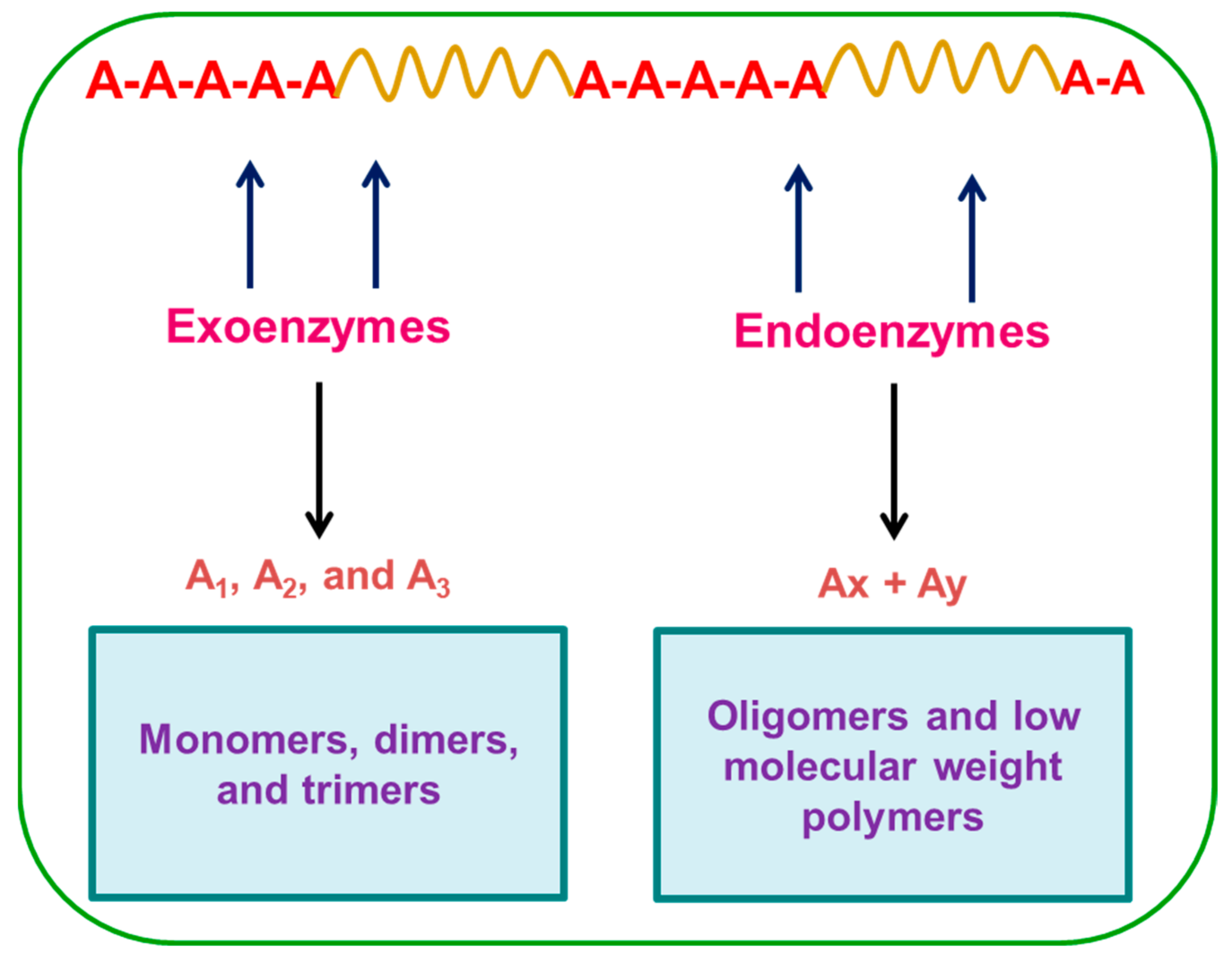
4. Enzymatic Degradation of Plastic

| Isolates | Enzyme Activity (IUml-1) | |||
|---|---|---|---|---|
| 40th DAI | ||||
| Cellulase | Amylase | Lipase | Protease | |
| Xtc1 | 0.54 ± 0.02 | 0.27 ± 0.01 | 2.91 ± 0.35 | 1.28 ± 0.12 |
| Xtc4 | 0.46 ± 0.04 | 0.15 ± 0.02 | 2.84 ± 0.41 | 0.89 ± 0.10 |
| Xtc8 | 0.34 ± 0.03 | 0.23 ± 0.01 | 1.75 ± 0.20 | 0.76 ± 0.08 |
| Xtc12 | 0.42 ± 0.02 | 0.17 ± 0.02 | 2.80 ± 0.29 | 1.01 ± 0.13 |
| Xtc20 | 0.39 ± 0.03 | 0.25 ± 0.03 | 2.37 ± 0.31 | 0.99 ± 0.10 |
| MTCC 3669 | 0.48 ± 0.02 | 0.31 ± 0.02 | 2.78 ± 0.25 | 1.14 ± 0.12 |
References
- Kubowicz, S.; Booth, A.M. Biodegradability of plastics: Challenges and misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060.
- Varyan, I.; Kolesnikova, N.; Xu, H.; Tyubaeva, P.; Popov, A. Biodegradability of polyolefin-based compositions: Effect of natural rubber. Polymers 2022, 14, 530.
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538.
- Di Mauro, E.; Rho, D.; Santato, C. Biodegradation of bio-sourced and synthetic organic electronic materials towards green organic electronics. Nat. Commun. 2021, 12, 3167.
- Marciniak, P.; Możejko-Ciesielska, J. What Is New in the Field of Industrial Wastes Conversion into Polyhydroxyalkanoates by Bacteria? Polymers 2021, 13, 1731.
- Witt, U.; Muller, R.J.; Deckwer, W.D. Biodegradation behaviour and material properties of aliphatic/aromatic polyesters of commercial importance. J. Environ. Poly. Degrad. 1997, 15, 81–89.
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335.
- Albinas, L.; Loreta, L.; Dalia, P. Micromycetes as deterioration agents of polymeric materials. Int. Biodeterior. Biodegra. 2003, 52, 233–242.
- Lee, B.; Pometto, A.L.; Fratzke, A.; Bailey, T.B. Biodegradation of degradable plastic polyethylene by Phanerochaete and Streptomyces species. Appl. Environ. Microbiol. 1991, 57, 678–685.
- Muhammad, I.A.; Perveen, Q.; Ahmad, B.; Javed, I.; Razi-Ul-Hussnain, R.; Andleeb, S.; Atique, N.; Ghumro, P.; Ahmed, S.; Hameed, A. Studies on biodegradation of cellulose blended polyvinyl chloride films. Int. J. Agriculture. Biol. 2009, 57, 9–175.
- Gupta, S.B.; Amrita, G.; Chowdhury, T. Isolation and selection of stress tolerant plastic loving bacterial isolates from old plastic wastes. World. J. Agri. Sci. 2010, 6, 138–140.
- Mergaert, J.; Swings, J. Biodeversity of microorganisms that degrade bacterial and synthetic polyesters. J. Ind. Microbiol. 1996, 17, 463–469.
- Orhan, Y.; Buyukgungor, H. Enhancement of biodegradability of disposable polyethylene in controlled biological soil. Int. Biodeter. Biodegrad. 2000, 45, 49–55.
- Volke-Sepulveda, T.; Castaneda, G.S.; Rojas, M.G.; Manzur, A.; Torres, E.F. Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J. Appl. Polym. Sci. 2002, 83, 305–314.
- Clutario, T.P.; Cuevus, V.C. Colonization of plastic by Xylaria sp. Phili. J. Sci. 2001, 130, 89–95.
- Thilagavathy, S.S.; Gomathi, V. Isolation of Decomposing Fungi with Plastic Degrading Ability. M.Sc Dissertation, Tamil Nadu Agricultural University, Coimbatore, India, 2014.
- Kim, D.Y.; Rhee, H.Y. Biodegradation of microbial and synthetic polyesters by fungi. Appl. Microbiol. Biotechnol. 2003, 61, 300–308.
- Griffin, G.L. Synthetic polymers and the living environment. Pure Appl. Chem. 1980, 52, 399–407.
- Saminathan, P.; Sripriya, A.; Nalini, K.; Sivakumar, T.; Thangapandian, V. Biodegradation of plastics by pseudomonas putida isolated from garden soil samples. J. Adv. Bot. Zoolo. 2014, 1, 2348–7313.
- Glass, J.E.; Swift, G. Agricultural and Synthetic Polymers, Biodegradation and Utilization; ACS Symposium Series, 433; American Chemical Society: Washington, DC, USA, 1989; Volume 37, pp. 9–64.
- Kamal, M.R.; Huang, B. Natural and artificial weathering of polymers. In Handbook of Polymer Degradation; Hamid, S.H., Ami, M.B., Maadhan, A.G., Eds.; Marcel Dekker: New York, NY, USA, 1992; Volume 36, pp. 68–127.
- Johnson, K.E.; Pometto, A.L.; Nikolov, Z.L. Degradation of degradable starch polythene plastics in a compost environment. J. Appl. Environ. Microbiol. 1993, 59, 1255–1261.
- Yabannavar, A.; Bartha, R. Biodegradability of some food packaging materials in soil. Soil. Biol. Biochem. 1993, 25, 1469–1475.
- Hamilton, J.D.; Reinert, K.H.; Hogan, J.V.; Lord, W.V. Polymers as solid waste in municipal landfills. J. Air Waste Manage. Assoc. 1995, 43, 247–251.
- Frazer, A.C. O-methylation and other transformations of aromatic compounds by acetogenicbacteria. In Acetogenesis; Drake, H.L., Ed.; Chapman & Hall: New York, NY, USA, 1994; Volume 120, pp. 445–483.
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotech. Adv. 2008, 26, 246–265.
- Pospisil, J.; Nespurek, S. Highlights in chemistry and physics of polymer stabilization. Macromol. Symp. 1997, 115, 143–163.
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl. Environ. Microbiol. 1998, 64, 62–67.
- Ikada, E. Electron microscope observation of biodegradation of polymers. J. Environ. Polym. Degrad. 1999, 7, 197–201.
- Prabhat, S.; Bhattacharyya, S.; Vishal, V.; Kalyan, R.K.; Vijai, K.; Pandey, K.N.; Singh, M. Studies on isolation and identification of active microorganisms during degradation of polyethylene/starch film. Int. Res. J. Environment Sci. 2013, 2, 83–88.
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128.
- Webb, E.C. Enzyme Nomenclature: Recommendations of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology on the Nomenclature and Classification of Enzymes; Academic Press Inc.: San Diego, CA, USA, 1992; Volume 7, pp. 1192–1194.
- Tokiwa, Y.; Suzuki, T. Hydrolysis of polyesters by lipases. Nature 1977, 270, 76–78.
- Sethuraman, A.; Akin, D.; Erikson, K. Plant-cell-wall-degrading enzymes produced by the white-rot fungus Ceriporiopsis subvermispora. Biotechnol. Appl. Biochem. 1998, 27, 37–47.
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic nanoparticle integrated ternary polymer blend of PVA/Starch/Glycerol: A promising antimicrobial food packaging material. Polymers 2022, 14, 1379.
- Chien, H.-L.; Tsai, Y.-T.; Tseng, W.-S.; Wu, J.-A.; Kuo, S.-L.; Chang, S.-L.; Huang, S.-J.; Liu, C.-T. Biodegradation of PBSA films by elite aspergillus isolates and farmland soil. Polymers 2022, 14, 1320.
- Friné, V.-C.; Hector, A.-P.; Sergio Manuel, N.-D.; Estrella, N.-D.; Antonio, G.J. Development and characterization of a biodegradable PLA food packaging hold monoterpene–cyclodextrin complexes against Alternaria alternata. Polymers 2019, 11, 1720.
- Prema, S.; Uma Maheswari Devi, P. Degradation of poly lactide plastic by mesophilic bacteria isolated from compost. Int. J. Rese. Pure. App. Microbiol. 2013, 3, 121–126.
