Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ruxandra Mare | -- | 2141 | 2022-10-20 21:00:55 | | | |
| 2 | Vivi Li | -5 word(s) | 2136 | 2022-10-21 03:47:16 | | | | |
| 3 | Vivi Li | Meta information modification | 2136 | 2022-10-24 09:56:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mare, R.; Sporea, I. Gastrointestinal and Liver Complications in Diabetes Mellitus. Encyclopedia. Available online: https://encyclopedia.pub/entry/30529 (accessed on 08 February 2026).
Mare R, Sporea I. Gastrointestinal and Liver Complications in Diabetes Mellitus. Encyclopedia. Available at: https://encyclopedia.pub/entry/30529. Accessed February 08, 2026.
Mare, Ruxandra, Ioan Sporea. "Gastrointestinal and Liver Complications in Diabetes Mellitus" Encyclopedia, https://encyclopedia.pub/entry/30529 (accessed February 08, 2026).
Mare, R., & Sporea, I. (2022, October 20). Gastrointestinal and Liver Complications in Diabetes Mellitus. In Encyclopedia. https://encyclopedia.pub/entry/30529
Mare, Ruxandra and Ioan Sporea. "Gastrointestinal and Liver Complications in Diabetes Mellitus." Encyclopedia. Web. 20 October, 2022.
Copy Citation
The number of diabetes mellitus patients has increased in developing countries, along with obesity and sedentary lifestyle. Besides macroangiopathy and microangiopathy, damage to the nerve fibers of the peripheral nervous system is the most common chronic complication of diabetes. Digestive complications in diabetic patients represent a consequence of diabetic autonomic neuropathy involving the gastrointestinal tract, but unfortunately not always evaluated by diabetologists. Aside from the complications encountered in the digestive tract, patients with diabetes mellitus are prone to developing liver diseases.
diabetes mellitus
digestive complications
liver disease
1. Introduction
Diabetes is one of the diseases of the modern world, with an increasing prevalence in recent decades, especially in developed countries. The global prevalence of type 2 diabetes is estimated at 10.5% [1] which has increased during the last two decades in tandem with obesity and sedentary lifestyle.
The diabetologist carefully monitors the diabetic patient for the typical complications of this disease, such as micro and macroangiopathy. However, recently, mounting data have suggested that diabetic patients, particularly those with type 2 diabetes, may experience a variety of digestive complications, including those connected to gastrointestinal or hepatic disease. Gastrointestinal (GI) complications of diabetes are often caused by abnormal GI motility, which is a consequence of diabetic autonomic neuropathy involving the GI tract. Up to 75% of diabetes patients may experience GI symptoms, and this is a consequence of poor blood glucose control and not necessarily due to the duration of diabetes [2]. GI conditions caused by diabetes include gastroparesis and intestinal enteropathy.
Gastroparesis is a well-recognized GI manifestation of diabetes, most frequently encountered in women [3]. The prevalence of gastroparesis varies widely in specialized centers; up to 40% of patients with type 1 diabetes mellitus (T1DM) [4] and up to 30% of patients with type 2 diabetes mellitus (T2DM) [5] have gastroparesis. Enteropathy is a less recognized GI manifestation of diabetes and clinical presentation includes diarrhea, constipation, and fecal incontinence, which mainly is nocturnal [2]. Over the last few years, it was demonstrated that there is a bidirectional relationship between diabetes, mainly T2DM, and non-alcoholic fatty liver disease (NAFLD). The presence of NAFLD increases the incidence of T2DM and accelerates the development of complications in them, while T2DM increases the probability of the progression of non-alcoholic fatty liver to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma [6]. If an increase in the frequency of gallstones in diabetics has long been known and reported, other associations between diabetes mellitus and the presence of Helicobacter pylori and between diabetes and the risk of colon cancer are less known. Additionally, there is more and more evidence that there is a link between diabetes and hemochromatosis, and according to the latest guideline of the American Diabetes Association, all adult patients with T1DM should be screened for celiac disease in the presence of gastrointestinal symptoms, signs, or laboratory manifestations suggestive of celiac disease [7].
2. Diabetic Autonomic Neuropathy of the Gastrointestinal Tract
Neuropathy of the gastrointestinal tract leads to the development of numerous complications, such as the exacerbation of gastroesophageal reflux disease (GERD), gastroparesis, and enteropathy.
2.1. Gastroesophageal Reflux Disease (GERD)
In diabetes patients, GERD is caused by abnormal peristalsis, spontaneous contractions, impaired lower esophageal sphincter (LES) tone, and an increased number of transient LES relaxation [8]. The clinical manifestations of GERD in this category of patients are pyrosis and regurgitation. The diagnosis of GERD can be based on clinical symptoms, and upper endoscopy or Ph-metry should be reserved only for cases that are resistant to treatment and in the presence of extra-esophageal symptoms. Treatment should focus on the normalization of body weight, the avoidance of coffee, alcohol, fried and roasted products, and proton pump inhibitors [9].
2.2. Diabetic Gastroparesis
Gastroparesis is characterized by delayed gastric emptying in the absence of mechanical obstruction [10]. Diabetic gastroparesis is thought to be caused by impaired vagal control, abnormal myenteric neurotransmission, the impairment of inhibitory nitric oxide-containing nerves, damage to the interstitial cells of Cajal, and underlying smooth muscle dysfunction [3][11]
The prevalence of diabetic gastroparesis varies between studies and between centers and this is due to the fact that most population-based studies have focused on symptoms rather than gastric scintigraphy findings. In such investigations, 5–12% of patients with diabetes report symptoms consistent with gastroparesis [2][12], and up to 20–40% of patients [9][13] have gastroparesis assessed by gastric emptying studies mostly performed in tertiary centers. The prevalence of gastroparesis is higher in women than in men, especially during the luteal phase of the menstrual cycle [14][15] mainly because gastric muscle contractility is reduced by progesterone. Patients with gastroparesis can present with early satiety, nausea, vomiting, bloating, postprandial fullness, upper abdominal pain, and, in severe cases, with weight loss [16]. The predominant symptom may vary based on the underlying etiology. For example, in a study that included 416 patients with gastroparesis, those with diabetic gastroparesis had more severe retching and vomiting as compared with patients with idiopathic gastroparesis [17]. Either way, gastroparesis should be suspected in diabetic patients presenting with this symptomatology and evaluation should begin with a history and physical examination, completed by laboratory studies (complete blood count, thyroid-stimulating hormone test, metabolic panel, amylase test if the patient has abdominal pain, and pregnancy test if appropriate) [11]. Furthermore, in order to rule out a mechanical obstruction, patients should undergo an upper gastrointestinal endoscopy, a computed tomographic enterography (CT), or a magnetic resonance (MR) enterography to exclude mechanical obstruction from a small bowel mass or superior mesenteric artery syndrome. In patients with suspected gastroparesis and no evidence of a mechanical obstruction on imaging or upper endoscopy, an assessment of gastric motility is mandatory to establish the diagnosis of gastroparesis. There are many tests that can be used for the assessment of gastric motility (Table 1), but gastric emptying scintigraphy is considered to be the gold standard and is recommended by the American Gastroenterological Association to confirm the diagnosis of gastroparesis [11]. The first-line treatment for diabetic gastroparesis should include dietary modifications, glycemic control, and the restoration of fluids and electrolytes but this nutritional approach will not be enough to control the symptoms of gastroparesis as the disease progresses. In addition to this, many patients will also require pharmacological, endoscopic, or surgical treatments. Table 2 summarizes the pharmacological, endoscopic, and surgical therapies available to treat gastroparesis.
Table 1. Tests used for the assessment of gastric motility.
| Tests | Comments |
|---|---|
| Scintigraphic gastric emptying | The gold standard, most cost-effective, simple, and available technique able to assess liquid and solid emptying; minimal radiation exposure [9] |
| Wireless motility capsule | Measures simultaneously phasic pressure amplitudes, temperature, and Ph as it passes through the GI tract [18] |
| 13 C breath testing | Non-invasive, non-radiation exposure. Acetate breath testing, octanoic acid breath test, or spirulin have been used to assess gastric emptying [19]. |
| Electrogastrography | Noninvasive method that measures gastric myoelectrical activity [20]. |
| Antroduodenal manometry | Invasive procedure requiring expertise to perform and interpret. Assess fasting and postprandial phases [21]. |
Table 2. Treatment options for gastroparesis.
| Treatment | Mechanism | Comments |
|---|---|---|
| Metoclopramide (10 mg four times daily) | Improves gastric emptying by enhancing gastric antral contractions and decreasing postprandial fundus relaxation | First line therapy Symptoms improved in 25 to 62% of patients [11] Risk of tardive dyskinesia |
| Domperidone (10 mg three times daily) | Similar with Metoclopramide | Used when symptoms fail to respond to Metoclopramide Risk of cardiac arrhythmias [22] |
| Erythromycin (250 mg three times daily) | Motilin receptor agonist Induces high amplitude gastric propulsive contractions that increase gastric emptying |
Used when symptoms fail to respond to Metoclopramide and Domperidone Duration: no more than 4 weeks Risk of tachyphylaxis [23] |
| Tricyclic agents | Reduce perception of pain at different levels of the brain–gut axis |
Medication for visceral pain [23] |
| Gastric per-oral endoscopic myotomy (G-POEM) | Induces dumping syndrome | Pooled analysis including open-label and retrospective studies suggest a reduction in post-procedure GCSI scores and improved gastric emptying, with 6.8% overall adverse events Indication: only in refractory gastroparesis in tertiary centers [23] |
| Gastric electrical stimulation | Electric stimulation with high-energy, long-duration pulses | Reserved for compassionate treatment in patients with refractory symptoms (e.g., nausea and vomiting, without pain) [23] |
| Surgery | Pyloroplasty, gastrectomy | Most studies are non-randomized, unblended, or case series [11][24] |
3. Non-Alcoholic Fatty Liver Disease and Diabetes
Inactivity and an imbalanced diet (high in fat and sugar content) are two major contributors to non-alcoholic fatty liver disease (NAFLD), one of the most prevalent causes of chronic liver disease. NAFLD is commonly classified into two phenotypes, nonalcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). Of these, NASH is associated with an increased risk of hepatic morbidity and mortality due to the risk of the development of severe fibrosis, cirrhosis, and hepatocellular carcinoma [25]. Previously, NAFLD has been considered as a hepatic component of metabolic syndrome (MetS), but recently, an association between NAFLD and type 2 diabetes mellitus has been described [26]. Insulin resistance, especially in adipose tissue and liver, lipotoxicity, and inflammation, is part of the common pathophysiological mechanisms of NAFLD and T2DM. Over the years, it has been demonstrated that NAFLD contributes to the development of T2DM by increasing hepatic glucose production and exacerbating hepatic insulin resistance as a result of the activation of hepatic protein kinases Cε and liver-secreted proteins with diabetogenic properties, such as fetuin A, fetuin B, RBP4, selenoprotein P, DPP4, and HFREP1 [27][28][29][30][31][32]. Furthermore, intrahepatic fat accumulation activates liver inflammation and further promotes the development of atherogenic dyslipidemia (an increase in small and dense lipoprotein particles -LDL, triglycerides, and a decrease in HDL cholesterol) and hypertension (activation of the renin–angiotensin–aldosterone system). Additionally, it triggers a systemic inflammatory state (increased protein C reactive, Interleukin 6, tumor necrosis factor, and reactive oxygen species) as well as a coagulation mechanism (increased fibrinogen, factor VII, and PAI-1) [33]. All procedures play important roles in the development of diabetic macrovascular and microvascular complications. On the other hand, T2DM and systemic insulin resistance promote an increase in the flux of free fatty acids from peripheral tissues to the liver, leading to the development and progression of NAFLD. Moreover, T2DM promotes the development of NAFLD through a number of mechanisms, such as direct hepatocyte lipotoxicity, hepatocellular oxidative stress brought on by an increase in the oxidation of free fatty acids, endoplasmic reticulum stress, causing the release of inflammatory cytokines by hepatic Kupffer cells and peripheral adipocytes, and hepatocellular apoptosis and necrosis, respectively [33]. A better representation of the relationship between T2DM and NAFLD is presented in Figure 1.
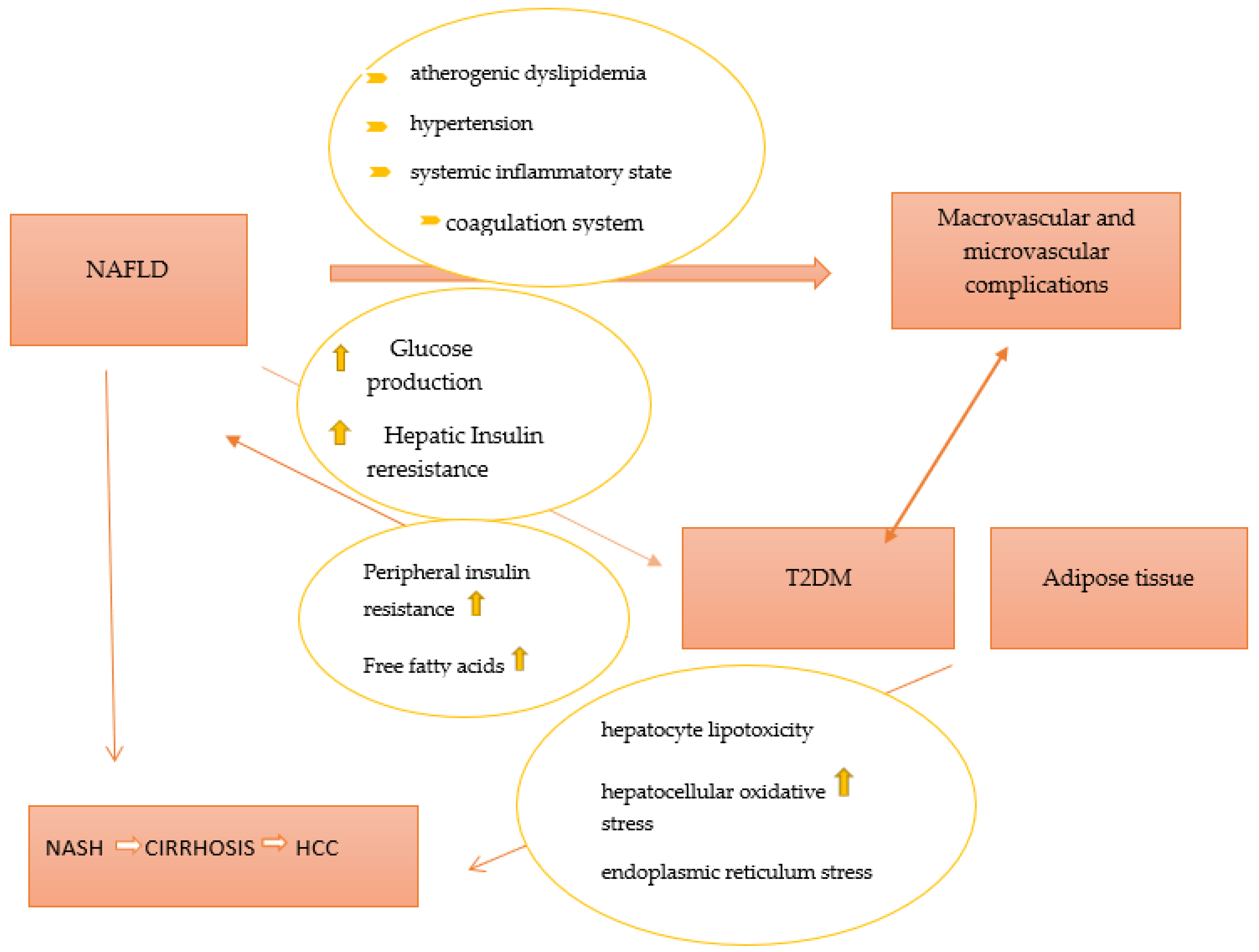
Figure 1. The pathophysiological link between NAFLD and T2DM. Abbreviations: T2DM—type 2 diabetes mellitus; NAFLD—non-alcoholic fatty liver disease; NASH—non-alcoholic steatohepatitis; HCC—hepatocellular carcinoma.
Two recent reviews explain the bidirectional relationship between NAFLD and insulin resistance and underline the clinical implications associated with these two conditions on the cardiovascular (CV), renal, and peripheral nervous systems. [34][35]. A meta-analysis revealed that patients with NAFLD had a greater risk of fatal and/or non-fatal CV events by up to 1.64 times (95% CI 1.26–2.13) than those without NAFLD [36]. Additionally, patients with T2DM and NAFLD have been found to have a higher prevalence of coronary, cerebrovascular, and peripheral vascular disease than those without NAFLD [37]. Similarly, to the association between NAFLD and coronary heart disease, several studies have studied the association between chronic kidney disease (CKD) and NAFLD [38][39]. A recent meta-analysis found a risk of CKD in NAFLD patients 1.43 times higher, even after adjustment for other factors such as age, sex, obesity, hypertension, and diabetes [38]. Another study [39] that included 4746 patients showed that liver fibrosis was associated with an increased prevalence of albuminuria and CKD.
While the association of T2DM with its microvascular and macrovascular complications is well established, the association of T2DM with NAFLD is more recently recognized. Furthermore, because patients are usually asymptomatic and routine blood tests are often normal, it may be an overlooked diagnosis in patients with T2DM. T2DM is one of the strongest clinical predictors of NAFLD progression to NASH and liver cirrhosis. The presence of diabetes increases the risk of NASH two to three times [40]. A recent study performed on T2DM patients using liver biopsy revealed that NASH was present in 96.8% of patients with T2DM, suggesting that the latter might be one of the early complications encountered in T2DM patients due to its pathophysiological correlation with insulin resistance [41].
The prevalence of diabetes in patients with NAFLD and NASH is estimated to be 22.5% and 43.6% [42], respectively, which is much higher than the prevalence of diabetes in the general population (8.5%), while the prevalence of NAFLD and NASH among patients with T2DM is 55.5% and 37.3% [43]. Nowadays, few data are available regarding the prevalence of NAFLD in people with T1DM. Some studies reported that ultrasound-diagnosed NAFLD was present in nearly 20–30% of adult patients with T1DM [44][45]. Clinically, patients will not have any symptoms besides fatigue, but in advanced stages due to the development of liver cirrhosis, they can develop ascites, esophageal varices, and jaundice. NAFLD is defined as the presence of hepatic steatosis, documented either by imaging or by histology, in the absence of significant alcohol consumption, the long-term use of steatogenic medication, or hereditary disorders [46]. Over the years, in the absence of histology, the presence of NASH was considered when patients had increased levels of transaminases, but recent studies have shown that approximately 56% of individuals with histologically proven NASH have normal liver enzymes [47][48].
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119.
- Bytzer, P.; Talley, N.J.; Leemon, M.; Young, L.J.; Jones, M.P.; Horowitz, M. Prevalence of Gastrointestinal Symptoms Associated with Diabetes Mellitus: A Population-Based Survey of 15,000 Adults. Arch. Intern. Med. 2001, 161, 1989–1996.
- Koch, K.L.; Calles-Escandón, J. Diabetic Gastroparesis. Gastroenterol. Clin. N. Am. 2015, 44, 39–57.
- Horowitz, M.; O’Donovan, D.; Jones, K.L.; Feinle, C.; Rayner, C.K.; Samsom, M. Gastric Emptying in Diabetes: Clinical Significance and Treatment. Diabet. Med. 2002, 19, 177–194.
- Intagliata, N.; Koch, K.L. Gastroparesis in Type 2 Diabetes Mellitus: Prevalence, Etiology, Diagnosis, and Treatment. Curr. Gastroenterol. Rep. 2007, 9, 270–279.
- Williamson, R.M.; Price, J.F.; Glancy, S.; Perry, E.; Nee, L.D.; Hayes, P.C.; Frier, B.M.; Van Look, L.A.F.; Johnston, G.I.; Reynolds, R.M.; et al. Prevalence of and Risk Factors for Hepatic Steatosis and Nonalcoholic Fatty Liver Disease in People with Type 2 Diabetes: The Edinburgh Type 2 Diabetes Study. Diabetes Care 2011, 34, 1139–1144.
- American Diabetes Association Professional Practice Committee 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S46–S59.
- Rayner, C.K.; Samsom, M.; Jones, K.L.; Horowitz, M. Relationships of Upper Gastrointestinal Motor and Sensory Function with Glycemic Control. Diabetes Care 2001, 24, 371–381.
- Kuźnik, E.; Dudkowiak, R.; Adamiec, R.; Poniewierka, E. Diabetic Autonomic Neuropathy of the Gastrointestinal Tract. Prz. Gastroenterol. 2020, 15, 89–93.
- Young, C.F.; Moussa, M.; Shubrook, J.H. Diabetic Gastroparesis: A Review. Diabetes Spectr. 2020, 33, 290–297.
- Parkman, H.P.; Hasler, W.L.; Fisher, R.S.; American Gastroenterological Association. American Gastroenterological Association Technical Review on the Diagnosis and Treatment of Gastroparesis. Gastroenterology 2004, 127, 1592–1622.
- Maleki, D.; Locke, G.R.; Camilleri, M.; Zinsmeister, A.R.; Yawn, B.P.; Leibson, C.; Melton, L.J. Gastrointestinal Tract Symptoms among Persons with Diabetes Mellitus in the Community. Arch. Intern. Med. 2000, 160, 2808–2816.
- Bharucha, A.E.; Camilleri, M.; Forstrom, L.A.; Zinsmeister, A.R. Relationship between Clinical Features and Gastric Emptying Disturbances in Diabetes Mellitus. Clin. Endocrinol. 2009, 70, 415–420.
- Gill, R.C.; Murphy, P.D.; Hooper, H.R.; Bowes, K.L.; Kingma, Y.J. Effect of the Menstrual Cycle on Gastric Emptying. Digestion 1987, 36, 168–174.
- Datz, F.L.; Christian, P.E.; Moore, J. Gender-Related Differences in Gastric Emptying. J. Nucl. Med. 1987, 28, 1204–1207.
- Soykan, I.; Sivri, B.; Sarosiek, I.; Kiernan, B.; McCallum, R.W. Demography, Clinical Characteristics, Psychological and Abuse Profiles, Treatment, and Long-Term Follow-up of Patients with Gastroparesis. Dig. Dis. Sci. 1998, 43, 2398–2404.
- Parkman, H.P.; Yates, K.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Koch, K.L.; Calles, J.; Abell, T.L.; et al. Similarities and Differences between Diabetic and Idiopathic Gastroparesis. Clin. Gastroenterol. Hepatol. 2011, 9, 1056–1064, quiz e133-134.
- Fritz, T.; Hünseler, C.; Broekaert, I. Assessment of Whole Gut Motility in Adolescents Using the Wireless Motility Capsule Test. Eur. J. Pediatr. 2022, 181, 1197–1204.
- Bonfrate, L.; Grattagliano, I.; Palasciano, G.; Portincasa, P. Dynamic Carbon 13 Breath Tests for the Study of Liver Function and Gastric Emptying. Gastroenterol. Rep. 2015, 3, 12–21.
- Koch, K.L. Chapter 15—Electrogastrography for Suspected Gastroparesis. In Gastroparesis; McCallum, R.W., Parkman, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 189–205. ISBN 978-0-12-818586-5.
- Patcharatrakul, T.; Gonlachanvit, S. Technique of Functional and Motility Test: How to Perform Antroduodenal Manometry. J. Neurogastroenterol. Motil. 2013, 19, 395–404.
- Drolet, B.; Rousseau, G.; Daleau, P.; Cardinal, R.; Turgeon, J. Domperidone Should Not Be Considered a No-Risk Alternative to Cisapride in the Treatment of Gastrointestinal Motility Disorders. Circulation 2000, 102, 1883–1885.
- Lacy, B.E.; Tack, J.; Gyawali, C.P. AGA Clinical Practice Update on Management of Medically Refractory Gastroparesis: Expert Review. Clin. Gastroenterol. Hepatol. 2022, 20, 491–500.
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic Gastroparesis. Endocr. Rev. 2019, 40, 1318–1352.
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023.
- Lonardo, A.; Nascimbeni, F.; Mantovani, A.; Targher, G. Hypertension, Diabetes, Atherosclerosis and NASH: Cause or Consequence? J. Hepatol. 2018, 68, 335–352.
- Pal, D.; Dasgupta, S.; Kundu, R.; Maitra, S.; Das, G.; Mukhopadhyay, S.; Ray, S.; Majumdar, S.S.; Bhattacharya, S. Fetuin-A Acts as an Endogenous Ligand of TLR4 to Promote Lipid-Induced Insulin Resistance. Nat. Med. 2012, 18, 1279–1285.
- Meex, R.C.; Hoy, A.J.; Morris, A.; Brown, R.D.; Lo, J.C.Y.; Burke, M.; Goode, R.J.A.; Kingwell, B.A.; Kraakman, M.J.; Febbraio, M.A.; et al. Fetuin B Is a Secreted Hepatocyte Factor Linking Steatosis to Impaired Glucose Metabolism. Cell Metab. 2015, 22, 1078–1089.
- Norseen, J.; Hosooka, T.; Hammarstedt, A.; Yore, M.M.; Kant, S.; Aryal, P.; Kiernan, U.A.; Phillips, D.A.; Maruyama, H.; Kraus, B.J.; et al. Retinol-Binding Protein 4 Inhibits Insulin Signaling in Adipocytes by Inducing Proinflammatory Cytokines in Macrophages through a c-Jun N-Terminal Kinase- and Toll-Like Receptor 4-Dependent and Retinol-Independent Mechanism. Mol. Cell. Biol. 2012, 32, 2010–2019.
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A Liver-Derived Secretory Protein, Selenoprotein P, Causes Insulin Resistance. Cell Metab. 2010, 12, 483–495.
- Baumeier, C.; Schlüter, L.; Saussenthaler, S.; Laeger, T.; Rödiger, M.; Alaze, S.A.; Fritsche, L.; Häring, H.-U.; Stefan, N.; Fritsche, A.; et al. Elevated Hepatic DPP4 Activity Promotes Insulin Resistance and Non-Alcoholic Fatty Liver Disease. Mol. Metab. 2017, 6, 1254–1263.
- Wu, H.-T.; Ou, H.-Y.; Hung, H.-C.; Su, Y.-C.; Lu, F.-H.; Wu, J.-S.; Yang, Y.-C.; Wu, C.-L.; Chang, C.-J. A Novel Hepatokine, HFREP1, Plays a Crucial Role in the Development of Insulin Resistance and Type 2 Diabetes. Diabetologia 2016, 59, 1732–1742.
- Xia, M.-F.; Bian, H.; Gao, X. NAFLD and Diabetes: Two Sides of the Same Coin? Rationale for Gene-Based Personalized NAFLD Treatment. Front. Pharmacol. 2019, 10.
- Caturano, A.; Acierno, C.; Nevola, R.; Pafundi, P.C.; Galiero, R.; Rinaldi, L.; Salvatore, T.; Adinolfi, L.E.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease: From Pathogenesis to Clinical Impact. Processes 2021, 9, 135.
- Rinaldi, L.; Pafundi, P.C.; Galiero, R.; Caturano, A.; Morone, M.V.; Silvestri, C.; Giordano, M.; Salvatore, T.; Sasso, F.C. Mechanisms of Non-Alcoholic Fatty Liver Disease in the Metabolic Syndrome. A Narrative Review. Antioxidants 2021, 10, 270.
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-Alcoholic Fatty Liver Disease and Risk of Incident Cardiovascular Disease: A Meta-Analysis. J. Hepatol. 2016, 65, 589–600.
- Targher, G.; Bertolini, L.; Padovani, R.; Poli, F.; Scala, L.; Tessari, R.; Zenari, L.; Falezza, G. Increased Prevalence of Cardiovascular Disease in Type 2 Diabetic Patients with Non-Alcoholic Fatty Liver Disease. Diabet. Med. 2006, 23, 403–409.
- Mantovani, A.; Petracca, G.; Beatrice, G.; Csermely, A.; Lonardo, A.; Schattenberg, J.M.; Tilg, H.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Risk of Incident Chronic Kidney Disease: An Updated Meta-Analysis. Gut 2022, 71, 156–162.
- Ciardullo, S.; Ballabeni, C.; Trevisan, R.; Perseghin, G. Liver Fibrosis Assessed by Transient Elastography Is Independently Associated with Albuminuria in the General United States Population. Dig. Liver Dis. 2021, 53, 866–872.
- Portillo-Sanchez, P.; Bril, F.; Maximos, M.; Lomonaco, R.; Biernacki, D.; Orsak, B.; Subbarayan, S.; Webb, A.; Hecht, J.; Cusi, K. High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 2015, 100, 2231–2238.
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver Biopsy in Type 2 Diabetes Mellitus: Steatohepatitis Represents the Sole Feature of Liver Damage. PLoS ONE 2017, 12, e0178473.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84.
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801.
- de Vries, M.; El-Morabit, F.; van Erpecum, K.J.; Westerink, J.; Bac, S.T.; Kaasjager, H.A.H.; de Valk, H.W. Non-Alcoholic Fatty Liver Disease: Identical Etiologic Factors in Patients with Type 1 and Type 2 Diabetes. Eur. J. Intern. Med. 2022, 100, 77–82.
- Sviklāne, L.; Olmane, E.; Dzērve, Z.; Kupčs, K.; Pīrāgs, V.; Sokolovska, J. Fatty Liver Index and Hepatic Steatosis Index for Prediction of Non-Alcoholic Fatty Liver Disease in Type 1 Diabetes. J. Gastroenterol. Hepatol. 2018, 33, 270–276.
- Chartampilas, E. Imaging of Nonalcoholic Fatty Liver Disease and Its Clinical Utility. Hormones 2018, 17, 69–81.
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569.
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated with Long-Term Outcomes of Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.e10.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Gastrointestinal Disease
Revisions:
3 times
(View History)
Update Date:
24 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




