
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexandru Deaconu | -- | 1551 | 2022-10-20 13:02:14 | | | |
| 2 | Vivi Li | Meta information modification | 1551 | 2022-10-21 03:32:38 | | |
Video Upload Options
Ventricular arrhythmias (VA) are a major cause of sudden cardiac death (SCD). Echocardiography is the first widely available imaging tool which guides VA management strategies. Along with other invasive and noninvasive imaging techniques, it provides essential information for identification of VA substrate such as differentiation between ischemic and non-ischemic etiology and identification of structural heart disease. Both classic as well as novel echocardiographic techniques such as left ventricular strain measurement and mechanical dispersion assessment provide prognostic information and assist in risk stratification. Furthermore, intracardiac echocardiography may have an adjunctive role for the VA ablation by providing real-time visualization of cardiac structures, continuous monitoring of catheter location and early recognition of procedural complications.
1. Introduction
2. Role of Echocardiography in Identification of VA Substrate
2.1. VA in Structural Heart Disease
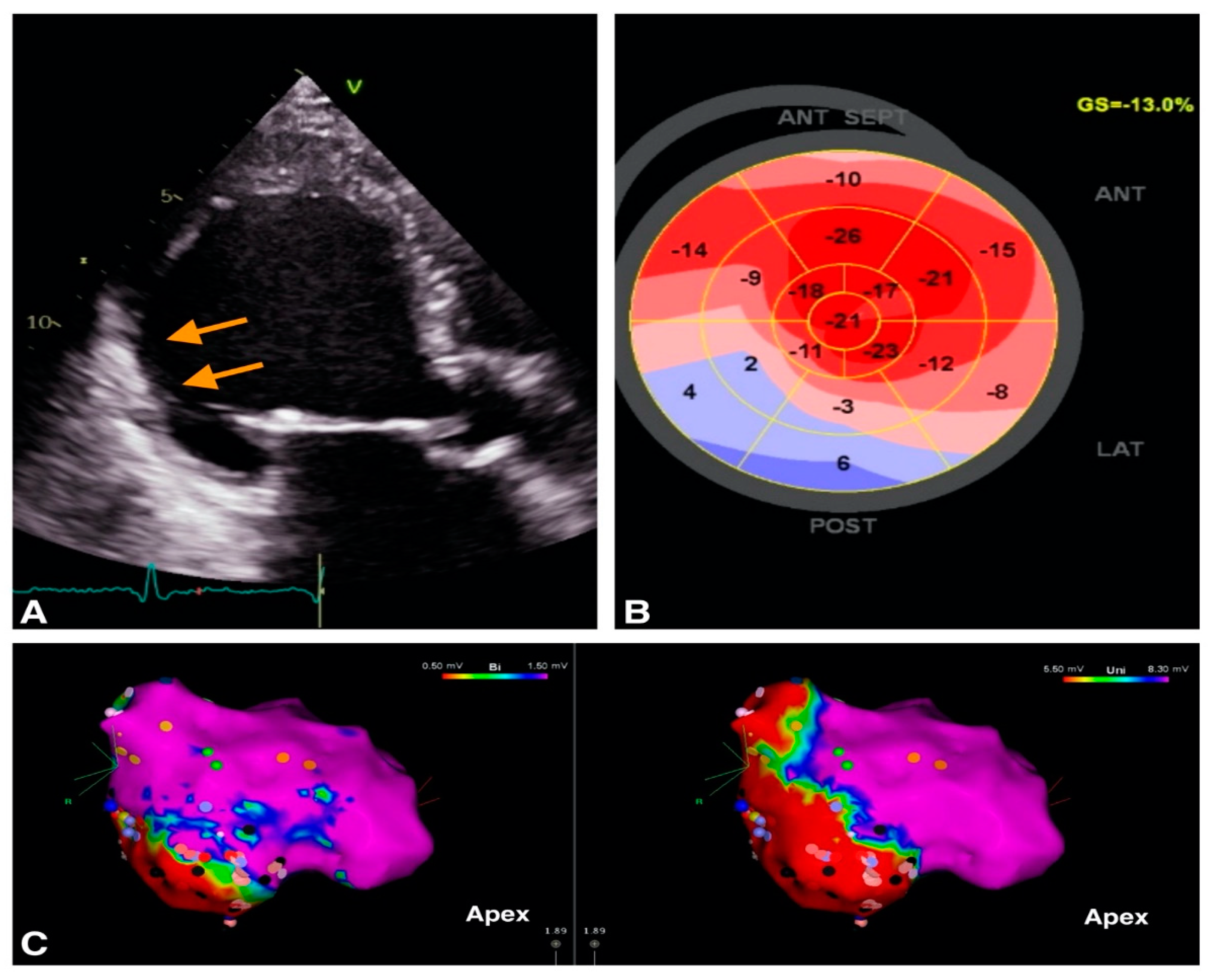
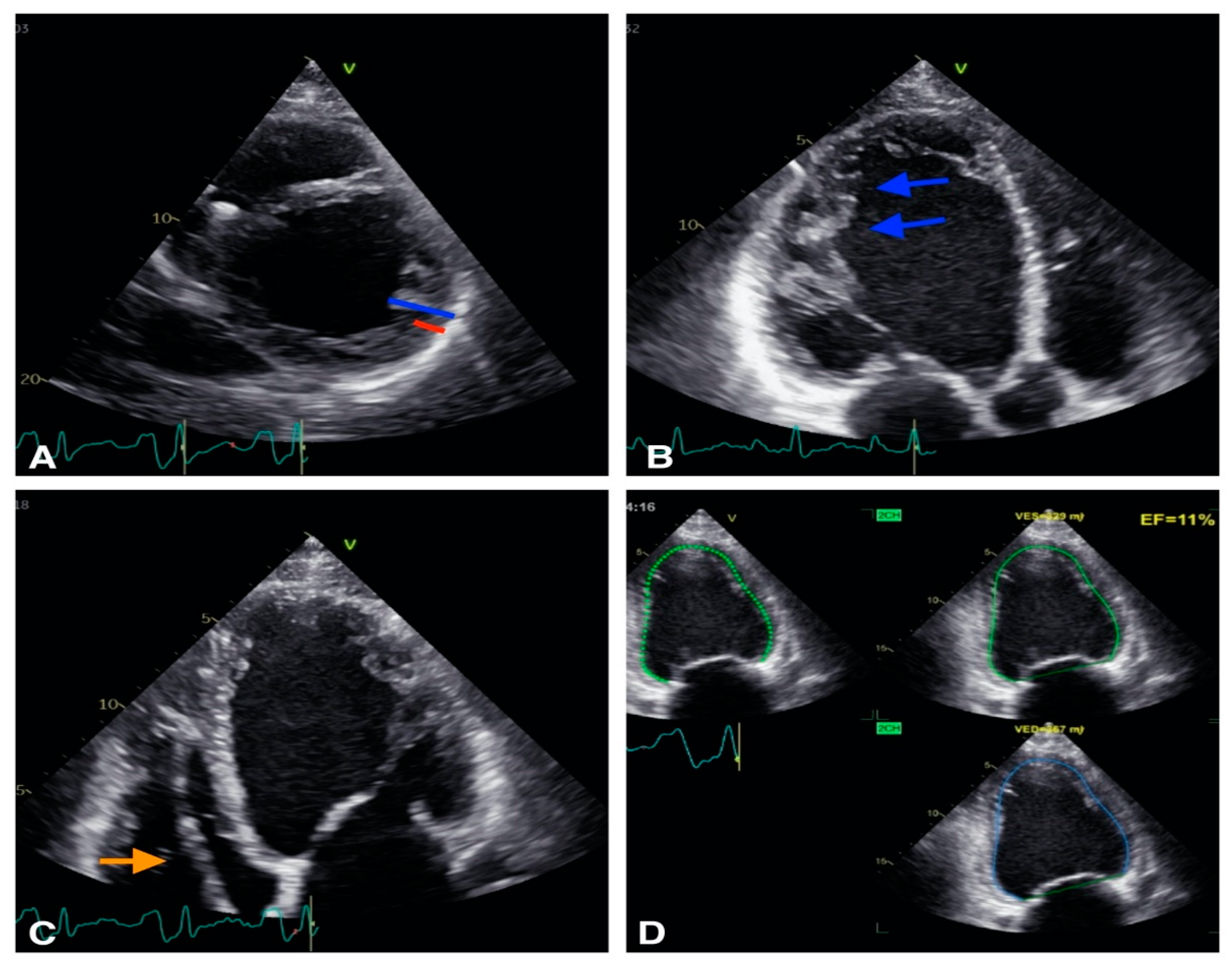
| Cardiomyopathy | Echocardiographic Parameters |
|---|---|
| HCM [7][8][9] |
|
| ARVC [10] |
|
| NCCM [11] |
|
| DCM [12] |
|
2.2. VA in Structurally Normal Hearts
| VA in Structural Heart Disease | VA in Structurally Normal Heart |
|---|---|
| IHD pARVC HCM DCM NCCM Valvular heart disease (i.e., aortic stenosis, mitral valve prolapse) Congenital heart disease |
Outflow tract VT Fascicular VT Interfascicular reentrant VT (Belhassen Tachycardia) Papillary muscle VA Channelopathies:
|
References
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac deathThe Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867.
- Papadopoulos, C.H.; Oikonomidis, D.; Lazaris, E.; Nihoyannopoulos, P. Echocardiography and cardiac arrhythmias. Hell. J. Cardiol. 2018, 59, 140–149.
- Saric, M.; Armour, A.C.; Arnaout, M.S.; Chaudhry, F.A.; Grimm, R.A.; Kronzon, I.; Landeck, B.F.; Maganti, K.; Michelena, H.I.; Tolstrup, K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J. Am. Soc. Echocardiogr. 2016, 29, 1–42.
- Therrien, J.; Siu, S.C.; Harris, L.; Dore, A.; Niwa, K.; Janousek, J.; Williams, W.G.; Webb, G.; Gatzoulis, M.A. Impact of Pulmonary Valve Replacement on Arrhythmia Propensity Late After Repair of Tetralogy of Fallot. Circulation 2001, 103, 2489–2494.
- Koyak, Z.; Harris, L.; de Groot, J.R.; Silversides, C.K.; Oechslin, E.N.; Bouma, B.J.; Budts, W.; Zwinderman, A.H.; Van Gelder, I.C.; Mulder, B.J.M. Sudden Cardiac Death in Adult Congenital Heart Disease. Circulation 2012, 126, 1944–1954.
- Mahida, S.; Sacher, F.; Dubois, R.; Sermesant, M.; Bogun, F.; Haïssaguerre, M.; Jaïs, P.; Cochet, H. Cardiac Imaging in Patients With Ventricular Tachycardia. Circulation 2017, 136, 2491–2507.
- Williams, L.K.; Frenneaux, M.P.; Steeds, R.P. Echocardiography in hypertrophic cardiomyopathy diagnosis, prognosis, and role in management. Eur. J. Echocardiogr. 2009, 10, iii9–iii14.
- Hagège, A.A.; Dubourg, O.; Desnos, M.; Mirochnik, R.; Isnard, G.; Bonne, G.; Carrier, L.; Guicheney, P.; Bouhour, J.-B.; Schwartz, K.; et al. Familial hypertrophic cardiomyopathy. Cardiac ultrasonic abnormalities in genetically affected subjects without echocardiographic evidence of left ventricular hypertrophy. Eur. Heart J. 1998, 19, 490–499.
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779.
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.P.J.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814.
- Chebrolu, L.H.; Mehta, A.M.; Nanda, N.C. Noncompaction cardiomyopathy: The role of advanced multimodality imaging techniques in diagnosis and assessment. Echocardiography 2017, 34, 279–289.
- Mathew, T.; Williams, L.; Navaratnam, G.; Rana, B.; Wheeler, R.; Collins, K.; Harkness, A.; Jones, R.; Knight, D.; O’Gallagher, K.; et al. Diagnosis and assessment of dilated cardiomyopathy: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2017, 4, G1–G13.
- Scheirlynck, E.; Malderen, S.V.; Motoc, A.; Lie, Ø.H.; de Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; Droogmans, S. Contraction alterations in Brugada syndrome; association with life-threatening ventricular arrhythmias. Int. J. Cardiol. 2020, 299, 147–152.
- Mitroi, C.; García-Izquierdo, E.; García-Lunar, I.; Castro-Urda, V.; Toquero-Ramos, J.; Moñivas-Palomero, V.; Mingo-Santos, S.; Cavero, M.A.; Brugada, J.; Fernández-Lozano, I. Right ventricular function and dyssynchrony in Brugada syndrome: Highlighting the importance of the mechanical substrate in the right ventricular outflow tract. Int. J. Cardiol. 2021, 333, 233–238.
- Scheirlynck, E.; Chivulescu, M.; Lie, Ø.H.; Motoc, A.; Koulalis, J.; de Asmundis, C.; Sieira, J.; Chierchia, G.-B.; Brugada, P.; Cosyns, B.; et al. Worse Prognosis in Brugada Syndrome Patients With Arrhythmogenic Cardiomyopathy Features. JACC Clin. Electrophysiol. 2020, 6, 1353–1363.
- Perin, E.C.; Silva, G.V.; Sarmento-Leite, R.; Sousa, A.L.S.; Howell, M.; Muthupillai, R.; Lambert, B.; Vaughn, W.K.; Flamm, S.D. Assessing Myocardial Viability and Infarct Transmurality With Left Ventricular Electromechanical Mapping in Patients With Stable Coronary Artery Disease. Circulation 2002, 106, 957–961.
- Njeim, M.; Desjardins, B.; Bogun, F. Multimodality Imaging for Guiding EP Ablation Procedures. JACC Cardiovasc. Imaging 2016, 9, 873–886.




