Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Song Wang | -- | 2143 | 2022-10-20 03:49:42 | | | |
| 2 | Peter Tang | -2 word(s) | 2141 | 2022-10-20 04:04:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, S.; Hu, W.; Liu, F. Autophagy in the Lifetime of Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/30275 (accessed on 14 January 2026).
Wang S, Hu W, Liu F. Autophagy in the Lifetime of Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/30275. Accessed January 14, 2026.
Wang, Song, Weiming Hu, Fen Liu. "Autophagy in the Lifetime of Plants" Encyclopedia, https://encyclopedia.pub/entry/30275 (accessed January 14, 2026).
Wang, S., Hu, W., & Liu, F. (2022, October 20). Autophagy in the Lifetime of Plants. In Encyclopedia. https://encyclopedia.pub/entry/30275
Wang, Song, et al. "Autophagy in the Lifetime of Plants." Encyclopedia. Web. 20 October, 2022.
Copy Citation
Autophagy is a highly conserved self-degradation mechanism in eukaryotes. Excess or harmful intracellular content can be encapsulated by double-membrane autophagic vacuoles and transferred to vacuoles for degradation in plants. Current research shows three types of autophagy in plants, with macroautophagy being the most important autophagic degradation pathway. More than 40 autophagy-related (ATG) proteins have been identified in plants that are involved in macroautophagy, and these proteins play an important role in plant growth regulation and stress responses.
autophagy
abiotic stress
biotic stress
vegetative growth
reproductive growth
1. Introduction
Autophagy, also known as self-eating, is an evolutionarily conserved process that occurs in eukaryotic cells and involves the degradation of organelles, protein complexes, and macromolecules [1]. Generally, the degraded material is sequestered into autophagic vesicles that are transported to the vacuole for breakdown. Under normal conditions, autophagy is a housekeeping process that degrades unwanted cytoplasmic content and maintains cellular homeostasis [2]. Under stress conditions (starvation, oxidative and abiotic stress, and pathogen infection), autophagy proteins are up-regulated and help in recycling damaged or non-essential cellular material [3].
Until now, three different types of autophagy in plants have been discovered, including microautophagy, macroautophagy, and mega-autophagy [4]. Microautophagy is the direct packaging of cargo into the vacuole for degradation through the invagination or protrusion of the vacuolar membrane [5]. Although the concept of microautophagy has been present for many years, little is known about the mechanism by which it occurs. In plants, microautophagy is found to play an important role in anthocyanin aggregates [6]. In addition, microautophagy is also involved in the degradation progress of damaged chloroplast, namely, chlorophagy [7]. The most well-studied type of autophagy in plants is macroautophagy, in which autophagosomes form and then fuse with vacuoles to degrade cargoes [8]. Until now, more than 40 ATG proteins have been found to be involved in the biological process of macroautophagy [1]. There is also a more direct type of autophagy, namely, mega-autophagy. Here, the tonoplast membrane ruptures to release the vacuolar hydrolases directly into the cytoplasm, where it degrades cytoplasmic materials [9][10]. Mega-autophagy often occurs during programmed cell death (PCD) including plant development or in response to pathogens [1].
In plants, autophagy is a feature in diverse biological processes such as development, nutrient recycling, and biotic and abiotic stresses [2] (Figure 1).
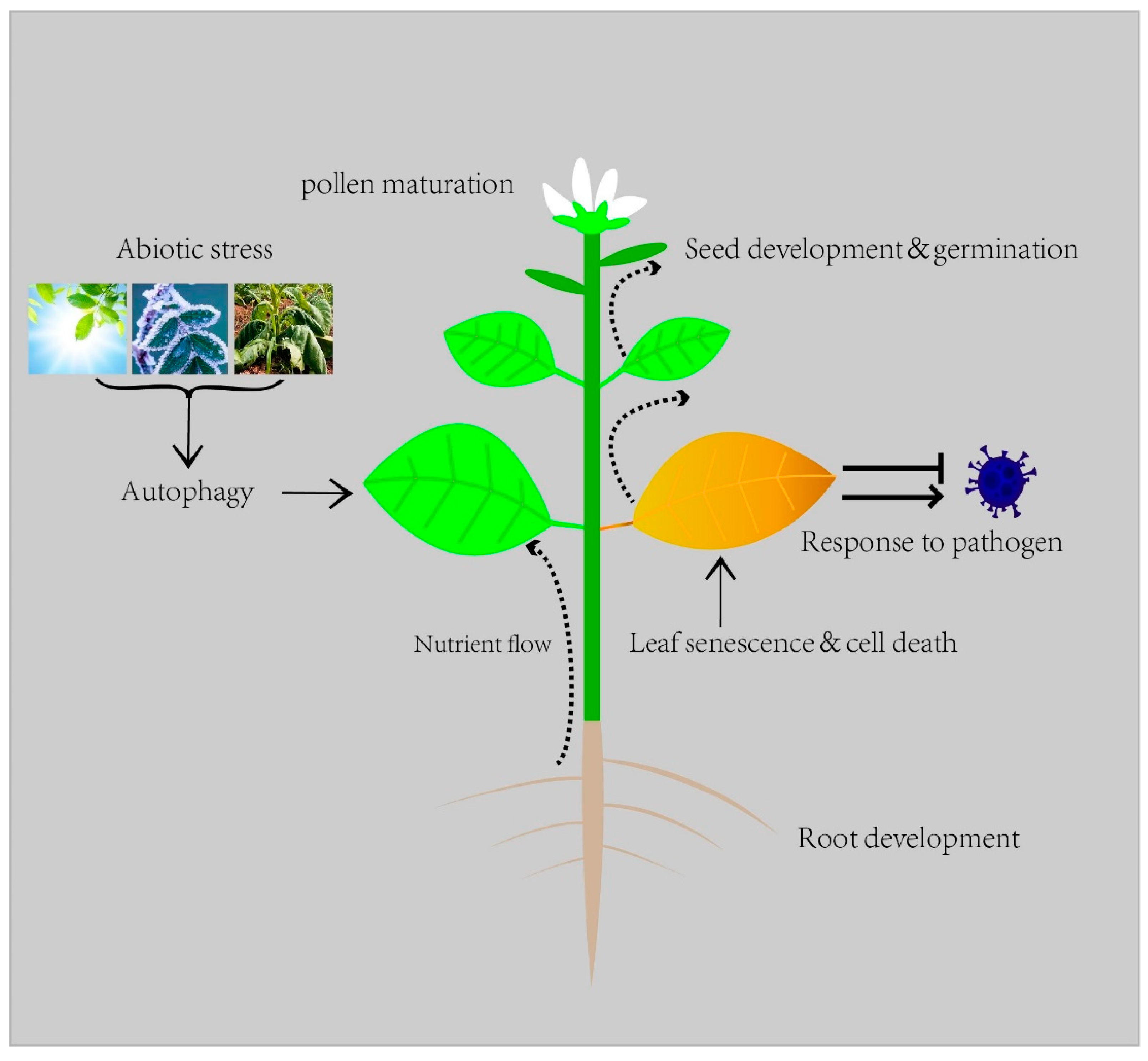
Figure 1. Autophagy in plant life.
2. Autophagy in Vegetative Growth
2.1. Seed Development
For flowering plants, the cycle of life begins with a seed. Generally, atg mutations in plants produce fewer seeds compared to WT plants, suggesting that autophagy may function during plant seed development [11]. In Arabidopsis, several atg mutations show decreased seed production [2], and some ATG genes are up-regulated during seed maturation [12]. Similar results are shown in maize, such as ATG1a, Atg18e, Atg18e, Atg18f, and Atg18h, which are expressed in endosperm instead of other tissues [13]. However, the role of autophagy in seed development has not been explained at the mechanism level. Some studies show that autophagy may contribute to the transport of seed storage proteins [14][15]. Both total protein and 12S globulins are accumulated in atg5 seeds, indicating that autophagy affects the seed protein content [15]. In addition to seed protein accumulation, autophagy also contributes to seed germination. In Arabidopsis, the overexpression of Atg8-interacting proteins (ATI1 and ATI2) can stimulate seed germination under ABA conditions [16].
2.2. Root Development
Structurally, plant roots are divided into three zones: the meristematic zone, elongation zone, and maturation zone [17]. A cross-section of roots includes three levels: dermal, cortex (ground tissue), and vascular tissues [18]. During root development, autophagy plays an important role in its establishment and functional differentiation.
ATG8 genes are mainly located in the root caps and maturation zone, which correspond to relevant protein degradation [19]. The role of autophagy in the root tips may be related to programmed cell death (PCD), but there is not enough evidence to prove this speculation [18].
In addition to root development, autophagy also plays a vital role in root senescence [20]. The up-regulation of ATG genes (ATG8C, ATG8D, and ATG8G) is characterized by the senescence of absorptive roots [20]. During the first stage of senescence, autophagy counteracts transient cell death and maintains cellular homeostasis [18]. Additionally, autophagy is also involved in the remobilization process, which is a key step in the senescence process [18].
2.3. Leaf Senescence
Leaf senescence is a late stage of plant vegetative growth. In Arabidopsis, premature leaf senescence is one of the common phenotypes in autophagy mutants. Most ATG genes are up-regulated under leaf senescence, while other autophagy genes are mainly expressed in leaf development. In barley, the transcript levels of both ATG7 and ATG18f are up-regulated during leaf senescence [21]. In rice, compared to the wild-type, the atg7 mutant not only reduces the plant height, root length, tiller number, and leaf area, but also has obvious premature leaf senescence [22]. In Arabidopsis, SAG12 (senescence-associated gene 12) is a marker gene for the onset of senescence, which is abundantly induced in atg2 and atg5 mutants [23]. Premature leaf senescence of these mutants can be alleviated by blocking SA (salicylic acid) biosynthesis or signal transduction. For example, the overexpression of SA hydroxylase NahG (salicylate hydroxylase) can inhibit the premature senescence phenotype of atg2 and atg5, and the administration of the SA analog BTH (benzothiadiazole) restores the normal phenotype of these mutants [23]. The regulation of autophagy in premature plant senescence may be attributed to the effect on the redistribution of plant nutrients, especially nitrogen. For example, rice atg7–1 mutant leaves prematurely senesce, and the nitrogen content of senescent leaves is higher than that of wild-type leaves, which reduces the nitrogen reuse efficiency [22]. In apples, the overexpression of ATG18a greatly improves resistance to low nitrogen stress and up-regulates the expression of nitrogen uptake and assimilation-related genes NIA2, NRT2.1, NRT2.4, and NRT2.5 [24]. Some new evidence suggests that SAG12 regulates plant senescence through involvement in protein degradation and N remobilization [25][26]. In future studies, it will be interesting to determine whether autophagy cooperates with senescence-associated proteases to cycle cellular components.
3. Autophagy in Reproductive Growth
Autophagy is not only involved in regulating the vegetative growth, but also regulates the reproductive growth in plants. Members of the PI3K complex (atg6, vps15, and vps34) are reported to regulate Arabidopsis pollen maturation, and none of them can produce mature pollen after mutation [27][28][29]. In the rice atg7 mutant, lipid and starch components in pollen grains cannot be accumulated normally during flowering, resulting in reduced pollen viability and sporophytic male sterility [30]. Autophagy also plays a key role in tobacco pollen germination. Autophagy flux is significantly increased in the early stage of pollen germination to degrade the cytoplasm in the germinal pores [31]. Cytoplasmic degradation of germinal pores during pollen germination is also inhibited after the silencing of ATG2, ATG5, and ATG7 in tobacco [32]. New research shows that autophagy is also involved in pollen tube elongation. In this process, a core protein SH3-domain-containing protein 2 (SH3P2) colocalizes with ATG proteins and participates in regulating mitophagosomes [32]. Down-regulation of SH3P2 expression significantly impairs pollen germination and pollen tube growth [32].
4. Autophagy in Abiotic Stress
Plants are exposed to various abiotic stresses during growth, such as salt, heat, cold, drought, and nutrition stress. Autophagy, a process that maintains cellular homeostasis, plays an important role in the defense of abiotic stresses.
4.1. Autophagy under Nutrient Starvation
Nutrient starvation triggers a strong induction of autophagy, and ATG mutants exhibit premature senescence upon carbon/nitrogen starvation. Using sucrose starvation in suspension-cultured cells shows that 30% to 50% of the total protein is degraded over a two day period [33], and the decrease in total proteins stems from non-selective degradation, rather than degradation of specific proteins [34]. Fusion between autophagosomes and central vacuoles is observed in cells treated with E-64c (cysteine protease inhibitors). Thus, during classical autophagy, the partially degraded cytoplasm in the autophagosome is likely to be released into the central vacuole for further degradation [34]. Using bafilomycin A1 and concanamycin A, which inhibits the activity of vacuolar hydrolase, it is observed that even non-degradable autophagosomes are still expelled into the central vacuole [35]. Nutrient stress also affects target of rapamycin (TOR) signaling and ultimately activates autophagy production. For example, autophagy induced by nutrient stress is inhibited in TOR-overexpressing plants, while the application of the TOR inhibitor AZD8055 in wild-type plants and raptor1a/b mutants leads to the production of structural autophagy [36]. Studies found that, although the ATG1 complex is involved in autophagy induced by nitrogen starvation and short-term carbon starvation, there is an ATG1-independent autophagy initiation pathway under long-term carbon starvation in Arabidopsis, in which the SnRK1 catalytic subunit KIN10 can directly phosphorylate the ATG6 to initiate autophagy [37].
A new study shows that autophagy is also involved in the regulation of sulfur starvation in plants [38]. Sulfur (S) remigration from the rosette to the seed is impaired in atg5 mutants compared to the wild-type [38]. These studies demonstrate that autophagy plays an indispensable role in maintaining cell homeostasis in plants under nutrient starvation.
4.2. Drought Stress
Drought stress increases the expression of many ATG genes in crops, such as ATG2 in peppers [39], ATG8a in millet [40], ATG6 in barley [41], and ATG3 and ATG18a in apples [22][42]. In tomatoes overexpressing HsfA1a, the silencing of ATG10 and ATG18f reduces HsfA1a-induced drought tolerance and autophagosome formation [43]. Conversely, the overexpression of MdATG18a in tomatoes degrades protein aggregation, limits oxidative damage, and ultimately improves drought tolerance [44]. Under drought stress, MtCAS31 promotes degradation of the MtPIP2;7 protein by autophagy, a negative regulator of drought, leading to a decrease in root hydraulic conductivity, thereby reducing water loss and improving drought tolerance [45]. In Arabidopsis, a plant-specific gene COST1, which is a negative regulator of drought, negatively regulates drought resistance by influencing the autophagy pathway [46]. COST1 co-localizes with ATG8e and the autophagy linker NBR1 on autophagosomes, suggesting a critical role in the direct regulation of autophagy [46]. A previous study found that mitochondrial alternative oxidase (AOX) may regulate autophagy through mitochondrial ROS during drought stress in tomatoes [47].
4.3. Heat and Cold Stress
Plants accumulate a large amount of oxidized and insoluble proteins at unsuitable temperatures. In this case, plants can eliminate these toxic proteins by inducing autophagy to improve plant resistance. ATG gene expression is up-regulated in various plants, and more autophagosomes are accumulated under heat stress [39][48][49]. On the contrary, silencing ATG5 or ATG7 in Arabidopsis and tomatoes under heat stress leads to heat sensitivity [48][49][50]. Plants degrade related proteins through NBR1-mediated selective autophagy under heat stress. Under heat stress, the expression of NBR1 is up-regulated and more puncta accumulate in the cytoplasm compared to the wild-type [51]. Furthermore, more NBR1 puncta accumulate in WT plants during the heat stress recovery stage, and the accumulation of the NBR1 protein is significantly higher in atg mutants than in WT plants [51][52]. Furthermore, the NBR1-mediated selective autophagy pathway degrades HSP90.1 and ROF1 to reduce plant resistance to heat stress memory [53].
Compared to heat stress, there are few studies on the regulation of cold stress by autophagy in plants. In rice, OsATG6b is down-regulated under cold stress, while OsATG6c expression is up-regulated [41]. In barley, the expression of HvATG6 is up-regulated under low temperatures [54]. This may suggest that ATG6 plays an important role in response to low plant temperature. In addition, NBR1-mediated selective autophagy also appears to be involved in plant responses to cold stress. In tomatoes, BRs (Brassinosteroids) and the positive regulator BZR1 induce autophagy and accumulation of the selective autophagy receptor NBR1 under cold stress [55].
4.4. Salt Stress
High concentrations of NaCl lead to a reduced photosynthetic rate, as well as excessive energy consumption and accumulation of excess reactive oxygen species (ROS) [56]. As an important regulator of cellular homeostasis, autophagy is also involved in the pathway of plant salt tolerance. Several autophagy genes are up-regulated under salt treatment in wheat seedlings [57]. Silencing metacaspase TaMCA-Id can reduce the tolerance of wheat seedlings to NaCl by promoting ROS production, which further participates in the regulation of autophagy and PCD triggered by NaCl treatment [58]. Within 3 h of salt treatment, accumulation of oxidized proteins in atg2 and atg7 is higher than that in the WT, and the mutants are highly sensitive to salt stress [59]. The Arabidopsis PI3K complex positively regulates salt tolerance by promoting the internalization of PIP2;1 from the plasma membrane into the vacuole under salt stress, thereby reducing root water permeability [60]. In addition, the overexpression of MdATG10 leads to increased autophagy activity in roots and enhances salt tolerance in apples [61]. Another study demonstrates spermidine (Spd), a kind of polyamine, activation of ATG gene expression and autophagosome formation under salt stress in cucumbers [62]. All of the above results indicate the role of autophagy in plants under salt stress.
5. Autophagy in Biotic Stress
Besides abiotic stresses, biotic stresses can influence autophagy. In plants, depending on the lifestyle of the pathogen, infecting the plant and activating autophagy is shown to lead to different outcomes [63]. The plant immune system is a complex mechanism, the most well-known of which is the hypersensitive response (HR)-related programmed cell death (PCD) [64]. It is reported that autophagy is involved in plant immunity by negatively regulating PCD [65].
References
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208.
- Liu, Y.; Bassham, D.C. Autophagy: Pathways for Self-Eating in Plant Cells. Annu. Rev. Plant Biol. 2012, 63, 215–237.
- Su, W.; Bao, Y.; Yu, X.; Xia, X.; Liu, C.; Yin, W. Autophagy and Its Regulators in Response to Stress in Plants. Int. J. Mol. Sci. 2020, 21, 8889.
- Van Doorn, W.G.; Papini, A. The ultrastructure of autophagy in plant cells: A review. Autophagy 2013, 9, 1922–1936.
- Sie’nko, K.; Poormassalehgoo, A.; Yamada, K.; Goto-Yamada, S. Microautophagy in Plants: Consideration of Its Molecular Mechanism. Cells 2020, 9, 887.
- Chanoca, A.; Kovinich, N.; Burkel, B.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell 2015, 27, 2545–2559.
- Nakamura, S.; Hidema, J.; Sakamoto, W.; Ishida, H.; Izumi, M. Selective elimination of membrane-damaged chloroplasts via microautophagy. Plant Physiol. 2018, 177, 1007–1026.
- Feng, Y.C.; He, D.; Yao, Z.Y.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41.
- Hatsugai, N.; Kuroyanagi, M.; Yamada, K.; Meshi, T.; Tsuda, S.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 2004, 305, 855–858.
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell. Biol. 2020, 21, 439–458.
- Minina, E.A.; Moschou, P.N.; Vetukuri, R.R.; Sanchez-Vera, V.; Cardoso, C.; Liu, Q.S.; Elander, P.H.; Dalman, K.; Beganovic, M.; Yilmaz, J.L.; et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018, 69, 1415–1432.
- Angelovici, R.; Fait, A.; Zhu, X.; Szymanski, J.; Feldmesser, E.; Fernie, A.R.; Galili, G. Deciphering transcriptional and metabolic networks associated with lysine metabolism during Arabidopsis seed development. Plant Physiol. 2009, 151, 2058–2072.
- Li, F.; Chung, T.; Pennington, J.G.; Federico, M.L.; Kaeppler, H.F.; Kaeppler, S.M.; Otegui, M.S.; Vierstra, R.D. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell 2015, 27, 1389–1408.
- Levanony, H.; Rubin, R.; Altschuler, Y.; Galili, G. Evidence for a novel route of wheat storage proteins to vacuoles. J. Cell Biol. 1992, 119, 1117–1128.
- Berardino, D.J.; Marmagne, A.; Berger, A.; Yoshimoto, K.; Cueff, G.; Chardon, F.; Masclaux-Daubresse, C.; Reisdorf-Cren, M. Autophagy controls resource allocation and protein storage accumulation in Arabidopsis seeds. J. Exp. Bot. 2018, 69, 1403–1414.
- Honig, A.; Avin-Wittenberg, T.; Ufaz, S.; Galili, G. A new type of compartment, defined by plant-specific Atg8-interacting proteins, is induced upon exposure of Arabidopsis plants to carbon starvation. Plant Cell 2012, 24, 288–303.
- Evert, R.F. Esau’s plant anatomy, meristems, cells, and tissues of the plant body: Their structure, function, and development. 3rd edn. Ann. Bot. 2007, 99, 785–786.
- Wojciechowska, N.; Michalak, K.M.; Bagniewska-Zadworna, A. Autophagy-an underestimated coordinator of construction and destruction during plant root ontogeny. Planta 2021, 254, 15.
- Sláviková, S.; Shy, G.; Yao, Y.; Glozman, R.; Levanony, H.; Pietrokovski, S.; Elazar, Z.; Galili, G. The autophagy-associated ATG8 gene family operates both under favourable growth conditions and under starvation stresses in Arabidopsis plants. J. Exp. Bot. 2005, 56, 2839–2849.
- Wojciechowska, N.; Marzec-Schmidt, K.; Kalemba, E.M.; Zarzyńska-Nowak, A.; Jagodziński, A.M.; Bagniewska-Zadworna, A. Autophagy counteracts instantaneous cell death during seasonal senescence of the fine roots and leaves in Populus trichocarpa. BMC Plant Biol. 2018, 18, 260.
- Hollmann, J.; Gregersen, P.L.; Krupinska, K. Identification of predominant genes involved in regulation and execution of senescence-associated nitrogen remobilization in flag leaves of field grown barley. J. Exp. Bot. 2014, 65, 3963–3973.
- Wada, S.; Hayashida, Y.; Izumi, M.; Kurusu, T.; Hanamata, S.; Kanno, K.; Kojima, S.; Yamaya, T.; Kuchitsu, K.; Makino, A.; et al. Autophagy supports biomass production and nitrogen use efficiency at the vegetative stage in rice. Plant Physiol. 2015, 168, 60–73.
- Yoshimoto, K.; Jikumaru, Y.; Kamiya, Y.; Kusano, M.; Consonni, C.; Panstruga, R.; Ohsumi, Y.; Shirasu, K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 2009, 21, 2914–2927.
- Sun, X.; Jia, X.; Huo, L.; Che, R.; Gong, X.; Wang, P.; Ma, F. MdATG18a overexpression improves tolerance to nitrogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2018, 41, 469–480.
- Poret, M.; Chandrasekar, B.; van der Hoorn, R.A.; Avice, J.C. Characterization of senescence-associated protease activities involved in the efficient protein remobilization during leaf senescence of winter oilseed rape. Plant Sci. 2016, 246, 139–153.
- James, M.; Poret, M.; Masclaux-Daubresse, C.; Marmagne, A.; Coquet, L.; Jouenne, T.; Chan, P.; Trouverie, J.; Etienne, P. SAG12, a major cysteine protease involved in nitrogen allocation during senescence for seed production in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 2052–2063.
- Wang, W.; Zhang, L.; Xing, S.; Ma, Z.; Liu, J.; Gu, H.; Qin, G.; Qu, L. Arabidopsis AtVPS15 plays essential roles in pollen germination possibly by interacting with AtVPS34. J. Genet. Genom. 2012, 39, 81–92.
- Fujiki, Y.; Yoshimoto, K.; Ohsumi, Y. An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol. 2007, 143, 1132–1139.
- Lee, Y.; Kim, E.; Choi, Y.; Hwang, I.; Staiger, C.J.; Chung, Y.; Lee, Y. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development. Plant Physiol. 2008, 147, 1886–1897.
- Kurusu, T.; Koyano, T.; Hanamata, S.; Kubo, T.; Noguchi, Y.; Yagi, C.; Nagata, N.; Yamamoto, T.; Ohnishi, T.; Okazaki, Y.; et al. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 2014, 10, 878–888.
- Zhao, P.; Zhou, X.; Zhao, L.; Cheung, A.Y.; Sun, M. Autophagy-mediated compartmental cytoplasmic deletion is essential for tobacco pollen germination and male fertility. Autophagy 2020, 16, 2180–2192.
- Yan, H.; Zhuang, M.; Xu, X.; Li, S.; Yang, M.; Li, N.; Du, X.; Hu, K.; Peng, X.; Huang, W.; et al. Autophagy and its mediated mitochondrial quality control maintain pollen tube growth and male fertility in Arabidopsis. Autophagy 2022, 18, 1–16.
- Takatsuka, C.; Inoue, Y.; Matsuoka, K.; Moriyasu, Y. 3-methyladenine inhibits autophagy in tobacco culture cells under sucrose starvation conditions. Plant Cell Physiol. 2004, 45, 265–274.
- Moriyasu, Y.; Ohsumi, Y. Autophagy in tobacco suspension-cultured cells in response to sucrose starvation. Plant Physiol. 1996, 111, 1233–1241.
- Yoshimoto, K.; Hanaoka, H.; Sato, S.; Kato, T.; Tabata, S.; Noda, T.; Ohsumi, Y. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell 2004, 16, 2967–2983.
- Pu, Y.; Luo, X.; Bassham, D.C. TOR-dependent and -independent pathways regulate autophagy in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1204.
- Huang, X.; Zheng, C.; Liu, F.; Yang, C.; Zheng, P.; Lu, X.; Tian, J.; Chung, T.; Otegui, M.S.; Xiao, S.; et al. Genetic analyses of the Arabidopsis ATG1 kinase complex reveal both kinase-dependent and independent autophagic routes during fixed-carbon starvation. Plant Cell 2019, 31, 2973–2995.
- Lornac, A.; Havé, M.; Chardon, F.; Soulay, F.; Clément, G.; Avice, J.; Masclaux-Daubresse, C. Autophagy Controls Sulphur Metabolism in the Rosette Leaves of Arabidopsis and Facilitates S Remobilization to the Seeds. Cells 2020, 9, 332.
- Zhai, Y.; Guo, M.; Wang, H.; Lu, J.; Liu, J.; Zhang, C.; Gong, Z.; Lu, M. Autophagy, a conserved mechanism for protein degradation, responds to heat, and other abiotic stresses in Capsicum annuum L. Front. Plant Sci. 2016, 7, 131.
- Li, W.; Chen, M.; Wang, E.; Hu, L.; Hawkesford, M.J.; Zhong, L.; Chen, Z.; Xu, Z.; Li, L.; Zhou, Y.; et al. Genome-wide analysis of autophagy-associated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genom. 2016, 17, 797.
- Zeng, X.; Zeng, Z.; Liu, C.; Yuan, W.; Hou, N.; Bian, H.; Zhu, M.; Han, N. A barley homolog of yeast ATG6 is involved in multiple abiotic stress responses and stress resistance regulation. Plant Physiol. Biochem. 2017, 115, 97–106.
- Wang, P.; Sun, X.; Jia, X.; Ma, F. Apple autophagy-related protein MdATG3s afford tolerance to multiple abiotic stresses. Plant Sci. 2017, 256, 53–64.
- Wang, Y.; Cai, S.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy 2015, 11, 2033–2047.
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557.
- Li, X.; Liu, Q.; Feng, H.; Deng, J.; Zhang, R.; Wen, J.; Dong, J.; Wang, T. Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 2020, 16, 862–877.
- Bao, Y.; Song, W.; Wang, P.; Yu, X.; Li, B.; Jiang, C.; Shiu, S.; Zhang, H.; Bassham, D.C. COST1 regulates autophagy to control plant drought tolerance. Proc. Natl. Acad. Sci. USA 2020, 117, 7482–7493.
- Zhu, T.; Zou, L.; Li, Y.; Yao, X.; Xu, F.; Deng, X.; Zhang, D.; Lin, H. Mitochondrial alternative oxidasedependent autophagy involved in ethylenemediated drought tolerance in Solanum lycopersicum. Plant Biotechnol. J. 2018, 16, 2063–2076.
- Zhou, J.; Wang, J.; Yu, J.; Chen, Z. Role and regulation of autophagy in heat stress responses of tomato plants. Front. Plant Sci. 2014, 5, 174.
- Cheng, F.; Yin, L.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y.; Foyer, C. Interactions between 2-Cys peroxiredoxins and ascorbate in autophagosome formation during the heat stress response in Solanum lycopersicum. J. Exp. Bot. 2016, 67, 1919–1933.
- Zhou, J.; Wang, J.; Cheng, Y.; Chi, Y.; Fan, B.; Yu, J.; Chen, Z. Correction: NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 2013, 9, e1004477.
- Jung, H.; Lee, H.N.; Marshall, R.S.; Lomax, A.W.; Yoon, M.J.; Kim, J.; Kim, J.H.; Vierstra, R.D.; Chung, T. Arabidopsis cargo receptor NBR1 mediates selective autophagy of defective proteins. J. Exp. Bot. 2020, 71, 73–89.
- Thirumalaikumar, V.P.; Gorka, M.; Schulz, K.; Masclaux-Daubresse, C.; Sampathkumar, A.; Skirycz, A.; Vierstra, R.D.; Balazadeh, S. Selective autophagy regulates heat stress memory in Arabidopsis by NBR1-mediated targeting of HSP90 and ROF1. Autophagy 2020, 16, 1–16.
- Rana, R.M.; Dong, S.; Ali, Z.; Huang, J.; Zhang, H.S. Regulation of ATG6/Beclin-1 homologs by abiotic stresses and hormones in rice (Oryza sativa L.). Genet. Mol. Res. 2012, 11, 3676–3687.
- Chi, C.; Li, X.; Fang, P.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Brassinosteroids act as a positive regulator of NBR1-dependent selective autophagy in response to chilling stress in tomato. J. Exp. Bot. 2020, 71, 1092–1106.
- Julkowska, M.M.; Testerink, C. Tuning plant signaling and growth to survive salt. Trends Plant Sci. 2015, 20, 586–594.
- Yue, W.; Nie, X.; Cui, L.; Zhi, Y.; Zhang, T.; Du, X.; Song, W. Genome-wide sequence and expressional analysis of autophagy gene family in bread wheat (Triticum aestivum L.). J. Plant Physiol. 2018, 229, 7–21.
- Yue, J.; Wang, Y.; Jiao, J.; Wang, W.; Wang, H. The Metacaspase TaMCA-Id Negatively Regulates Salt-Induced Programmed Cell Death and Functionally Links with Autophagy in Wheat. Front. Plant Sci. 2022, 13, 904933.
- Luo, L.; Zhang, P.; Zhu, R.; Fu, J.; Su, J.; Zheng, J.; Wang, Z.; Wang, D.; Gong, Q. Autophagy Is Rapidly Induced by Salt Stress and Is Required for Salt Tolerance in Arabidopsis. Front. Plant Sci. 2017, 8, 1459.
- Ueda, M.; Tsutsumi, N.; Fujimoto, M. Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 474, 742–746.
- Huo, L.; Guo, Z.; Jia, X.; Sun, X.; Wang, P.; Gong, X.; Ma, F. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. 2020, 294, 110444.
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.J.; Cao, K.; Shah Jahan, M.; Shu, S.; Wang, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890.
- Leary, A.Y.; Sanguankiattichai, N.; Duggan, C.; Tumtas, Y.; Pandey, P.; Segretin, M.E.; Linares, J.S.; Savage, Z.D.; Yow, R.J.; Bozkurt, T.O. Modulation of plant autophagy during pathogen attack. J. Exp. Bot. 2018, 69, 1325–1333.
- Coll, N.S.; Epple, P.; Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 2011, 18, 1247–1256.
- Liu, Y.; Schiff, M.; Czymmek, K.; Tallóczy, Z.; Levine, B.; Dinesh-Kumar, S.P. Autophagy regulates programmed cell death during the plant innate immune response. Cell 2005, 121, 567–577.
- Yang, M.; Liu, Y. Autophagy in plant viral infection. FEBS Lett. 2022, 596, 2152–2162.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
872
Revisions:
2 times
(View History)
Update Date:
20 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




