
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shiguo Sun | -- | 18087 | 2022-10-19 13:47:17 | | | |
| 2 | Shiguo Sun | -2456 word(s) | 6602 | 2022-10-20 12:49:50 | | | | |
| 3 | Shiguo Sun | + 162 word(s) | 6764 | 2022-10-20 16:28:15 | | | | |
| 4 | Shiguo Sun | Meta information modification | 6764 | 2022-10-20 16:30:31 | | | | |
| 5 | Shiguo Sun | + 503 word(s) | 7267 | 2022-10-20 16:35:49 | | | | |
| 6 | Shiguo Sun | Meta information modification | 7267 | 2022-10-20 16:36:51 | | | | |
| 7 | Peter Tang | -31 word(s) | 7236 | 2022-10-21 02:54:58 | | |
Video Upload Options
As a mental disease in modern society, depression shows an increasing occurrence, with low cure rate and high recurrence rate. It has become the most disabling disease in the world. At present, the treatment of depression is mainly based on drug therapy combined with psychological therapy, physical therapy, and other adjuvant therapy methods. Antidepressants are primarily administered peripherally (oral and intravenous) and have a slow onset of action. Antidepressant active ingredients, such as neuropeptides, natural active ingredients, and some chemical agents, are limited by factors such as the blood–brain barrier (BBB), first-pass metabolism, and extensive adverse effects caused by systemic administration. The potential anatomical link between the non-invasive nose–brain pathway and the lesion site of depression may provide a more attractive option for the delivery of antidepressant active ingredients.
1. Introduction
Depression is a very common central nervous system (CNS) disorder disease worldwide. Clinical symptoms include chronic depression, apathy, loss of appetite, and loss of interest. According to the World Health Organization, more than 350 million people worldwide suffer from depression, hundreds of thousands commit suicide each year, and the number is rising rapidly. More than 75% of patients in countries with a shortage of medical resources and healthcare personnel go untreated [1]. The clinical treatment of depression is still characterized by a low cure rate, high recurrence rate, residual symptoms, dysfunction, and high risk of self-injury and suicide, which imposes a serious burden on physical and mental health and economic levels around the world [2][3]. After decades of research, the most widely accepted treatment strategy for depression is a combination of medication, psychotherapy, and physical therapy. Due to the social environment, psychological burden, and other reasons, the use of antidepressants is generally accepted by patients. There are several antidepressants on the market, most of which are administered orally in tablets or capsules (Selegiline in the transdermal patch, Esketamine in the nasal spray, and Brexanolone in the intravenous infusion). Antidepressants are absorbed by different untargeted tissues and organs in the gastrointestinal tract or after absorption into the blood circulatory system, resulting in systemic clearance (in oral and parenteral route) and widespread adverse reactions such as drowsiness, weight gain, constipation, dry mouth, and dizziness [4][5][6][7]. Invasive drug administration of the brain can cause great discomfort to patients. Some depressive patients have marked relief or disappearance of depressive symptoms after taking antidepressants for some time. However, there is also a subset of patients (major depressive disorder, MDD) who do not respond to two or more first-line antidepressants and have acute or severe suicidal thoughts. Drug therapy is limited because of the complexity of the pathogenesis of depression and the variability of individual patients [8]. The current clinical situation is that depressive symptoms are relieved after 2 to 3 weeks or even longer after oral first-line antidepressants. The early stage after taking antidepressants is also the stage where adverse reactions are prominent. During this incubation period, patients are at increased risk of disability or suicide (especially in patients ≤ 24 years of age), aggravated disease, and decreased medication compliance. Therefore, there is an urgent need to find new antidepressant active ingredients and routes of administration with a fast onset of action and fewer side effects.
Due to BBB, extensive metabolism, high protein binding rate, and systemic side effects, many ingredients with antidepressant activity cannot exert effective therapeutic effects by oral or injection administration. The BBB is mainly composed of microvascular endothelial cells, mural cells, and glial cell astrocytes (Figure 1). In contrast to inner cells in other tissues, brain endothelial cells are connected by tight junctions (TJs) and have very little vesicle-mediated transcellular transport. The endothelium of the brain contains a variety of enzymes that inactivate certain neurotransmitters, antidepressants, and toxins, preventing them from entering the brain. In addition, efflux transporters, which are located on the blood side of the endothelial cells, use energy to transport passively diffused lipophilic molecules back into the blood, especially the P-glycoprotein (P-gp) [9][10][11]. Preclinical studies have shown that many antidepressants are substrates of P-gp, which may affect the distribution of antidepressants to their target of action [12][13]. The effects of ABCB1 polymorphisms on P-gp expression and antidepressant transport were significantly different between individuals, which may lead to treatment variability [14].
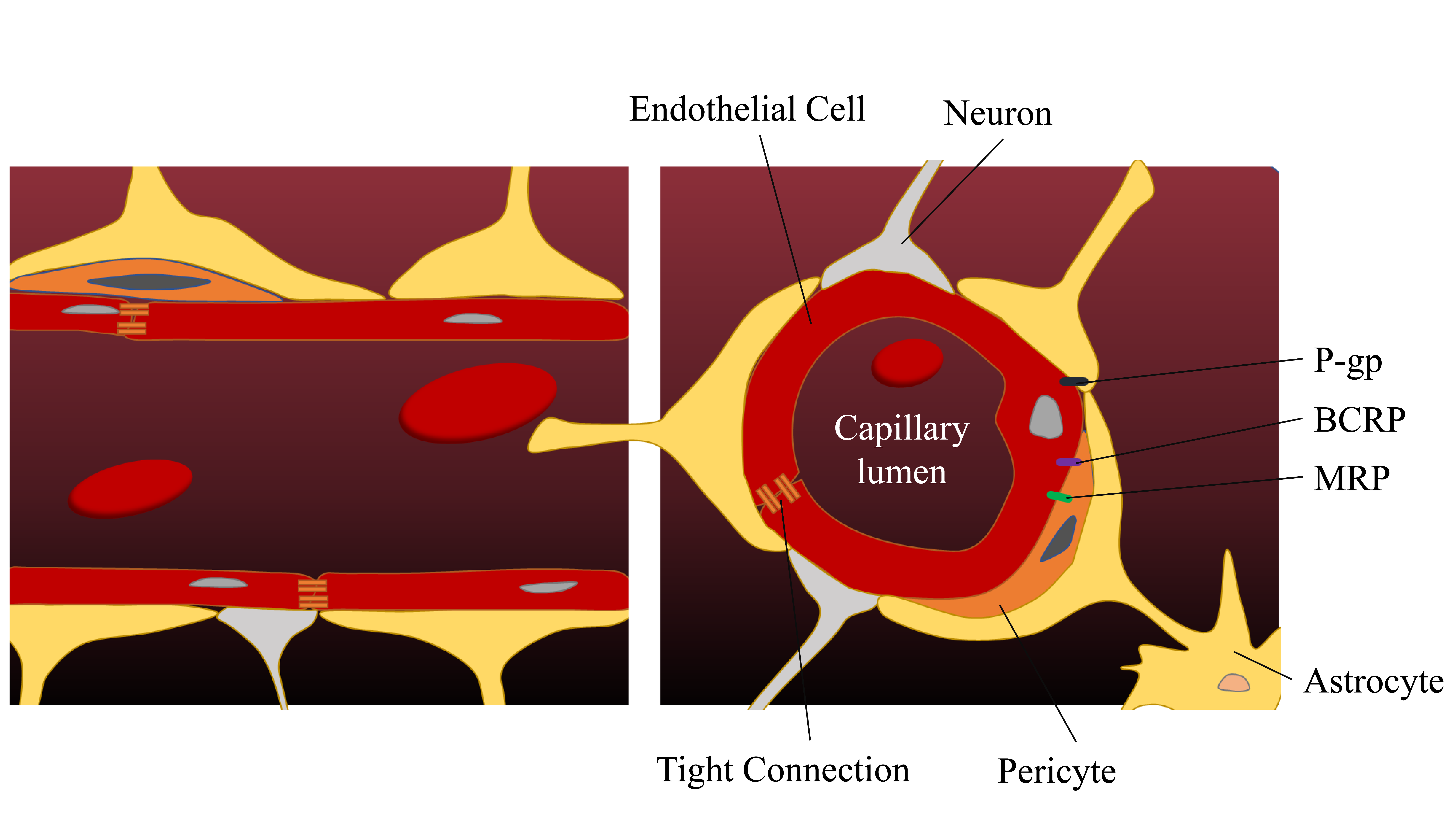
Figure 1. The blood–brain barrier. P-gp, P-glycoprotein; BCRP, breast cancer resistance protein; MRP, multidrug resistance-associated protein.
Some first-line antidepressants, off-label drugs, peptides, natural active ingredients, and other substances with antidepressant activity are limited by the choice of route of administration because of their properties. Even when administered intravenously, some drugs still enter the liver for degradation and are restricted by the BBB. Intracerebral or spinal injections are the most direct methods of delivering drugs to the brain but are invasive and cause great discomfort to patients. Invasive drug delivery is not suitable for depression, which requires long-term treatment. Subsequent increasing studies have shown that intranasal administration can treat brain diseases through the bypassing of BBB, olfactory nerve pathways, trigeminal nerve pathway, and mucosal epithelial pathways, to get the drugs to the CNS [15][16][17][18]. Compared with traditional antidepressant administration routes (oral administration, intravenous administration, intramuscular injection, etc.), intranasal has the advantages of avoiding first-pass effect, improving bioavailability, short onset time, small dose of administration, less toxic and adverse reactions on the body, and good patient compliance [17]. The direct non-invasive pathway between the nose and the brain is undoubtedly one of the best options for the limited antidepressant active ingredients to enter the CNS to exert antidepressant effects. The purpose of this research is to present the unique anatomical and physiological link between the nose–brain pathway and the lesion site of depression. For example, the olfactory system has a high degree of overlap with areas that process emotions and memory functions [19][20]. Furthermore, this research also summarizes studies of antidepressant active ingredients (off-label drugs, peptides and natural active ingredients, etc.), in addition to first-line antidepressants and the delivery carriers in intranasal administration.
2. Unique Advantages of Intranasal Administration in the Treatment of Depression
The nose is the starting part of the respiratory pathway and olfactory organs. The nose is divided into two different compartments by the nasal septum. The surface area of the human nasal mucosa is 150 cm2~160 cm2, the thickness of nasal mucosa is about 2~5 mm, and the average pH value of nasal mucus is 5.5~6.5 [18][21]. The nasal cavity can be divided into three areas according to their structure and function: the nasal vestibule, olfactory region, and respiratory region.
2.1. Direct Pathway
Olfactory sensory neurons (OSNs) are bipolar neurons. Axons from OSNs expressing the same odorant receptors aggregate in LP to form axon bundles that pass through the ethmoid plate and terminate in glomeruli formed within the olfactory bulb (Figure 2) [22].
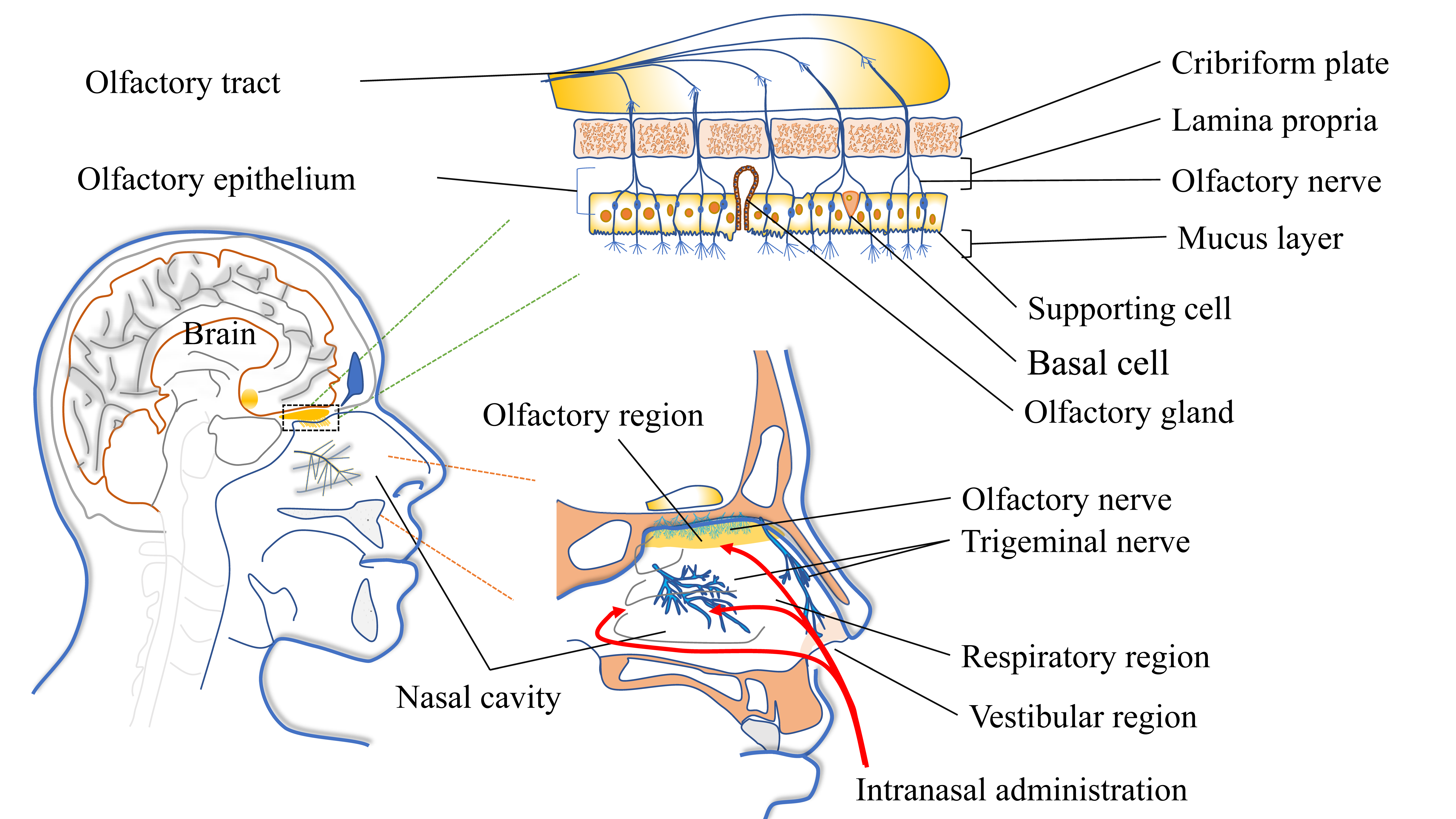
Figure 2. Anatomical schematic of the non-invasive pathway between nose and brain. Reprinted/adapted with permission from Ref. [23]. © 2017 Elsevier Ltd.
The glomerulus of the olfactory bulb is the only transit point between the peripheral and central olfactory systems. OSNs synapse with mitral cells and tuft cells to project secondary and tertiary olfactory structures. Olfactory information is transmitted to secondary olfactory structures, particularly the piriform cortex and amygdala cortex. Tertiary olfactory structures include the thalamus, hypothalamus, amygdala, hippocampus, orbitofrontal cortex, and insular cortex. These regions are closely associated with the expression of mood and memory function in depression [19][20]. Depressed patients are often associated with reduced olfactory bulb volume and olfactory dysfunction, and people with olfactory dysfunction are at higher risk for depression [24][25]. The causal relationship between the olfactory system and depression is unclear, but the correlation between the severity of olfactory disturbance and the severity of depression have been established. Melatonin MT1 and MT2 receptors are expressed in the glomerular layer of the olfactory bulb and coupled to Gi protein. Studies have shown that modulation of melatonin receptors expressed in the olfactory bulb can ameliorate 6-hydroxydopamine-induced depression-like behaviors [26][27]. By intranasal administration, the olfactory bulb can serve as a target for the antidepressant effects of melatonin and melatonin analogs.
Olfactory nerve cilia, located on the surface of the olfactory mucosa, can internalize antidepressants, which are then slowly transported in the axoplasm to synapses within the olfactory bulb that connect with secondary olfactory neurons. Afterward, antidepressants are delivered to the prefrontal cortex, hippocampus, and other parts along the olfactory conduction pathway, possibly by repeating this process. Antidepressants need to be transported through the axons of neurons in the olfactory system at least through the tertiary neurons to reach the hippocampus. There are complex possibilities for interneuronal transfer and axoplasmic transport. Further studies are needed on drug delivery in the perineuronal and intraneuronal spaces and transfer between neurons.
The axons of olfactory neurons are surrounded by a series of olfactory ensheathing cells, and their outer layers are covered by additional neuro fibroblasts (ONFs). The ONFs layer is continuous to the meninges that cover the brain, which means that the perineural space formed between the olfactory sheath cell layer and the neuro fibroblasts are continuous to the subarachnoid space [28]. Antidepressants can enter LP through transcellular or paracellular pathways of the olfactory epithelium, and then along the perineural space into the olfactory bulb and cerebrospinal fluid [30]. Antidepressants need to pass through TJs to reach LP, where OSNs undergo apoptosis and replacement in about 30 to 60 days to protect the brain from airborne contaminants, bacteria, and so on. During this period, a potential delay between lysis and regrowth of OSNs leaves gaps between the tightly connected nasal epithelial cells, resulting in increased permeability [28][29]. In addition, increased permeability of drug absorption is associated with phosphorylation of closed-protein, such as protein kinase signaling or certain substances [30][31]. The characteristic of this pathway is that its effect is faster than that of the olfactory nerve pathway. As the olfactory bulb is located below and anterior to the orbital surface of the frontal lobe of the cerebral hemisphere, antidepressants can be rapidly distributed to the close prefrontal cortex.
The trigeminal nerve is another nerve pathway that connects the nose to the brain, innervating the olfactory and respiratory mucosa [7][32][33]. The trigeminal nerve originates from the pons, where the ocular and maxillary branches supply the nasal cavity. Unlike the axonal bundles of the OSNs, the trigeminal nerve is mainly composed of the myelin sheath made of Schwann cells. The non-myelinated branches of the trigeminal nerve supply olfactory mucosal vessels and regulate blood flow. Trigeminal nerve endings (and their associated arteries) are located below LP and nasal mucosa TJs and do not penetrate the epithelial surface like OSNs [34][35]. Insulin, which has antidepressant activity, was administered intranasally, and fluorescently labeled insulin was found to reach the brain through the extracellular space around the trigeminal nerve [36]. Drugs entering LP may also reach other brain areas, such as the brainstem, through the trigeminal branch [34].
The locus coeruleus and raphe nuclei located in the brainstem are the major sites for the synthesis of norepinephrine (NE) and serotonin (5-HT) in the brain. The locus coeruleus and raphe nuclei have a wide range of neuronal projections and play an important role in regulating neuronal activity in areas such as the prefrontal cortex, amygdala, hippocampus, lateral habenula, and anterior cingulate gyrus related to emotion and memory. Neuronal damage in the locus coeruleus and raphe nucleus is strongly associated with depression, especially MDD. The study found that after intranasal administration of radiolabeled immunoglobulin ([125I]-IgG), the highest signal in the olfactory bulb and brain stem was observed by radiography [37] Pang et al. evaluated the pharmacokinetics of intranasal insulin in the rat brain and showed that the highest levels were in the brainstem of the brain, followed by the olfactory bulb, cerebellum, hippocampus, hypothalamus, and striatum [38]. Exogenous active ingredients may be directly distributed to the brainstem through the trigeminal nerve and its surrounding space to repair damaged neurons.
According to previous studies, the convection in the brain perivascular space driven by arterial pulsation is thought to be the main reason for the rapid and widespread distribution of antidepressant active ingredients after entering the brain from the nasal cavity [39][40]. As the olfactory bulb is located below and anterior to the orbital surface of the frontal lobe, antidepressants can be rapidly distributed to the adjacent prefrontal cortex through the olfactory bulb. The cerebrospinal fluid and the subarachnoid space have many vascular punctures deep into the brain, separated from the brain parenchyma by the pia mater. The tiny spaces between these blood vessel punctures and the pia mater allow for the circulation of cerebrospinal fluid, known as the cerebrospinal fluid microcirculation. Antidepressants are better distributed in the brain through perineural, perivascular, and cerebrospinal fluid flow.
2.2. Indirect Pathway
The olfactory area, about 130 square centimeters, acts to warm and humidify the inhaled air as well as filter particles and pathogenic microorganisms. The mucosa is the stratified or pseudostratified columnar ciliated epithelium, and the cilia mainly move from the front to the back of the nasopharynx. The mucosa is rich in secretory glands and goblet cells, producing a large number of secretions. The mucosal surface is covered with a layer of mucus blanket, which moves backward with the movement of cilia [41]. The abundant capillaries in the respiratory area make this area a nasal cavity, and when administered, antidepressants are absorbed into the system rather than directly into the brain.
The nasal mucosa is in direct contact with the external environment, where many pathogens exist. Nasal-associated lymphoid tissue (NALT) plays a vital role in maintaining the body’s immunity [42]. NALT is located in the LP and submucosal region of the nasal epithelium and connects to the cervical lymph nodes. This is also a potential pathway for the nose-to-brain antidepressant delivery.
Antidepressants can be absorbed by capillaries and lymphatics through the continuous porous endothelium, or enter the blood circulation in the olfactory area through the LP, avoiding first-pass metabolism [43]. Small lipophilic molecules pass more easily. However, the indirectly transported substances still need to pass through the BBB to enter the CNS or brain tissue; so, the substances that enter the brain through the circulation are usually small molecular weight and strong lipophilic substances [44][45].
From the perspective of physiological anatomy and pathological correlation, intranasal administration may have a more direct antidepressant effect than other routes of administration. Results from different studies suggest that intranasal drug delivery may be through a single route or a combination of different routes. Compared with the traditional administration route, intranasal administration can effectively avoid the extensive metabolism and low permeability of the blood–brain barrier caused by the gastrointestinal route. but also avoid the antidepressants in the circulation of the blood as a result of higher plasma protein binding and distribution in the other route, targeting the organization and improve the concentration of antidepressants in the CNS [2][46]. Non-invasive intranasal administration can effectively shorten the onset time and improve compliance in patients with depression requiring long-term treatment [2][47].
3. Challenges of Intranasal Administration
Intranasal administration offers an excellent strategy to overcome the challenge of the complex pathophysiology of brain diseases and drug penetration into the brain. However, the physiological characteristics of the nose, the physicochemical properties of antidepressant active ingredients, and even intranasal drug delivery devices can influence the nose-to-brain delivery of antidepressants [18][48].
TJs of the olfactory and respiratory epithelium and their protective mucus lining act as selective filters, reducing antidepressants diffusion and permeability [49]. Although most lipophilic compounds are more permeable to the nasal mucosa, peptides, macromolecules, and small hydrophilic molecule compounds are generally less permeable [34][50]. Permeation enhancers have been shown to improve the absorption of high molecular weight antidepressants by facilitating the production of hydrophilic pores, and increasing membrane fluidity and permeability of TJs [34][51]. Common Permeation enhancers include cyclodextrin, chitosan (CN), surfactant, Cremophor RH40, saponins, etc. [50][52][53]. There are many cilia on the surface of the olfactory and respiratory areas, which block particles from the external environment from entering the nasal cavity. The olfactory region cilia do not move, and the respiratory region cilia generally toward the pharynx at an average velocity of 5~8 mm per minute; so, the nasal administration of drug particles tends to be cleared in an average time of 20 to 30 min [54]. Inhibitory substances and mucoadhesive materials such as hyaluronan, poloxamer, carbopol, gellan gum, polycarbophil, and other polymers can effectively delay mucociliary clearance and increase the retention time of antidepressants in the nasal cavity, thus increasing drug intake in the brain by interacting with mucin or reversibly/irreversibly inhibiting cilia movement [50].
Although there are fewer enzymes in the nasal cavity, they still may affect antidepressant absorption, such as cytochrome P-450 enzyme system, glutathione S-transferase, proteolytic enzyme, and other “Pseudo-first-pass effect” on foreign substances. Enzyme inhibitors or some surfactants such as bestatin, amastatin, boroleucine, fusidic acids, and phospholipids can be used to avoid the metabolism of the compound, improve stability, and thus increase the absorption of the original drug [34].
Like the BBB, P-gp act as multidrug resistance pumps, expressed in nasal epithelial cells, and P-gp effusion restricts the entry of most substances into the brain. This effect can be modulated by the use of P-gp inhibitors [55]. For example, cyclosporine A and rifampin as P-gp inhibitors improve the permeability of verapamil after intranasal administration [56].
As the breathing area is much larger than the olfactory area, some of the antidepressants that are administered intranasally will still enter the brain via indirect routes. Therefore, studies have shown that the use of direct access can be increased by combining vasoconstrictors to reduce the absorption of antidepressants through the nasal vessels and to increase the retention time of antidepressants in the nasal mucosa [50][57]. Dhuria S. V. et al. administered an intranasal combination of phenylephrine and neuropeptides and measured the concentration of the drug in CNS tissues and blood. The addition of 1% phenyl epinephrine significantly reduced the absorption of HC and D-KTP in the blood and increased the delivery volume of the olfactory bulb [58]. Vasoconstrictors reduce the peripheral side effects of CNS antidepressants by restricting the absorption of nasal blood vessels into systemic circulation.
In addition to the physiological factors mentioned above, the physicochemical properties and formulation composition of antidepressant active ingredients also have an effect on the nose-to-brain delivery. The molecular weight of the drug is inversely proportional to the percentage absorbed. Lipophilic ingredients are more readily absorbed than hydrophilic ones. Lipophilic small molecule ingredients with a molecular weight of less than 1 kDa can be transported quickly. The absorption of ingredients of less than 300 Da was almost unaffected by molecular weight, while the absorption of antidepressants with molecular weight between 300 Da and 1 kDa was inversely correlated with molecular weight [59]. Currently, the most widely used and marketed antidepressants, such as Escitalopram, Fluoxetine, Duloxetine, and Venlafaxine, have molecular weights ranging from 200 to 400 Da [60][61]. The pH value of the formulation not only ensures the stability of the drug itself but also ensures the stability of the physiological conditions of the nasal cavity. The nasal pH range is 5.0~6.8, and intranasal administration should be close to this to avoid irritation of the nasal mucosa. The drug is always absorbed in a non-ionized state; so, the pKa of the drug should also be taken into account in determining the pH of the formulation [62]. However, changes in temperature, humidity, and some pathological conditions can cause changes in nasal pH [63]. In addition, the prescribed osmotic pressure should also be adapted to the physiological conditions of the nasal cavity; otherwise, it will affect the normal nasal mucosal cell morphology and ciliary movement and further affect the absorption of antidepressants [31][64].
The choice of drug delivery equipment also plays a very important role in whether the drug formulation can be utilized to the maximum extent and play its due therapeutic effect [34][48][65]. Treatment of CNS diseases such as depression requires antidepressants to be delivered to the olfactory region. Traditional pump sprays usually deposit antidepressants in the anterior nasal cavity, which are removed quickly by clearance of the nasal mucosa. The droppers may deposit the olfactory area above the nasal cavity better than nasal pump sprays, but this often requires the patient to lie on his or her back in a head-down, forward–forward position and even professional administration techniques, which can be very inconvenient [66]. Thus, the research and development of more convenient and efficient devices is one of the focuses of current research.
4. The Delivery Carriers and Nanocarriers for Intranasal Administration
4.1. Polymer-Based Carriers
The physiological characteristics of the nose–brain pathway and the physicochemical properties of ingredients lead to a certain degree of limitation for intranasal administration [67][68]. So far, many scholars have conducted studies on the nose-to-brain delivery system to improve the safety and effectiveness of ingredients, alongside the development in polymer technology and pharmaceutical technology. The delivery carriers and nanocarriers have made significant contributions to protecting antidepressants from protein degradation, enhancing olfactory mucosal uptake and CNS utilization and prolonging half-life (Figure 3).
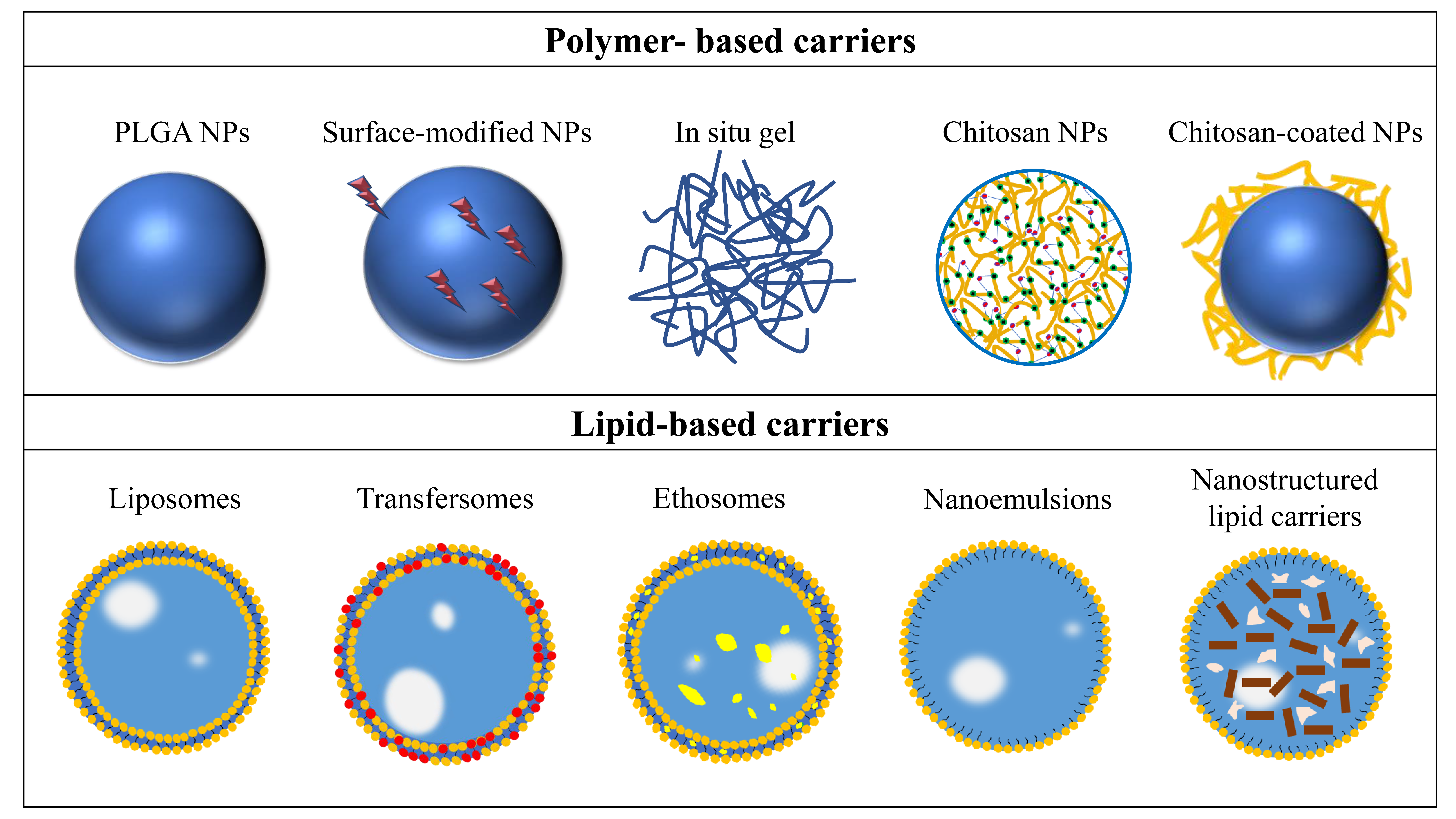
Figure 3. Carriers for intranasal administration of antidepressant active ingredients. Reprinted/adapted with permission from Ref. [24]. © 2017 Elsevier Ltd.
In situ gel refers to a kind of preparation that is transformed from liquid to non-chemical cross-linked semi-solid gel immediately after administration in solution state due to physiological conditions of the administration site or some stimulus factors in the external environment (pH, temperature, ionic strength, etc.) [69][70]. Compared with the traditional drug delivery system, in situ gels prepared with a variety of different polymers or containing different stimulus-induced release factors show good biocompatibility when exposed to the site of administration for a longer time to prolong the retention time of formulation and improve the bioavailability of antidepressants [71][72][73]. The combination of nanocarriers with in situ gels is also a promising strategy.
Poly (lactic-co-glycolic acid) (PLGA), which is approved for therapeutic applications by the U.S. Food and Drug Administration (FDA), is considered one of the most promising synthesized polymers as a drug delivery system due to its biodegradability, biocompatibility, controllable properties, well-defined formulation techniques, and great potential for targeting [31][74][75]. PLGA is a polymer synthesized by the interaction of glycolic acid and lactic acid monomer.
Chitosan nanoparticles (CN-NPs) not only can open TJs between cells transiently but also reduce the mucociliary clearance, prolonging the retention time of the compound as a biological adhesive material, which enhances the delivery of antidepressants [76][77]. CN is a natural linear polysaccharide cationic and hydrophilic polymer by alkaline hydrolysis of chitin, which is the second most abundant biopolymer in nature. It is an important component of the shells of many lower animals, especially arthropods such as shrimp, crabs, and insects. It also exists in the cell walls of lower plants such as bacteria, algae, and fungi. CN consists of randomly distributed β-(1, 4)-linked d-glucosamine (deacetylated) and N-acetyl-d-glucosamine (acetylated) units. Due to its properties of adhesivity, biocompatibility, and biodegradability, CN has been well used in the field of medical engineering [78].
Alginate nanoparticles have also been studied and reported by many researchers in nose-to-brain drug delivery [79][80]. Alginate is extracted from brown Marine algae and then processed several times before it can be used as a polymer in pharmaceutical preparations. Alginates are mainly composed of β-D-mannuronic acid and α-L-guluronic acid, and have properties such as biocompatibility, biodegradability, low toxicity, and pH sensitivity [81][82]. Alginate contains a large number of carboxyl groups and is a hydrophilic anionic polymer, showing a certain adhesion. Divalent cations, such as Ca2+, exchange ions with cations on α-L-guluronic to form a cross-linked network structure, thus forming alginate hydrogels [83].
Nanoemulsion (NE) is a liquid nano-dispersion system formed by two kinds of insoluble liquid stabilized by surfactant and co-surfactant (O/W; W/O). NEs have been widely used in nasal and brain delivery of insoluble antidepressants due to excellent solubility, thermodynamic stability, and easy preparation [84][85][86]. CN is often added to NEs to reduce clarity and prolong the retention time of antidepressants in the nasal cavity [84][87].
4.2. Lipid-Based Carriers
Compared with polymer carriers, lipid carriers have better biocompatibility than synthetic polymers because the materials of lipid carriers are basically derived from natural materials [88]. The degradation of polymer carriers in vivo is often accompanied by an increase in toxicity, while the degradation products of lipid carriers have low immunogenicity. Lipid carriers can effectively avoid being cleared by the immune system in the body and achieve long cycles. Liposome is a kind of structure similar to the cell membrane, mainly composed of phospholipids’ double-layer synthetic membrane. In water-soluble solvents, the hydrophobic tails are clustered close to each other inside the bilayer phospholipids, while the hydrophilic heads are exposed outward to the aqueous phase, forming vesicles with bilayer molecular structure [89]. Liposomes can effectively protect the stability of encapsulated antidepressants, reduce drug toxicity, and play a slow release, prolonging the action time of antidepressants [90]. In addition, some studies have used altered phospholipid vesicles, such as transferosomes, ethosomes, and phospholipid magnesomes, for intranasal administration. Adding glycerol and ethanol makes the phospholipid vesicles softer, enhancing the permeability to the nose–brain pathway [91][92][93].
It is noteworthy that due to the traditional lipid carrier containing unsaturated chains, it is easy to fuse, oxidize, and hydrolyze as a solution form; so, the half-life of classic liposomes is short and the stability is poor. In order to overcome the defects of liposome preparations, new lipid-based forms for drug delivery have been gradually developed. For instance, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) have been widely used in drug delivery [94][95]. SLNs are nanoparticles made using one or more lipids—such as triglycerides, lecithin, fatty acids, etc.—as carrier materials and combined with surfactants as stabilizers to form solid nanoparticles at body temperature and room temperature. Compared with traditional liposomes, SLNs have lower cytotoxicity, higher stability, and bioavailability and can more effectively maintain drug stability and control drug release, as well as mature the industrial production process [89][94][95][96][97]. Although SLNs have many advantages as nanoscale drug carriers, its disadvantages should not be ignored. For example, SLNs still have the problem of gelation in the dispersed phase and expulsion of encapsulated antidepressants resulting from β-modification during storage, and low encapsulation rates and drug loads due to the fact that cavities are not allowed to occur within the lipoid nucleus during crystallization [97]. NLCs are the second generation of lipid nanomaterials. Different from SLNs, NLCs are composed of liquid lipids and solid lipids combined with surfactants as stabilizer to form nanoparticles that are also solid at room temperature and body temperature. NLCs significantly enhance drug loading due to the existence of liquid lipids, imperfect crystal order and loose structure of nanoparticles, and reduced drug leakage caused by β-modification during storage. The combined use of different carriers, or the functional modification of classical carriers, has aroused extensive interest in facilitating intranasal drug delivery and has promising prospects.
5. Intranasal Administration of Antidepressant Active Ingredients
Under the action of different factors, the incidence of depression in today’s society is becoming more and more serious. Drug treatment for depression is mainly oral administration, which is challenged by extensive first-pass metabolism, the BBB, and systemic side effects. Some antidepressant active ingredients, such as peptides, natural active ingredients, etc., cannot achieve their high brain bioavailability due to the limitations of their own physical and chemical properties. The direct pathway between the nasal cavity and the brain provides a reliable guarantee for improving the bioavailability of antidepressant active ingredients and reducing side effects. As the olfactory system highly overlaps with areas that process emotion and memory functions, intranasal administration (especially to the olfactory area) may be a potential route for treating depression. Antidepressant active ingredients can enter the brain directly through the olfactory sensory nerve, trigeminal nerve, and olfactory mucosal epithelial pathways, or indirectly through the rich capillaries in the respiratory area and lymphatic tissue (Table 1).
Table 1. Summary of studies on antidepressant active ingredients and new dosage forms for intranasal administration.
|
Types |
Ingredients |
Dosage Form |
Characterization |
Ex Vivo/In Vivo Studies |
Relevant Outcomes |
Ref. |
|
Antidepressants |
Venlafaxine |
Poly lactic-co-glycolic acid nanoparticles (PLGA-NPs); Peptide-modified nanoparticles |
PS = 206.3 ± 3.7 nm; PI = 0.041 ± 0.017; ZP = −26.5 ± 0.5 mV; around 200 nm after lyophilization process; DL = 10–12%; EE = 48–50%. |
In vitro cell viability and cellular uptake (hCMEC/D3 cells); Permeability assay and transport studies; Biodistribution studies (C57/bl6 mice). |
Cell viability of h-CMEC/D3 cells is more than 85% in the MTT assay. In vivo biodistribution studies showed higher concentrations of plain fluorescent NPs than functionalized NPs in the brain after 30 min of administration. |
[98] |
|
Chitosan nanoparticles (CN-NPs) |
PS = 167 ± 6.5 nm; PI = 0.367 ± 0.045; ZP = +23.83 ± 1.76 mV; DL = 32.25 ± 1.63%; EE = 79.3 ± 2.6%; Yield = 71.42 ± 3.24%. |
Ex vivo permeation studies using porcine nasal mucosal membrane (Franz cells); Pharmacodynamic studies in Wistar rats (modified forced swim test, locomotor activity test); Qualitative localization and biodistribution studies by confocal laser scanning microscopy; Pharmacokinetic analysis. |
The cumulative drug permeability after 24 h in VLF CN-NPs was nearly 3 times compared with VLF solution. VLF CN-NPs showed a more significant antidepressant effect than VLF solution on chronic depression rats by forced swimming method. DTE (%)/DTP (%): NPs = 508.59/80.34 |
[99] |
||
|
Desvenlafaxine |
Chitosan-coated poly lactic-co-glycolic acid nanoparticles (PLGA-CN-NPs) |
PS = 172.5 ± 10.2 nm; PI = 0.254 ± 0.02; ZP = +35.63 ± 8.25 mV; DL = 30.8 ± 3.1%; EE = 76.4 ± 4.2%; Release (24 h) = 77.21 ± 3.87% (pH 7.4) and 76.32 ± 3.54% (pH 6.0). |
Ex vivo permeation studies on porcine nasal mucosa; Pharmacodynamic studies (Wistar rats); Stress-induced model (forced swimming test); Drug-induced model (reserpine reversal test); Biochemical estimation of serotonin, Blood and brain pharmacokinetic studies. |
In a rodent model of depression, compared with intranasal DVF solution and oral administration, increased levels of 5-HT and NE in the brain showed a more pronounced antidepressant effect. Pharmacokinetic parameters such as concentration, half-life, and AUC in the brain after intranasal administration were higher than those of through intravenous. DTE (%)/ DTP (%) = 544.23/81.62 (DVF-NP s) DTE (%)/ DTP (%) = 202.41/50.59 (DVF) |
[100] |
|
|
Agomelatine |
Nanoemulsion thermosensitive in situ gel + 0.5% Chitosan |
Gelling point = 28 ± 1 °C; Mucoadhesive strength = 6246.27 dynes/cm2; NEs: PS = 206.3 ± 3.7 nm; Micelles of P-407: PS = 142.58 ± 4.21 nm; Ago-NE-gel + 0.5%chitosan: Viscosity = 2439 ± 23 cP (35 ± 1 °C); pH = 5.8 ± 0.2. |
In vitro gel erosion study; Ex vivo drug permeation through the bovine nasal mucosa; Nasal toxicity study; Pharmacokinetic analysis: DTE (%) and DTP (%); Pharmacodynamic studies (Behavioral test; modified forced swim test and tail suspension test). |
Pharmacokinetic study in Wistar rats showed plasma concentration in the brain was 2.82 times higher than that of the intravenous suspension via the intranasal route. DTE (%)/DTP (%) = 344.9/71.0 |
[101] |
|
|
Solid lipid nanoparticles (SLNs) |
PS = 167.70 ± 0.42 nm; PI = 0.12 ± 0.10; ZP = −17.90 ± 2.70 mV; EE = 91.25 ± 1.70%; Release (1 h)/(8 h) = 35.40 ± 1.13%/80.87 ± 5.16%. |
Pharmacokinetic study (rats): assay of agM in plasma and brain; Pharmacokinetic analysis: DTE (%) and DTP (%). |
The nasal solid lipid nanoparticles prepared by Ahmed et al. were superior to the oral suspension in brain concentration, AUC 0–360 min, and absolute bioavailability (44.44%) DTE (%)/DTP (%) = 190.02/47.37 |
[102] |
||
|
Duloxetine |
Nanostructured lipid carriers (NLCs) |
PS = 137.2 ± 2.88 nm; ZP = -31.53 ± 11.21 mV; DL = 9.73 ± 3.22%; EE = 79.15 ± 4.17%. |
Biodistribution studies (Wistar rats); Pharmacokinetic study; Gamma-imaging study. |
Intranasal DLX-NLCs showed higher concentrations in blood and brain compared with DLX solution and oral route, which showed the same results in behavioral tests in mice. Intranasal NLCs were 8-fold higher in brain concentrations than intravenous DLX. DTE (%)/DTP (%) = 757.74/86.80 (DLX-NLC) DTE (%)/DTP (%) = 287.34/65.12 (DLX) |
||
|
Thiomer gel loaded with proniosomes |
20% w/v PF127, 5% w/v PF68; 3.76 lipid ratio; PS = 265.13 ± 9.85 nm; GT = 32 ± 0.05 °C; EE = 98.13 ± 0.50%; Release (3 h) = 33%. |
Pharmacokinetic analysis: DTE (%) and DTP (%); Stability study. |
Thiomer gel loaded with duloxetine proniosomes increased the retention time and sustained release and penetration of DLX in the nasal mucosa (1.96 times that of duloxetine proniosomes). DTE (%)/DTP (%) = 137.77/10.5 |
[105] |
||
|
Paroxetine |
Nanoemulsion (NEs) |
PS = 58.47 ± 3.02 nm; PDI = 0.339 ± 0.007; ZP = −33 mV; Transmittance = 100.60 ± 0.577%; Refractive index = 1.412 ± 0.003. |
Ex vivo permeation studies using porcine nasal mucosal membrane (Franz cells); Pharmacodynamic studies (Wistar rats; forced swimming test, locomotor activity test); Biochemical estimation: GSH and TBARS. |
The permeability of paroxetine NEs was 2.57 times higher than that of its suspension via permeation studies. Results of behavioral studies in rats showed that intranasal administration of paroxetine NEs significantly improved behavioral activity in depressed rats compared with the oral suspension of paroxetine. |
[106] |
|
|
Trazodone |
Microemulsion |
labelling yield = 91.23 ± 2.12%; In vitro stability of 131I-TZ = 6 h; Droplet size = 16.4 ± 2.5 nm; PDI = 0.11 ± 0.02; ZP = 3.83 ± 0.36; Viscosity (25 °C) = 261.7 ± 3.0; Viscosity (37 °C) = 157.3 ± 7.5. |
Biodistribution of 131I-TZ; The 131I-TZ uptake in organs and body fluids. |
Sayyed et al. radiolabeled trazodone and compared the pharmacokinetic parameters of intranasal delivery of 131I-TZ solution, 131I-TZ microemulsion, and intravenous injection of 131I-TZ solution. Intranasal 131I-TZ microemulsion had sustained and higher brain uptake at any time tested than the other two formulations and routes. In addition, the blood exposure of intranasal 131I-TZ microemulsion was lower than that of intravenous injection, reducing systemic toxicity. |
[107] |
|
|
Quetiapine fumarate |
Microemulsion Chitosan microemulsion (CH-ME) methyl-β-cyclodextrin microemulsion (MeβCD-ME) |
PS: QF-ME = 29.75 ± 0.99 nm; CH-ME = 35.31 ± 1.71 nm; MeβCD-ME = 46.55 ± 1.9 nm with; PDI: QF-ME = 0.221 ± 0.01; CH-ME = 0.249 ± 0.03; MeβCD-ME = 0.233 ± 0.02; ZP: QF-ME = 2.77 ± 0.51; CH-ME = 20.29 ± 1.23 MeβCD-ME = 8.43 ± 0.7; Viscosity: QF-ME = 17.5 ± 0.69 cP; CH-ME = 38.5 ± 1.26 cP; MeβCD-ME = 33.3 ± 0.93 cP. |
Ex vivo mucoadhesive strength; Ex vivo nasal and intestinal diffusion study (goat nasal mucosa and small intestine); Nasal mucosal toxicity test; Pharmacokinetic analysis: DTE (%) and DTP (%). |
The brain bioavailability of quetiapine fumarate of chitosan-coated microemulsion was 3.8-fold and 2.7-fold higher than that of drug solution and chitosan-free microemulsion, respectively. DTE (%)/DTP (%) = 371.20 ± 12.02/ 68.66 ± 6.84 (QF-ME) DTE (%)/DTP (%) = 453.69 ± 10.17/80.51 ± 6.46 (CH-ME) |
[108] |
|
|
Doxepin hydrochloride |
Thermoreversible biogels |
Gelation temperature = 37.4 °C; Gelation time = 7.32 min pH = 6.93. |
In vitro penetration test on sheep nasal mucosa; Stress-induced model (forced swimming test). |
Compared with doxepin hydrochloride solution, the thermoreversible biogel showed more advantages in immobility time and swimming activity count in mice after 13 days of drug administration. |
[109] |
|
|
Off-label drugs |
Ketamine/Esketamine |
Nasal spray |
N/A |
N/A |
Ketamine, whether administered intravenously or intranasally, has a higher bioavailability than the oral route, and has a more rapid and significant effect than traditional antidepressants with delayed onset of action. Due to the plasma elimination half-life of ketamine of 2–4 h and the discomfort associated with invasive administration, delivery of ketamine directly to the brain via the nasal cavity is a more advantageous strategy. |
|
|
Amisulpride |
Lipid-based poloxamer-gellan gum nanoemulgel AMS nanoemulsion (AMS-NE) AMS in situ nanoemulgel (AMS-NG) |
AMS-NE: PS = 92.15 ± 0.42 nm; PI = 0.46 ± 0.03; ZP = −18.22 mV; Transmittance = 99.57%; Mucoadhesive strength = 1.24 g; Release (4 h) = 99.99%; AMS-NG: PS = 106.11 ± 0.26 nm; PI = 0.51 ± 0.01; ZP = −16.01 mV; Transmittance = 98.47%; Mucoadhesive strength = 8.90 g; Release (4 h) = 98.96%. |
Ex vivo drug permeation study on freshly isolated sheep nasal mucosa; In vivo animal experiments (pharmacokinetic study, AMS in brain and blood plasma samples); Animal behavioral studies (induced locomotor activity test, paw test); In vivo safety assessment. |
Pharmacokinetic studies in Wister rats showed that the intranasal C(max) of the brain was 3.39 times higher than that of the intravenous administration and intranasal administration within one month did not affect blood leukocyte and granulocyte counts. DTE (%)/DTP (%) = 314.08/76.13 (AMS-NE) DTE (%)/DTP (%) = 1821.72/275.09 (AMS-NG) |
[112] |
|
|
Aripiprazole |
Mucoadhesive nanoemulsion |
PS = 121.8 ± 1.5 nm; PI = 0.248 ± 0.05; ZP = −18.89 ± 3.47 mV; Viscosity = 187.79 ± 5.35 cP (25% Carbopol); Viscosity = 626.32 ± 8.63 cP (1% Carbopol); Release (8 h) = 84.92%. |
Ex vivo permeation test and nasal ciliotoxicity on sheep nasal mucosa; In vitro cytotoxicity study (Vero cells, PC12 cells); In vivo pharmacokinetic study (DTE (%) and DTP (%)); Locomotor activity study. |
Pharmacokinetic studies with single-dose administration showed that the plasma concentration in the brain of intranasal ARP-MNE was 1.44 and 6.03 times higher than that of intranasal and intravenous ARP-NE, respectively, and the Tmax was smaller than that of intravenously administered ARP-NE. DTE (%)/ DTP (%) = 96.90/89.73 |
[113] |
|
|
Poly(caprolactone) nanoparticles |
PS = 199.2 ± 5.65 nm; ZP = -21.4 ± 4.6 mV; EE = 69.2 ± 2.34%; Release (8 h) = 90 ± 2.69%. |
Ex vivo diffusion studies on goat nasal mucosa; Nasal toxicity study (goat nasal mucosa); In vivo pharmacokinetics study (DTE (%) and DTP (%)). |
The AUC 0–8 h of Aripiprazole in the rat brain administered by the intranasal route of APNPs was approximately twice that of the intravenous route. DTE (%)/DTP (%) = 64.11/74.34 |
[114] |
||
|
Selegiline |
Chitosan nanoparticle |
PS = 341.6 ± 56.91 nm; PI = 0.317 ± 0.29; ZP = −13.4 ± 0.04 mV; EE = 92.20 ± 7.15%; Release (8 h) = 90 ± 2.69%. |
Ex vivo drug diffusion on sheep nasal mucosa; Pharmacokinetics and pharmacodynamics studies; Behavioral testing; Biochemical analyses: dopamine level, catalase activity, reduced glutathione (GSH) content. |
The Cmax of plain solution of selegiline in the brain and plasma by intranasal administration (Tmax = 5 min) was 20 and 12 times higher, respectively, compared with oral administration (Tmax = 15 min). Furthermore, intranasal administration of selegiline-loaded CN-NPs and mucoadhesive thermosensitive gel showed superior formulation advantages compared with the AUC 0–24 h of plain solution. |
||
|
Peptides |
Insulin |
N/A |
N/A |
Pharmacokinetics study (insulin concentrations in brain and plasma via different delivery routes); AUC brain: plasma ratio; Repeated in insulin administration. |
The study found intranasal delivery of insulin showed a 2000-fold increased AUC brain: plasma ratio compared with subcutaneous administration, with no apparent effect on blood glucose levels. |
[117] |
|
Lixisenatide |
N/A |
N/A |
Chronic unpredictable mild stress depression model (rats); Behavioral studies (forced swim test, tail suspension test, open field test); Cells were labeled with BrdU and neurogenesis in the olfactory bulb and hippocampus was observed. |
Intranasal lixisenatide not only improved depressive and anxious behaviors in a chronic unpredictable mild stress model, but also improved olfactory system function. In addition, intranasal lixisenatide was demonstrated to play an antidepressant role by regulating cyclic-AMP response binding protein (CREB)-mediated neurogenesis. |
[118] |
|
|
GLP-2 |
PAS-CPPs-GLP-2 |
N/A |
Behavioral studies (forced swim test, tail suspension test, open field test); Distribution test (rats’ brain). |
Studies have found that intranasal PAS-CPP-GLP-2 exhibited antidepressant effects similar to intracerebroventricular injection in mouse models, but not intravenous injection. |
||
|
BDNF |
BDNF-HA2TAT/AAV |
Each step was qualified by specific restriction enzyme reactions and AGE; High expression of BDNF in infected Hela cells. |
Chronic unpredictable mild stress depression model (rats); Behavioral assessment (forced swim test, sucrose preference test, open field test); Body weight; Western-blotting analysis; Expression of BDNF mRNA. |
Western-blotting analysis showed that the content of BDNF in the hippocampus increased via intranasal administration. Compared with the control group and the AVV group, the BDNF-HA2TAT/AAV group significantly reversed the depressive behavior of the rats. |
||
|
NAP |
NT4-NAP/AAV |
Each step was qualified by specific restriction enzyme reactions and AGE; Expression of BDNF in infected PC12 cells. |
Behavioral assessment (forced swim test, sucrose preference test, open field test); Effect on plasma CORT; Expression of 5-HT and BNDF in hippocampus. |
Experiments have shown that the depressive symptoms of female mice are improved after ten days of administration. Although the effect is not significant, it also proves that intranasal administration from different targets, such as microtubules, provide new ideas for the treatment of depression. |
||
|
NPY/LCG-17/MCH/CST-14/NGF |
N/A |
N/A |
Behavioral assessment (forced swim test, sucrose preference test, open field test); Biochemical studies. |
These peptides bypass the blood–brain barrier via a non-invasive intranasal route of administration, improving bioavailability and brain targeting. The peptides both improve anxiety and depression behavior in animal models. The peptides also promote neuroplasticity in the central nervous system, especially the hippocampus and prefrontal cortex. |
||
|
Natural active ingredients |
Albiflorin |
Alginate nanogels |
PS = 45.6 ± 5.2 nm; PI < 0.20; ZP = −19.8 ± 0.9 mV; EE = ±7.15%; Release (12 h) = 99%; Gelling temperature = 28 °C. |
In vivo fluorescence distribution analysis of alginate nanogels (rats); Pharmacodynamic study; Antidepressant behavioral studies: tail suspension test; Transcriptome studies: cAMP, calcium ion, and cGMP PKG signal pathway. |
Fluorescent labeling showed that albiflorin could quickly reach the brain for distribution after intranasal administration (≤30 min). The authors observed through tail suspension experiments in mice that low-dose intranasal administration significantly shortened the chronic unpredictable mild stress model of mice compared with intragastric gavage and intravenous injection of albiflorin solution. Do not move time. The reduction of pro-inflammatory cytokine levels and the repair of neuronal damage in CUMS rats further suggest that albiflorin has an excellent potential for rapid antidepressant effects. |
[128] |
|
Berberine |
Cyclodextrin + thermosensitive hydrogel |
The berberine /HP-β-CD inclusion complex (1H-NMR-NMR showed good degree of inclusion); Gelling temperature = 30 °C; Release (6 h) = 83.29 ± 3.98%; Loading efficiency = 22.86%. |
Brain targeting of berberine study (Radioactive tracer of 125I); Pharmacokinetic analysis: berberine in hippocampus; Monoamine neurotransmitters in rats (reserpine-induced model). |
The relative intracerebral bioavailability of berberine showed that the intranasal formulation of berberine was 110 times higher than the oral inclusion complex of berberine–cyclodextrin. Pharmacological studies have found that the intranasal route, in addition to increasing the levels of monoamine neurotransmitters in the hippocampus compared with oral administration, exhibits a potential antidepressant mechanism by restoring sphingolipid and phospholipid abnormalities and mitochondrial dysfunction. |
[129] |
|
|
Berberine and Evodiamine |
Thermosensitive in situ hydrogels |
P407/P188/HP-β-CD/PEG 8000 = 20/0/8/1; Release = 93% (berberine); Release = 43% (evodiamine); Gelling temperature = 28 °C. |
Pharmacokinetic study (plasma and hippocampus); Antidepressant behavioral studies (open field test, tail suspension test); Monoamine neurotransmitters studies in rats. |
The bioavailability of intranasal hydrogels was more than 135- and 112-fold higher than that of gavage berberine and evodiamine solutions. The intranasal formulation significantly improved behavioral despair by modulating monoamine levels and related metabolic pathways in mice. |
[130] |
|
|
Cang-ai volatile oil |
Intranasal inhaler |
N/A |
Chronic unpredictable mild stress depression model (rats); Behavioral studies (open field test, forced swim test, and sucrose preference test); Expression of pro-inflammatory cytokines and monoamine neurotransmitters studies in prefrontal cortex. |
Studies have shown that Cang-ai volatile oil can inhibit microglia activation and kynurenine pathway to regulate 5-HT and play an antidepressant effect. The forced swim test, open field test, sucrose preference test, etc. confirmed that intranasal delivery of Cang-ai volatile oil can effectively regulate the metabolism of dopamine and 5-HT in the brain of CUMS rats and improve depressive behavior. |
||
|
Icariin |
Nanogel loaded thermosensitive hydrogel (NGSTH) |
PS = 73.80 ± 2.34 nm; PI < 0.15; ZP = −19.2 ± 1.14 mV; Loading efficiency = 2.03%; Release (36 h) = 70% (nanogel); Gelling temperature = 30 °C; Release (36 h) = 100% (NGSTH). |
In vivo distribution fluorescently labeled nanogels Behavioral testing (tail suspension test, forced swim test); Expression of pro-inflammatory cytokines and morphological changes in the hippocampus. |
ICA-NGSTH could be distributed in the brain in about half an hour and showed zero order kinetic release within 10 h. By comparing the oral route of ICA, intranasal ICA-NGSTH showed better behavior improvement ability in an animal model of depression. |
[133] |
|
|
White tea |
N/A |
N/A |
Chronic unpredictable mild stress depression model (rats); Behavioral testing (open-field test, sucrose preference test, buried food pellet test); Olfactory sensitivity test. |
High and low levels of white tea extracts could effectively reverse depressive behavior in mice. Olfactory avoidance tests and olfactory sensitivity tests showed its relief of olfactory dysfunction. Pharmacological studies found that white tea reduced mitochondrial and synaptic damage in the olfactory bulb and enhanced the content of BDNF. |
[134] |
Abbreviations: PS, globule size; ZP, zeta potential; PI, polydispersity index; DTE (%), drug targeting efficiency; DTP (%), nose-to-brain direct transport percentage; BDNF, brain-derived neurotrophic factor; CORT, corticosterone; P407, poloxamer 407; P188, poloxamer 188.
6. Summary and Outlook
With the continuous progress of medical technology and the pharmaceutical engineering industry, remarkable research achievements have been made in how to make more effective use of nose-to-brain drug delivery, but there are still some problems to be solved. The small size of the nasal space and olfactory area limits the amount of drug that needs to be delivered directly to the brain. Moreover, factors such as the close connection of olfactory epithelial cells, the clearance of nasal mucosa cilia and the degradation of antidepressant active ingredients by various enzymes all make fewer parts to be absorbed; so, the method of enhancing absorption is very important. At present, some remarkable achievements have been made in the research of promoting nasal mucous absorption. For example, some materials with good biocompatibility, biodegradation, and low toxicity, such as permeability enhancers, adhesives, enzyme inhibitors, and nanoparticles, have been well applied in promoting nose-to-brain delivery. Solvent enhancers, antioxidants, preservatives, moisturizers, buffers, and taste maskers are added to the formulation to ensure drug stability and patient compliance. However, the treatment of depression is often long-term, and these absorption-based materials can more or less cause irritation and side effects to the nasal cavity and other tissues and organs. On the other hand, the invention of some nasal delivery devices, such as Vianase™, has significantly improved the deposition of antidepressant active ingredients in the olfactory region of the upper nasal cavity compared with traditional delivery devices. However, the deposition rate in the olfactory area is still relatively low; the highest is only about 50%. Therefore, there is an urgent need to find better excipients and new devices for enhancing drug delivery in the nose and brain.
Current studies on intranasal administration of antidepressant active ingredients are mostly limited to the cellular level and model animal level. In addition, different stimulation methods may also lead to individual differences in the model. There are differences between the structure and physiological conditions of the human nasal cavity and experimental animals; thus, it is not effective in an animal model but in the human body. To further verify the drug, a clinical observation test is needed to evaluate its safety and effectiveness. In addition to intranasal administration, the development of new routes of administration, such as transdermal targeted administration, to overcome the disadvantages of oral and injectable administration is also one of the main exploration directions to improve the efficacy of antidepressant treatment in the future.
References
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2017, 48, 1560–1571. https://doi.org/10.1017/s0033291717003336.
- Panek, M.; Kawalec, P.; Pilc, A.; Lasoń, W. Developments in the discovery and design of intranasal antidepressants. Expert Opin. Drug Discov. 2020, 15, 1145–1164. https://doi.org/10.1080/17460441.2020.1776697.
- Depression and Other Common Mental Disorders: Global Health Estimates. Available online: https://www.who.int/publications/i/item/depression-global-health-estimates (accessed on 17 September 2022).
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. https://doi.org/10.1016/s0928-0987(00)00087-7.
- Kashyap, K.; Shukla, R. Drug Delivery and Targeting to the Brain Through Nasal Route: Mechanisms, Applications and Challenges. Curr. Drug Deliv. 2019, 16, 887–901. https://doi.org/10.2174/1567201816666191029122740.
- Mato, Y.L. Nasal route for vaccine and drug delivery: Features and current opportunities. Int. J. Pharm. 2019, 572, 118813. https://doi.org/10.1016/j.ijpharm.2019.118813.
- Giunchedi, P.; Gavini, E.; Bonferoni, M.C. Nose-to-brain delivery. Pharmaceutics 2020, 12, 138.
- O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Faster, better, stronger: Towards new antidepressant therapeutic strategies. Eur. J. Pharmacol. 2015, 753, 32–50. https://doi.org/10.1016/j.ejphar.2014.07.046.
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975.
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412.
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. https://doi.org/10.1016/j.nbd.2009.07.030.
- Zheng, Y.; Chen, X.; Benet, L.Z. Reliability of In Vitro and In Vivo Methods for Predicting the Effect of P-Glycoprotein on the Delivery of Antidepressants to the Brain. Clin. Pharmacokinet. 2015, 55, 143–167. https://doi.org/10.1007/s40262-015-0310-2.
- O'Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: Clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2011, 165, 289–312. https://doi.org/10.1111/j.1476-5381.2011.01557.x.
- Brückl, T.M.; Uhr, M. ABCB1 genotyping in the treatment of depression. Pharmacogenomics 2016, 17, 2039–2069. https://doi.org/10.2217/pgs.16.18.
- Bicker, J.; Fortuna, A.; Alves, G.; Falcão, A. Nose-to-brain delivery of natural compounds for the treatment of central nervous system disorders Curr. Pharm. Des. 2020, 26, 594–619.
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. https://doi.org/10.1016/j.phrs.2020.104795.
- Shringarpure, M.; Gharat, S.; Momin, M.; Omri, A. Management of epileptic disorders using nanotechnology-based strategies for nose-to-brain drug delivery. Expert Opin. Drug Deliv. 2020, 18, 169–185. https://doi.org/10.1080/17425247.2021.1823965.
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601.
- Kim, B.-Y.; Bae, J.H. Olfactory Function and Depression: A Meta-Analysis. Ear Nose Throat J. 2022. https://doi.org/10.1177/01455613211056553.
- Staszelis, A.; Mofleh, R.; Kocsis, B. The effect of ketamine on delta-range coupling between prefrontal cortex and hippocampus supported by respiratory rhythmic input from the olfactory bulb. Brain Res. 2022, 1791, 147996. https://doi.org/10.1016/j.brainres.2022.147996.
- Schwartz, J.S.; Tajudeen, B.A.; Kennedy, D.W. Diseases of the nasal cavity. Handb. Clin. Neurol. 2019, 164, 285–302. https://doi.org/10.1016/b978-0-444-63855-7.00018-6.
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. https://doi.org/10.1002/jps.21924.
- Samaridou, E.; Alonso, M.J. Nose-to-brain peptide delivery—The potential of nanotechnology. Bioorganic Med. Chem. 2018, 26, 2888–2905. https://doi.org/10.1016/j.bmc.2017.11.001.
- Rottstädt, F.; Han, P.; Weidner, K.; Schellong, J.; Wolff-Stephan, S.; Strauß, T.; Kitzler, H.; Hummel, T.; Croy, I. Reduced olfactory bulb volume in depression-A structural moderator analysis. Hum. Brain Mapp. 2018, 39, 2573–2582. https://doi.org/10.1002/hbm.24024.
- Rottstaedt, F.; Weidner, K.; Strauß, T.; Schellong, J.; Kitzler, H.; Wolff-Stephan, S.; Hummel, T.; Croy, I. Size matters—The olfactory bulb as a marker for depression. J. Affect. Disord. 2017, 229, 193–198. https://doi.org/10.1016/j.jad.2017.12.047.
- Cecon, E.; Ivanova, A.; Luka, M.; Gbahou, F.; Friederich, A.; Guillaume, J.; Keller, P.; Knoch, K.; Ahmad, R.; Delagrange, P.; et al. Detection of recombinant and endogenous mouse melatonin receptors by monoclonal antibodies targeting the C‐terminal domain. J. Pineal Res. 2018, 66, e12540. https://doi.org/10.1111/jpi.12540.
- Noseda, A.C.D.; Rodrigues, L.S.; Targa, A.D.S.; Ilkiw, J.L.; Fagotti, J.; Dos Santos, P.D.; Cecon, E.; Markus, R.P.; Solimena, M.; Jockers, R.; et al. MT(2) melatonin receptors expressed in the olfactory bulb modulate depressive-like behavior and olfaction in the 6-OHDA model of Parkinson's disease. Eur. J. Pharmacol. 2021, 891, 173722.
- Renner, D.B.; Svitak, A.L.; Gallus, N.J.; Ericson, M.E.; Frey, W.H., 2nd; Hanson, L.R. Intranasal delivery of insulin via the olfactory nerve pathway. J. Pharm. Pharmacol. 2012, 64, 1709–1714.
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expert Opin. Drug Deliv. 2020, 17, 839–853. https://doi.org/10.1080/17425247.2020.1762563.
- Altner, H.; Altner-Kolnberger, I. Freeze-fracture and tracer experiments on the permeability of the zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974, 154, 51–59. https://doi.org/10.1007/bf00221071.
- Durante, M.A.; Kurtenbach, S.; Sargi, Z.B.; Harbour, J.W.; Choi, R.; Kurtenbach, S.; Goss, G.M.; Matsunami, H.; Goldstein, B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020, 23, 323–326. https://doi.org/10.1038/s41593-020-0587-9.
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. https://doi.org/10.1016/j.lfs.2017.12.025.
- Trevino, J.; Quispe, R.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439.
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. https://doi.org/10.1016/j.addr.2011.11.002.
- Croy, I.; Hummel, T. Involvement of nasal trigeminal function in human stereo smelling. Proc. Natl. Acad. Sci. USA 2020, 117, 25979–25979. https://doi.org/10.1073/pnas.2016043117.
- Lochhead, J.J.; Kellohen, K.L.; Ronaldson, P.T.; Davis, T.P. Distribution of insulin in trigeminal nerve and brain after intranasal administration. Sci. Rep. 2019, 9, 1–9. https://doi.org/10.1038/s41598-019-39191-5.
- Kumar, N.N.; Lochhead, J.; Pizzo, M.; Nehra, G.; Boroumand, S.; Greene, G.; Thorne, R.G. Delivery of immunoglobulin G antibodies to the rat nervous system following intranasal administration: Distribution, dose-response, and mechanisms of delivery. J. Control. Release 2018, 286, 467–484. https://doi.org/10.1016/j.jconrel.2018.08.006.
- Pang, Y.; Fan, L.-W.; Carter, K.; Bhatt, A. Rapid transport of insulin to the brain following intranasal administration in rats. Neural Regen. Res. 2019, 14, 1046–1051. https://doi.org/10.4103/1673-5374.250624.
- Lochhead, J.; Wolak, D.J.; Pizzo, M.; Thorne, R.G. Rapid Transport within Cerebral Perivascular Spaces Underlies Widespread Tracer Distribution in the Brain after Intranasal Administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. https://doi.org/10.1038/jcbfm.2014.215.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. https://doi.org/10.1126/scitranslmed.3003748.
- Pardeshi, C.V.; Rajput, P.V.; Belgamwar, V.S.; Tekade, A.R. Formulation, optimization and evaluation of spray-dried mucoadhesive microspheres as intranasal carriers for Valsartan. J. Microencapsul. 2011, 29, 103–114. https://doi.org/10.3109/02652048.2011.630106.
- Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal nose-to-brain delivery: A review on architecture, physico-chemical characteristics and mucociliary cearance of the nasal olfactory mucosa. Pharmaceutics 2018, 10, 116.
- Schwarz, B.; Merkel, O.M. Nose-to-brain delivery of biologics Ther. Deliv. 2019, 10, 207–210.
- Smith, T.D.; Bhatnagar, K.P. Anatomy of the olfactory system. Handb. Clin. Neurol. 2019, 164, 17–28. https://doi.org/10.1016/b978-0-444-63855-7.00002-2.
- Olivares, J.; Schmachtenberg, O. An update on anatomy and function of the teleost olfactory system. PeerJ 2019, 7, e7808. https://doi.org/10.7717/peerj.7808.
- Palleria, C.; Roberti, R.; Iannone, L.F.; Tallarico, M.; Barbieri, M.A.; Vero, A.; Manti, A.; De Sarro, G.; Spina, E.; Russo, E. Clinically relevant drug interactions between statins and antidepressants. J. Clin. Pharm. Ther. 2019, 45, 227–239. https://doi.org/10.1111/jcpt.13058.
- Wyska, E. Pharmacokinetic considerations for current state-of-the-art antidepressants. Expert Opin. Drug Metab. Toxicol. 2019, 15, 831–847. https://doi.org/10.1080/17425255.2019.1669560.
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170.
- Ruigrok, M.J.; de Lange, E.C. Emerging insights for translational pharmacokinetic and pharmacokinetic-pharmacodynamic studies: Towards prediction of nose-to-brain transport in humans. AAPS J. 2015, 17, 493–505.
- Martins, P.P.; Smyth, H.D.; Cui, Z. Strategies to facilitate or block nose-to-brain drug delivery. Int. J. Pharm. 2019, 570, 118635. https://doi.org/10.1016/j.ijpharm.2019.118635.
- Iwasaki, S.; Yamamoto, S.; Sano, N.; Tohyama, K.; Kosugi, Y.; Furuta, A.; Hamada, T.; Igari, T.; Fujioka, Y.; Hirabayashi, H.; et al. Direct Drug Delivery of Low-Permeable Compounds to the Central Nervous System Via Intranasal Administration in Rats and Monkeys. Pharm. Res. 2019, 36, 1–14. https://doi.org/10.1007/s11095-019-2613-8.
- Marttin, E.; Verhoef, J.C.; Merkus, F.W.H.M. Efficacy, Safety and Mechanism of Cyclodextrins as Absorption Enhancers in Nasal Delivery of Peptide and Protein Drugs. J. Drug Target. 1998, 6, 17–36. https://doi.org/10.3109/10611869808997878.
- Li, Y.; Li, J.; Zhang, X.; Ding, J.; Mao, S. Non-ionic surfactants as novel intranasal absorption enhancers: In vitro and in vivo characterization. Drug Deliv. 2014, 23, 2272–2279. https://doi.org/10.3109/10717544.2014.971196.
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-beta-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control Release 2015, 201, 68–77.
- Ozsoy, Y.; Güngör, S. Nasal route: An alternative approach for antiemetic drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1439–1453. https://doi.org/10.1517/17425247.2011.607437.
- Liu, L.; Tian, C.; Dong, B.; Xia, M.; Cai, Y.; Hu, R.; Chu, X. Models to evaluate the barrier properties of mucus during drug diffusion. Int. J. Pharm. 2021, 599, 120415. https://doi.org/10.1016/j.ijpharm.2021.120415.
- Graff, C.L.; Pollack, G.M. Nasal Drug Administration: Potential for Targeted Central Nervous System Delivery. J. Curr. Chem. Pharm. Sci. 2005, 94, 1187–1195. https://doi.org/10.1002/jps.20318.
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Sakane, T.; Katsumi, H.; Yamamoto, A.; Yamashita, S. Nasal Delivery of P-gp Substrates to the Brain through the Nose–Brain Pathway. Drug Metab. Pharmacokinet. 2011, 26, 248–255. https://doi.org/10.2133/dmpk.dmpk-10-rg-108.
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. https://doi.org/10.1016/j.ejpb.2018.05.009.
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd; Ii, W.H.F. Novel Vasoconstrictor Formulation to Enhance Intranasal Targeting of Neuropeptide Therapeutics to the Central Nervous System. J. Pharmacol. Exp. Ther. 2008, 328, 312–320. https://doi.org/10.1124/jpet.108.145565.
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311.
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A case history of its discovery and preclinical development. Expert Opin. Drug Discov. 2014, 9, 567–578. https://doi.org/10.1517/17460441.2014.907790.
- Suwała, J.; Machowska, M.; Wiela-Hojeńska, A. Venlafaxine pharmacogenetics: A comprehensive review. Pharmacogenomics 2019, 20, 829–845. https://doi.org/10.2217/pgs-2019-0031.
- Patel, R.G. Nasal Anatomy and Function. Facial Plast. Surg. 2017, 33, 3–8.
- Kumar, A.; Pandey, A.N.; Jain, S.K. Nasal-nanotechnology: Revolution for efficient therapeutics delivery. Drug Deliv. 2014, 23, 671–683. https://doi.org/10.3109/10717544.2014.920431.
- Costa, C.P.; Moreira, J.; Amaral, M.H.; Lobo, J.M.S.; Silva, A. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. https://doi.org/10.1016/j.jconrel.2018.12.049.
- Quintana, D.S.; Westlye, L.T.; Rustan, G.Ø.; Tesli, N.; Poppy, C.L.; Smevik, H.; Tesli, M.; Røine, M.; Mahmoud, R.A.; Smerud, K.T.; et al. Low-dose oxytocin delivered intranasally with Breath Powered device affects social-cognitive behavior: A randomized four-way crossover trial with nasal cavity dimension assessment. Transl. Psychiatry 2015, 5, e602.
- Mittal, D.; Ali, A.; Shadab; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2013, 21, 75–86. https://doi.org/10.3109/10717544.2013.838713.
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2012, 3, 42–62. https://doi.org/10.1007/s13346-012-0108-9.
- Tong, X.; Dong, J.; Shang, Y.; Inthavong, K.; Tu, J. Effects of nasal drug delivery device and its orientation on sprayed particle deposition in a realistic human nasal cavity. Comput. Biol. Med. 2016, 77, 40–48. https://doi.org/10.1016/j.compbiomed.2016.08.002.
- Warnken, Z.N.; Smyth, H.D.; Watts, A.B.; Weitman, S.; Kuhn, J.G.; Williams, R.O. Formulation and device design to increase nose to brain drug delivery. J. Drug Deliv. Sci. Technol. 2016, 35, 213–222. https://doi.org/10.1016/j.jddst.2016.05.003.
- Chen, Y.; Liu, Y.; Xie, J.; Zheng, Q.; Yue, P.; Chen, L.; Hu, P.; Yang, M. Nose-to-Brain Delivery by Nanosuspensions-Based in situ Gel for Breviscapine. Int. J. Nanomed. 2020, 15, 10435–10451. https://doi.org/10.2147/ijn.s265659.
- Agrawal, M.; Saraf, S.; Saraf, S.; Dubey, S.K.; Puri, A.; Gupta, U.; Kesharwani, P.; Ravichandiran, V.; Kumar, P.; Naidu, V.; et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 2020, 327, 235–265. https://doi.org/10.1016/j.jconrel.2020.07.044.
- Kanwar, N.; Sinha, V.R. In Situ Forming Depot as Sustained-Release Drug Delivery Systems.. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 93–136. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2018025013.
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. https://doi.org/10.4155/tde.14.91.
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. https://doi.org/10.1615/critrevtherdrugcarriersyst.2016014850.
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. https://doi.org/10.1016/j.ijbiomac.2014.10.052.
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. https://doi.org/10.2174/1574888x13666180912142028.
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des. 2016, 22, 3399–3410. https://doi.org/10.2174/1381612822666160510125718.
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. https://doi.org/10.1081/ddc-120003853.
- Severino, P.; Da Silva, C.F.; Andrade, L.N.; de Lima Oliveira, D.; Campos, J.; Souto, E.B. Alginate Nanoparticles for Drug Delivery and Targeting. Curr. Pharm. Des. 2019, 25, 1312–1334. https://doi.org/10.2174/1381612825666190425163424.
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Tran, L.D.; Bach, G.L.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909–915. https://doi.org/10.1038/s41598-020-57666-8.
- Reig-Vano, B.; Tylkowski, B.; Montané, X.; Giamberini, M. Alginate-based hydrogels for cancer therapy and research. Int. J. Biol. Macromol. 2020, 170, 424–436. https://doi.org/10.1016/j.ijbiomac.2020.12.161.
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2016, 9, 1174–1183. https://doi.org/10.1039/c6nr07581a.
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. https://doi.org/10.3390/pharmaceutics12121230.
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for "nose-to-brain" drug delivery. Pharmaceutics 2019, 11, 1230.
- Rinaldi, F.; Oliva, A.; Sabatino, M.; Imbriano, A.; Hanieh, P.N.; Garzoli, S.; Mastroianni, C.M.; De Angelis, M.; Miele, M.C.; Arnaut, M.; et al. Antimicrobial Essential Oil Formulation: Chitosan Coated Nanoemulsions for Nose to Brain Delivery. Pharmaceutics 2020, 12, 678. https://doi.org/10.3390/pharmaceutics12070678.
- Pandey, V.; Kohli, S. Lipids and Surfactants: The Inside Story of Lipid-Based Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 99–155. https://doi.org/10.1615/critrevtherdrugcarriersyst.2018016710.
- Urquhart, A.J.; Eriksen, A.Z. Recent developments in liposomal drug delivery systems for the treatment of retinal diseases. Drug Discov. Today 2019, 24, 1660–1668. https://doi.org/10.1016/j.drudis.2019.04.004.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. https://doi.org/10.1021/acs.chemrev.5b00046.
- Natsheh, H.; Touitou, E. Phospholipid Magnesome—A nasal vesicular carrier for delivery of drugs to brain. Drug Deliv. Transl. Res. 2018, 8, 806–819. https://doi.org/10.1007/s13346-018-0503-y.
- Natsheh, H.; Touitou, E. Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959. https://doi.org/10.3390/molecules25132959.
- Touitou, E.; Duchi, S.; Natsheh, H. A new nanovesicular system for nasal drug administration. Int. J. Pharm. 2020, 580, 119243. https://doi.org/10.1016/j.ijpharm.2020.119243.
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. https://doi.org/10.1016/j.jconrel.2017.08.033.
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. https://doi.org/10.1016/j.ejps.2017.11.023.
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2018, 71, 156–166. https://doi.org/10.1016/j.pharep.2018.10.008.
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. https://doi.org/10.1016/j.biopha.2018.04.055.
- Cayero-Otero, M.D.; Gomes, M.J.; Martins, C.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Sarmento, B.; Martín-Banderas, L. In vivo biodistribution of venlafaxine-PLGA nanoparticles for brain delivery: Plain vs. functionalized nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 1413–1427.
- Haque, S.; Shadab; Fazil, M.; Kumar, M.; Sahni, J.K.; Ali, J.; Baboota, S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012, 89, 72–79. https://doi.org/10.1016/j.carbpol.2012.02.051.
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2016, 25, 844–851. https://doi.org/10.1016/j.jsps.2016.12.003.
- Ahmed, S.; Gull, A.; Aqil, M.; Ansari, M.D.; Sultana, Y. Poloxamer-407 thickened lipid colloidal system of agomelatine for brain targeting: Characterization, brain pharmacokinetic study and behavioral study on Wistar rats. Colloids Surfaces B: Biointerfaces 2019, 181, 426–436. https://doi.org/10.1016/j.colsurfb.2019.05.016.
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: Formulation, optimization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2017, 11, 1815–1825. https://doi.org/10.2147/DDDT.S102500.
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K. Intranasal infusion of nanostructured lipid carriers (NLC) containing CNS acting drug and estimation in brain and blood. Drug Deliv. 2013, 20, 247–251. https://doi.org/10.3109/10717544.2013.822945.
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. https://doi.org/10.1016/j.ijpharm.2014.05.004.
- Elsenosy, F.M.; Abdelbary, G.A.; Elshafeey, A.H.; Elsayed, I.; Fares, A.R. Brain Targeting of Duloxetine HCL via Intranasal Delivery of Loaded Cubosomal Gel: In vitro Characterization, ex vivo Permeation, and in vivo Biodistribution Studies. Int. J. Nanomed. 2020, 15, 9517–9537. https://doi.org/10.2147/ijn.s277352.
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2015, 27, 25102. https://doi.org/10.1088/0957-4484/27/2/025102.
- Motaleb, M.A.; Ibrahim, I.T.; Sayyed, M.E.; Awad, G.A.S. (131)I-trazodone: Preparation, quality control and in vivo biodis-tribution study by intranasal and intravenous routes as a hopeful brain imaging radiopharmaceutical. Rev. Esp. Med. Nucl. Imagen Mol. 2017, 36, 371–376.
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. https://doi.org/10.1016/j.ejps.2016.05.008.
- Naik, A.; Nair, H. Formulation and Evaluation of Thermosensitive Biogels for Nose to Brain Delivery of Doxepin. BioMed Res. Int. 2014, 2014, 1–10. https://doi.org/10.1155/2014/847547.
- Eduardo, T.Q.; Angela, A.; Mateo, L.; Melanie, L.Z.; Valentina, P.F.; David, C.; Estefania, C.; Natalia, R.S.; Andrés, V.C.; Angel, R.O.; et al. Ketamine for resistant depression: A scoping review. Actas Esp. Psiquiatr. 2022, 50, 144–159.
- Yavi, M.; Lee, H.; Henter, I.D.; Park, L.T.; Zarate, C.A., Jr. Ketamine treatment for depression: A review. Discov. Ment. Heal. 2022, 2, 1–14. https://doi.org/10.1007/s44192-022-00012-3.
- Gadhave, D.; Tupe, S.; Tagalpallewar, A.; Gorain, B.; Choudhury, H.; Kokare, C. Nose-to-brain delivery of amisul-pride-loaded lipid-based poloxamer-gellan gum nanoemulgel: In vitro and in vivo pharmacological studies. Int. J. Pharm. 2021, 607, 121050. https://doi.org/10.1016/j.ijpharm.2021.121050.
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Antipsychotic Potential and Safety Profile of TPGS-Based Mucoadhesive Aripiprazole Nanoemulsion: Development and Optimization for Nose-To-Brain Delivery. J. Pharm. Sci. 2021, 110, 1761–1778. https://doi.org/10.1016/j.xphs.2021.01.021.
- Sawant, K.; Pandey, A.; Patel, S. Aripiprazole loaded poly(caprolactone) nanoparticles: Optimization and in vivo pharma-cokinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 230–243. https://doi.org/10.1016/j.msec.2016.04.089.
- Sridhar, V.; Gaud, R.; Bajaj, A.; Wairkar, S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson's disease. Nanomedicine 2018, 14, 2609–2618.
- Sridhar, V.; Wairkar, S.; Gaud, R.; Bajaj, A.; Meshram, P. Brain targeted delivery of mucoadhesive thermosensitive nasal gel of selegiline hydrochloride for treatment of Parkinson's disease. J. Drug Target 2018, 26, 150–161.
- Nedelcovych, M.T.; Gadiano, A.J.; Wu, Y.; Manning, A.A.; Thomas, A.G.; Khuder, S.S.; Yoo, S.-W.; Xu, J.; McArthur, J.C.; Haughey, N.J.; et al. Pharmacokinetics of Intranasal versus Subcutaneous Insulin in the Mouse. ACS Chem. Neurosci. 2017, 9, 809–816. https://doi.org/10.1021/acschemneuro.7b00434.
- Ren, G.; Xue, P.; Wu, B.; Yang, F.; Wu, X. Intranasal treatment of lixisenatide attenuated emotional and olfactory symptoms via CREB-mediated adult neurogenesis in mouse depression model. Aging 2021, 13, 3898–3908. https://doi.org/10.18632/aging.202358.
- Sasaki-Hamada, S.; Nakamura, R.; Nakao, Y.; Akimoto, T.; Sanai, E.; Nagai, M.; Horiguchi, M.; Yamashita, C.; Oka, J.-I. Antidepressant-like effects exerted by the intranasal administration of a glucagon-like peptide-2 derivative containing cell-penetrating peptides and a penetration-accelerating sequence in mice. Peptides 2017, 87, 64–70. https://doi.org/10.1016/j.peptides.2016.11.013.
- Ma, X.-C.; Liu, P.; Zhang, X.-L.; Jiang, W.-H.; Jia, M.; Wang, C.-X.; Dong, Y.-Y.; Dang, Y.-H.; Gao, C.-G. Intranasal Delivery of Recombinant AAV Containing BDNF Fused with HA2TAT: A Potential Promising Therapy Strategy for Major Depressive Disorder. Sci. Rep. 2016, 6, 22404. https://doi.org/10.1038/srep22404.
- Chen, C.; Dong, Y.; Liu, F.; Gao, C.; Ji, C.; Dang, Y.; Ma, X.; Liu, Y. A Study of Antidepressant Effect and Mechanism on Intranasal Delivery of BDNF-HA2TAT/AAV to Rats with Post-Stroke Depression. Neuropsychiatr. Dis. Treat. 2020, 16, 637–649. https://doi.org/10.2147/ndt.s227598.
- Liu, F.; Liu, Y.-P.; Lei, G.; Liu, P.; Chu, Z.; Gao, C.-G.; Dang, Y.-H. Antidepressant effect of recombinant NT4-NAP/AAV on social isolated mice through intranasal route. Oncotarget 2016, 8, 10103–10113. https://doi.org/10.18632/oncotarget.14356.
- Ma, X.-C.; Chu, Z.; Zhang, X.-L.; Jiang, W.-H.; Jia, M.; Dang, Y.-H.; Gao, C.-G. Intranasal Delivery of Recombinant NT4-NAP/AAV Exerts Potential Antidepressant Effect. Neurochem. Res. 2016, 41, 1375–1380. https://doi.org/10.1007/s11064-016-1841-0.
- Jiang, J.; Peng, Y.; Liang, X.; Li, S.; Chang, X.; Li, L.; Chang, M. Centrally administered cortistation-14 induces antidepres-sant-like effects in mice via mediating ghrelin and GABA(A) receptor signaling pathway. Front. Pharmacol. 2018, 9, 767.
- Serova, L.; Laukova, M.; Alaluf, L.; Pucillo, L.; Sabban, E. Intranasal neuropeptide Y reverses anxiety and depressive-like behavior impaired by single prolonged stress PTSD model. Eur. Neuropsychopharmacol. 2014, 24, 142–147. https://doi.org/10.1016/j.euroneuro.2013.11.007.
- Oh, J.-Y.; Liu, Q.F.; Hua, C.; Jeong, H.J.; Jang, J.-H.; Jeon, S.; Park, S.J.A.H.-J. Intranasal Administration of Mela-nin-Concentrating Hormone Reduces Stress-Induced Anxiety- and Depressive-Like Behaviors in Rodents. Exp. Neurobiol. 2020, 29, 453–469. https://doi.org/10.5607/en20024.
- Shi, C.-G.; Wang, L.-M.; Wu, Y.; Wang, P.; Gan, Z.-J.; Lin, K.; Jiang, L.-X.; Xu, Z.-Q.; Fan, M. Intranasal Administration of Nerve Growth Factor Produces Antidepressant-Like Effects in Animals. Neurochem. Res. 2010, 35, 1302–1314. https://doi.org/10.1007/s11064-010-0183-6.
- Xu, D.; Qiao, T.; Wang, Y.; Wang, Q.-S.; Cui, Y.-L. Alginate nanogels-based thermosensitive hydrogel to improve antide-pressant-like effects of albiflorin via intranasal delivery. Drug Deliv. 2021, 28, 2137–2149. https://doi.org/10.1080/10717544.2021.1986604.
- Wang, Q.-S.; Li, K.; Gao, L.-N.; Zhang, Y.; Lin, K.-M.; Cui, Y.-L. Intranasal delivery of berberine via in situ thermoresponsive hydrogels with non-invasive therapy exhibits better antidepressant-like effects. Biomater. Sci. 2020, 8, 2853–2865. https://doi.org/10.1039/c9bm02006c.
- Xu, D.; Qiu, C.; Wang, Y.; Qiao, T.; Cui, Y.-L. Intranasal co-delivery of berberine and evodiamine by self-assembled ther-mosensitive in-situ hydrogels for improving depressive disorder. Int. J. Pharm. 2021, 603, 120667. https://doi.org/10.1016/j.ijpharm.2021.120667.
- Zhang, K.; Lei, N.; Li, M.; Li, J.; Li, C.; Shen, Y.; Guo, P.; Xiong, L.; Xie, Y. Cang-Ai Volatile Oil Ameliorates Depressive Be-havior Induced by Chronic Stress Through IDO-Mediated Tryptophan Degradation Pathway. Front. Psychiatry 2021, 12, 791991. https://doi.org/10.3389/fpsyt.2021.791991.
- Chen, B.; Li, J.; Xie, Y.; Ming, X.; Li, G.; Wang, J.; Li, M.; Li, X.; Xiong, L. Cang-ai volatile oil improves depressive-like behaviors and regulates DA and 5-HT metabolism in the brains of CUMS-induced rats. J. Ethnopharmacol. 2019, 244, 112088. https://doi.org/10.1016/j.jep.2019.112088.
- Xu, D.; Lu, Y.-R.; Kou, N.; Hu, M.-J.; Wang, Q.-S.; Cui, Y.-L. Intranasal delivery of icariin via a nanogel-thermoresponsive hydrogel compound system to improve its antidepressant-like activity. Int. J. Pharm. 2020, 586, 119550. https://doi.org/10.1016/j.ijpharm.2020.119550.
- Hu, W.; Xie, G.; Zhou, T.; Tu, J.; Zhang, J.; Lin, Z.; Zhang, H.; Gao, L. Intranasal administration of white tea alleviates the olfactory function deficit induced by chronic unpredictable mild stress. Pharm. Biol. 2020, 58, 1230–1237. https://doi.org/10.1080/13880209.2020.1855213.




