Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Silas Leavesley | -- | 3719 | 2022-10-18 15:02:56 | | | |
| 2 | Rita Xu | + 54 word(s) | 3773 | 2022-10-19 05:54:04 | | | | |
| 3 | Rita Xu | -12 word(s) | 3761 | 2022-10-19 05:55:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Browning, C.M.; Cloutier, R.; Rich, T.C.; Leavesley, S.J. Endoscopy Lifetime Systems Architecture. Encyclopedia. Available online: https://encyclopedia.pub/entry/29981 (accessed on 08 February 2026).
Browning CM, Cloutier R, Rich TC, Leavesley SJ. Endoscopy Lifetime Systems Architecture. Encyclopedia. Available at: https://encyclopedia.pub/entry/29981. Accessed February 08, 2026.
Browning, Craig M., Robert Cloutier, Thomas C. Rich, Silas J. Leavesley. "Endoscopy Lifetime Systems Architecture" Encyclopedia, https://encyclopedia.pub/entry/29981 (accessed February 08, 2026).
Browning, C.M., Cloutier, R., Rich, T.C., & Leavesley, S.J. (2022, October 19). Endoscopy Lifetime Systems Architecture. In Encyclopedia. https://encyclopedia.pub/entry/29981
Browning, Craig M., et al. "Endoscopy Lifetime Systems Architecture." Encyclopedia. Web. 19 October, 2022.
Copy Citation
Systems engineering captures the desires and needs of the customer to conceptualize a system from the overall goal down to the small details prior to any physical development. While many systems projects tend to be large and complicated (i.e., cloud-based infrastructure, long-term space travel shuttles, missile defense systems), systems engineering can also be applied to smaller, complex systems. The system of interest is the endoscope, a standard biomedical screening device used in laparoscopic surgery, screening of upper and lower gastrointestinal tracts, and inspection of the upper airway.
endoscopy
system architecture
system lifecycle
hyperspectral
subsystem trends
model-based systems engineering (MBSE)
1. Introduction
Systems engineering (SE) is a holistic engineering skillset and mindset structured to decompose large, complex systems down to nuts and bolts and ones and zeros prior to “breaking ground” on design and fabrication. The documentation produced from SE procedures is comparable to an instruction manual that traces those bolts and bytes into components, assays, and subsystems culminating to the final system. Simultaneously, the “manual” provides parameters and verification metrics that should be met at every level of decomposition to ensure the end product’s output is productive, safe, and correct for all stakeholders involved. A note of importance in the SE process is that the documentation produced should maintain a level of abstraction to allow for creative, inventive, and cost-effective design when producing physical aspects of the system. For example, a future smart city system needs to dynamically transmit data to the populous of autonomous vehicles on the street at X Mb/s. The requirement does not dictate that it should be Bluetooth or 5G link; it could be a new method of data transfer.
Systems engineering also tracks a system throughout its lifecycle, from conception to retirement and disposal. Researchers coin a new term called “system lifetime”, which considers many different lifecycles of a system throughout history, in other words, many generations of development of a system. Noteworthy models for lifecycle processes are the waterfall and spiral model [1]. These models are primarily used for software systems, but are also exemplary models for the iterative process of producing a physical system to meet the requirements set forth in the conceptual phase. These models have been beneficial to the structuring of this work considering the lifetime of a system. Considering the waterfall or spiral in a three-dimensional space with lengths of time between new waterfalls or spirals provided a unique concept to organize the many lifecycles of system development over the course of the system lifetime. Furthermore, Hossain and colleagues have recently detailed a review of systems thinking topics through a bibliometric analysis to highlight past trends and determine current gaps in knowledge of systems thinking [2], and researchers made use of some of the approaches presented in this review to analyze the development of endoscope systems and potential future directions. While SE is beneficial for new, large, complicated systems such as smart cities, green energy infrastructure, and digital medical recording techniques, it can also be utilized to review and optimize smaller, complex, existing technology such as the endoscope.
Endoscopy is a medical screening process by which internal (normally hollow) organs are imaged by the insertion of a scope with illumination and imaging capabilities. Through visualization, clinicians can optically diagnose infection, inflammation, or lesion growth and resect portions of tissue for pathological diagnostics. There are four major endoscopic techniques widely used today: white light endoscopy (WLE), narrow-band imaging (NBI), Fujifilm flexible spectral imaging color enhancement (FICE), and virtual endoscopy (VE). WLE is the gold standard technique used for decades to capture a typical RGB (red–green–blue) image providing reflectance-based images of the luminal wall [3][4][5]. NBI illuminates body cavities with blue and green light to harness the light absorption of the vasculature at these wavelengths providing additional contrast to the image [6][7]. FICE is a post-acquisition process that divides the RGB image into the respective three colors and digitally alters wavelengths to enhance the contrast [8][9]. VE uses coherent tomography scanning (CT scan) or magnetic resonance imaging (MRI) to render a 2D or 3D model of the hollow cavities traditionally imaged with an endoscope [10]. Pathologies of the gastrointestinal (GI) tract can at times be difficult to differentiate from the surrounding mucosa [4][11][12][13]. While current techniques provide several complementary modes for visualizing internal body cavities, the contrast and definition between healthy and afflicted tissue is limited, especially in early-stage cancer. The limited contrast between some cancers and the surrounding mucosa can have downstream consequences on detection accuracy and patient outcomes, for example in colorectal cancer, which is the third-ranking cancer in the United States for incidence and mortality rates [14][15][16][17]. Neoplasia (abnormal cellular growth) can be difficult to observe within the mucosal lining. If missed, neoplasia can become invasive and malignant (cancerous); in essence, researchers have let a cellular vehicle run a red light without getting ticketed. Tumor growth (1) can approximately double in volume annually [18][19], and (2) the standard of care for interval routine endoscopic screenings is 5 years [5]. Further, (3) a missed colorectal tumor could approximate a minimum 32× volume increase (assuming a constant exponential growth rate) before being detected at a subsequent colonoscopy. Therefore, it is important to develop improved technologies that provide high contrast and the ability to visualize neoplasia or early-stage cancer when viewing the hollow organs endoscopically.
2. Presenting, the Endoscope
Current state-of-the-art endoscope systems utilize a combination of broadband light sources (xenon arc lamps, halogens, or LEDs), bandwidth filtering, and digital analyses to produce WLE (gold standard), NBI, or FICE. One limitation of WLE is that small and subtle changes within the lumen may not generate sufficient contrast to be detected. In addition, abnormal, irritated, inflamed, or neoplastic tissue may appear very similar to normal tissue, resulting in difficulty determining potential areas of risk, especially for patients with underlying inflammatory conditions. To improve detection sensitivity and specificity, a range of correlating factors are often considered, such as: irregular mucosal patterns, condensed vasculature, and definitive redness. Introduced briefly before, NBI and FICE are two complementary modalities to WLE that can provide enhanced contrast of tissue structures between mucosa and lesions. NBI filters utilize narrow spectral bands in the blue and green regions to illuminate the tissue, harnessing the absorption of blood at those wavelengths and creating an image that contrasts vasculature as brown [6]. Condensed vasculature has been associated with lesional tissue due to its invasive, nutrient-draining nature. FICE is an image algorithm that uses the RGB image acquired through normal screenings and processes individual color channels into unique wavelengths (within the respective color range) that accentuates tissue differences greater than the original colored image [8]. This method has defined mucosal irregularities and tissue irritation more effectively than traditional WLE due to post-imaging processing. These techniques are included in aspects of the current architecture (Figure 1).
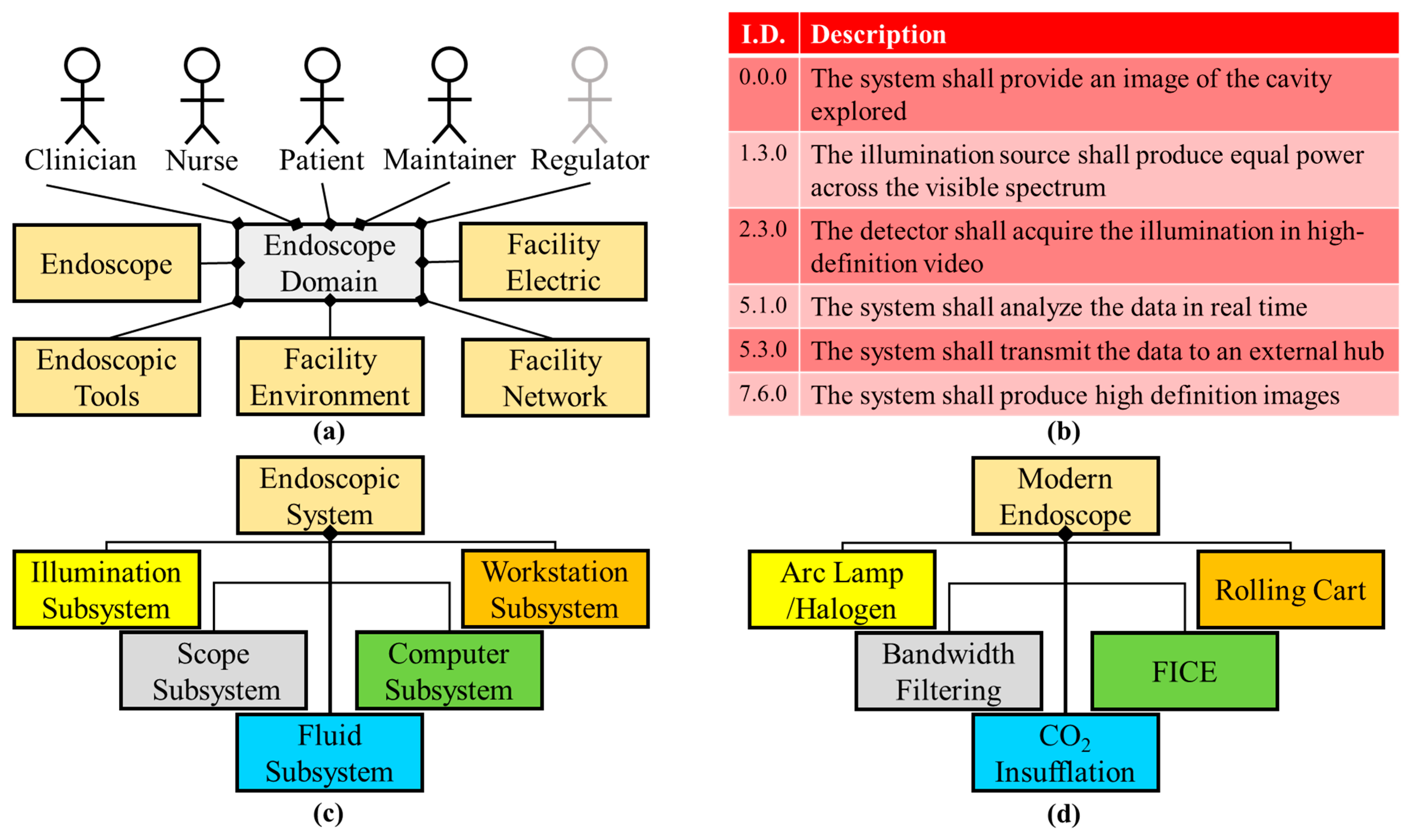
Figure 1. Current endoscope condensed system architecture. (a) The facility network external system was added to the domain diagram of the current system, compared to that of the imaging milestone domain, because of the use of the internet and cloud storage. (b) High-definition and spectral aspects are stated in the requirements for the current system. (c) The logical diagram remains the same as the previous milestone. (d) Physical components and software are bandwidth filters and FICE software for this architecture milestone.
Another modern endoscopic screening technique is virtual endoscopy (VE) or computed tomography (CT) endoscopy [10][20]. Using CT creates a volumetric image of the entire colon or tracheobronchial tree with a noninvasive technique. VE is a beneficial screening for patients with occlusions prohibiting traditional WLE, providing a complete image of the respective body cavity and the best option for older patients or patients who are contraindicated from standard endoscopic procedures. CT data provides cross-sectional views of the organs and three-dimensional reconstruction to create a virtual scan that resembles a WLE procedure.
Another alternative to WLE is capsule endoscopy [21][22]. Capsule endoscopy is more invasive than VE, but still requires less hospital procedure time than standard WLE colonoscopy. An endoscopic camera and illumination source in capsule form is ingested and transmits video feed to wearable receiver for clinicians to view post ingestion. Current models are intuitive to their location in the gastrointestinal tract with automated data acquisition rates depending on the rate of capsule movement (data acquisition would decrease in the stomach and if the capsule slowed or stopped) and have two wide-angle cameras to ensure a full view of the colon. The fact that the capsule primarily images the small and large intestines highlights that capsule endoscopy is currently utilized primarily for colonoscopy imaging and the optimal scenario to image or view the small intestine.
Modern endoscopic techniques are a definite advancement from Bozzini’s original endoscope. Current state-of-the-art endoscopes are focused on contrasting and detecting minute tissue changes to diagnose early, screening processes that are minimally invasive, and providing as much information (the “big picture”) to benefit the patient. In the previous section, the history of the endoscope was detailed and the system upgrades were traced. Next, individual subsystems of the endoscope system were analyzed to view the trends through the endoscope lifetime and discuss where components or subsystems are currently optimal and can be improved or upgraded.
2.1. What’s Trending
Systematic decomposition of previous and existing endoscopic systems highlights the improvements of subsystems and elements within the system, as well as areas for potential upgrades. Here, the current milestone logical architecture is highlighted and the trends in technology are shown for particular subsystems (Figure 2). A primary advancement is the illumination source from candles to incandescent bulbs to LEDs (Figure 2a). Light sources have shown a substantial improvement in luminosity, decreased power consumption, and increased component longevity. Some literature theorizes that illumination technology is reaching maximum potential in white light luminous efficacy [23].
With regard to the computational subsystem, researchers examined the trend in the literature of endoscope-related computer science publications. Researchers searched the Scopus database for publications in the field of computer science using the keywords “gastroenterology” or “endoscopy” and a date range of 1990–2019, divided into five-year increments (Figure 2b). Results indicated that the last two decades have shown a 50X increase in the number of publications fitting these parameters. Examples of the increased computational demand of such components begin with the image sensor digital signal processor (DSP) to convert photons to digital signals [24] and expand to three-dimensional (VE and optical coherence tomography—OCT) [25] and wireless or self-contained (capsule endoscopy) or enhanced channel contrast (FICE).
The optical light path which carries illumination to the patient cavity and the image back to the user or imager has also been optimized through the years (Figure 2c). The Lichtleiter with a length of ~10 cm increased to the current colonoscopes with a length over 1.5 m, capable of spanning the entire large intestine and a portion of the small intestine. These depths would not be possible if fiber optics were not introduced, creating flexible endoscopes. Furthermore, a smaller fiber optic bundle and overall endoscopic diameter allowed for the development of smaller systems such as bronchoscopes, cystoscopes, laparoscopes, and ureteroscopes for lungs, bladder, small surgical openings, and ureter, respectively [26]. Flexible endoscopy has not extended the working length further into the small intestine due to the tortuous and compact nature of the organ [27]. Maneuvering a flexible scope through the small intestine could perforate the mucosal lining or damage the fiber optics of the endoscope. Furthermore, the amount of articulation and force that could be applied to the endoscope tip decreases with increased length and depth into the lower GI. Smaller endoscopes are limited in illumination and detection due to size constraints. Therefore, the output of these smaller systems has lower spatial resolution and potentially lower contrast between normal and abnormal tissue.
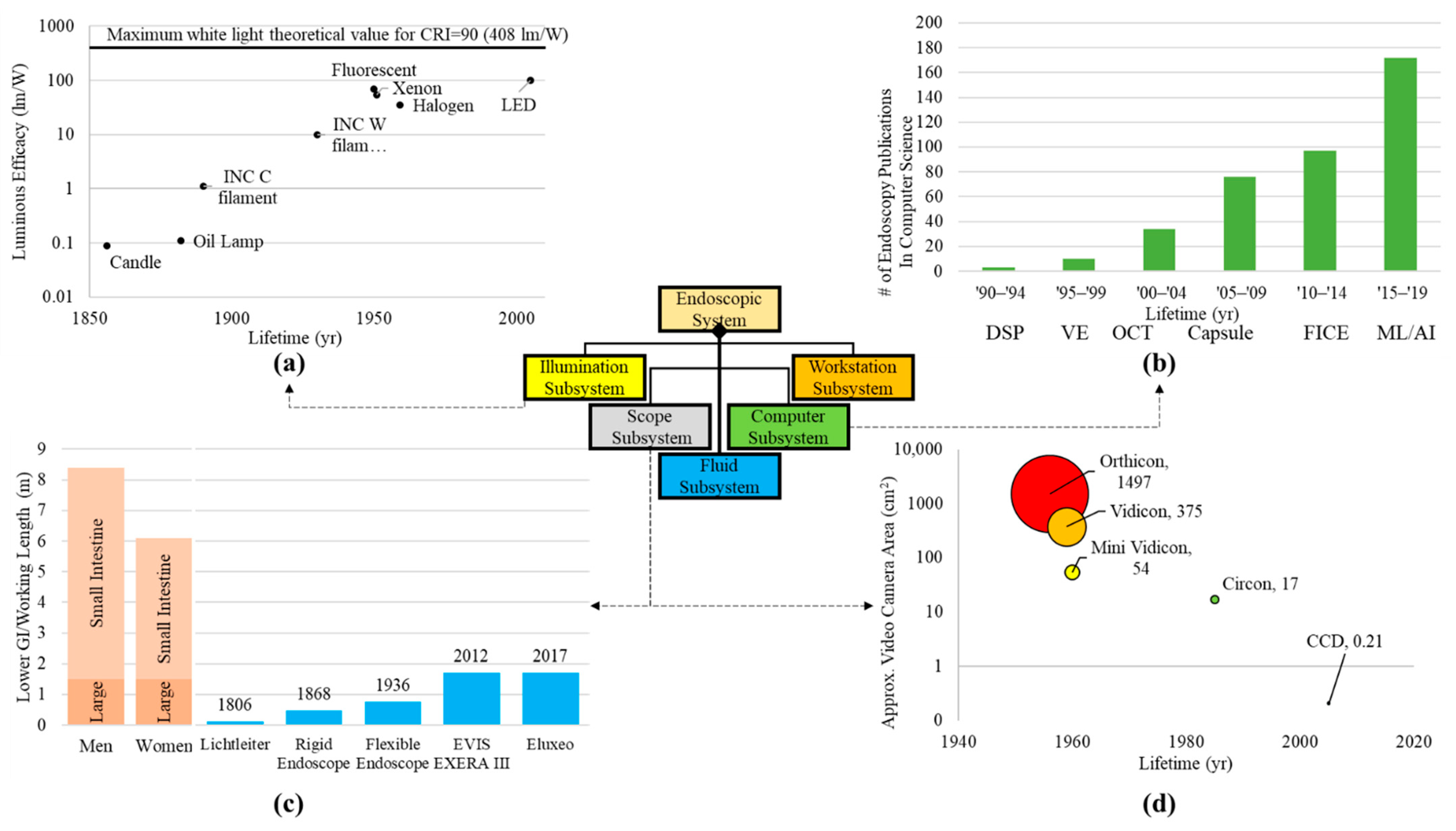
Figure 2. Trends for components within subsystems. (a) Light sources in the illumination subsystem presenting the luminous efficacy from the candle to arc lamps and LEDs. Illumination data were extracted from Azevedo et al.’s paper on solid state lighting [23]. (b) Advancements in computational aspects of endoscope systems were visualized by plotting the increase in endoscope-related publications within the computer science field (publication search for “endoscopy” in the computer science category per quinquennium—Source: http://www.scopus.com, accessed on 28 July 2022) [28]. Some examples of imaging processing technologies that were found include: digital signal processing (DSP), virtual endoscopy (VE), optical coherence tomography (OCT), capsule endoscopy (Capsule), Fujinon’s flexible spectral imaging color enhancement (FICE), and machine learning (ML) [29]. (c) Optical pathway (working length) [30][31][32] for the scope subsystem showing the depth the endoscope has traversed throughout the milestones compared to the length of human body intestinal tract [33]. (d) Approximate camera/detector area for various cameras (both film and digital) throughout imaging in endoscopy. Camera and detector areas were assumed from dimensions given in literature [30][34][35][36][37].
Creating physical records of endoscopic screenings is one of the most important aspects of the field today. The transition from clinician hand-drawn images to film photography lessened the workload and provided an objective image to support diagnosis. Ironically, cameras continued to improve by creating clearer pictures while becoming smaller components within the system (Figure 2d). Film-based cameras were large additions to system on the proximal end. Now, digital image sensors are miniaturized on the distal end of scopes providing real-time, high-definition images and video.
2.2. Endoscopy: The Next Generation
Using systems engineering architecture as a tool for review, researchers can exhaustively survey the needs of the range of subsystems and components, as well as environmental constraints and current technologies. Researchers can predict which technologies may need to evolve and what a next generation endoscope would provide. Based on Figure 2, illumination is at a current maximum, digital sensors can accommodate any endoscope diameter with the caveat of limited resolution for smaller sensor sizes, the working length of the scope cannot get any longer due to highly condensed and tortuous nature of the small intestine and there is a high interest in the computational capabilities of the endoscopic technologies. Reviewing past inventions that were implemented into endoscopy, Figure 3 shows the importance of looking at off-the-shelf components and technology.
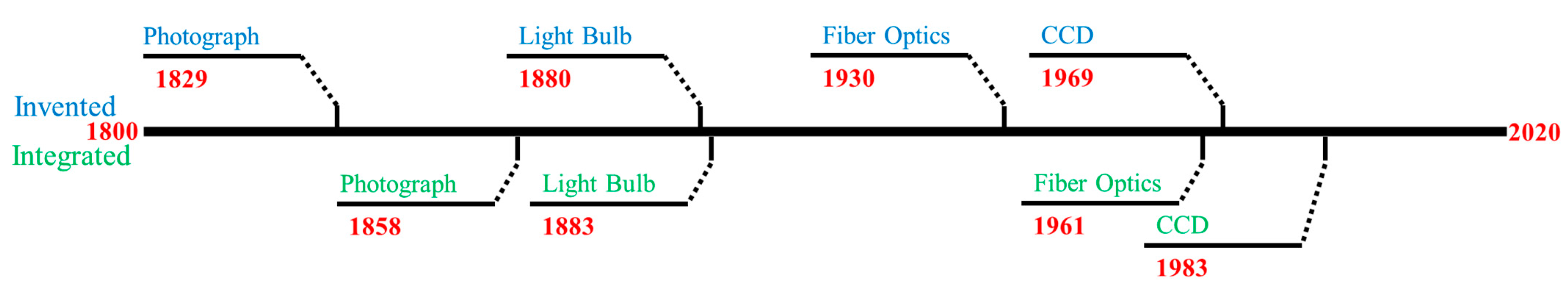
Figure 3. Timeline comparing technology invention dates to the time it was integrated into endoscopy. Blue text denotes invention and green for integration.
The endoscopic community was quick (3 years) to integrate incandescent light bulbs to the endoscope design, but it was over 30 years between invention and implementation of the fiber optics, creating the flexible endoscope. Therefore, a technology might already exist with the potential to benefit the output of endoscopy. Researchers reference the previous upgrades and potential gaps in the system while acknowledging the technologies that exist outside the field of endoscopy. To begin justification for these possible upgrades, a mind map was constructed to outline the avenues (Figure 4).
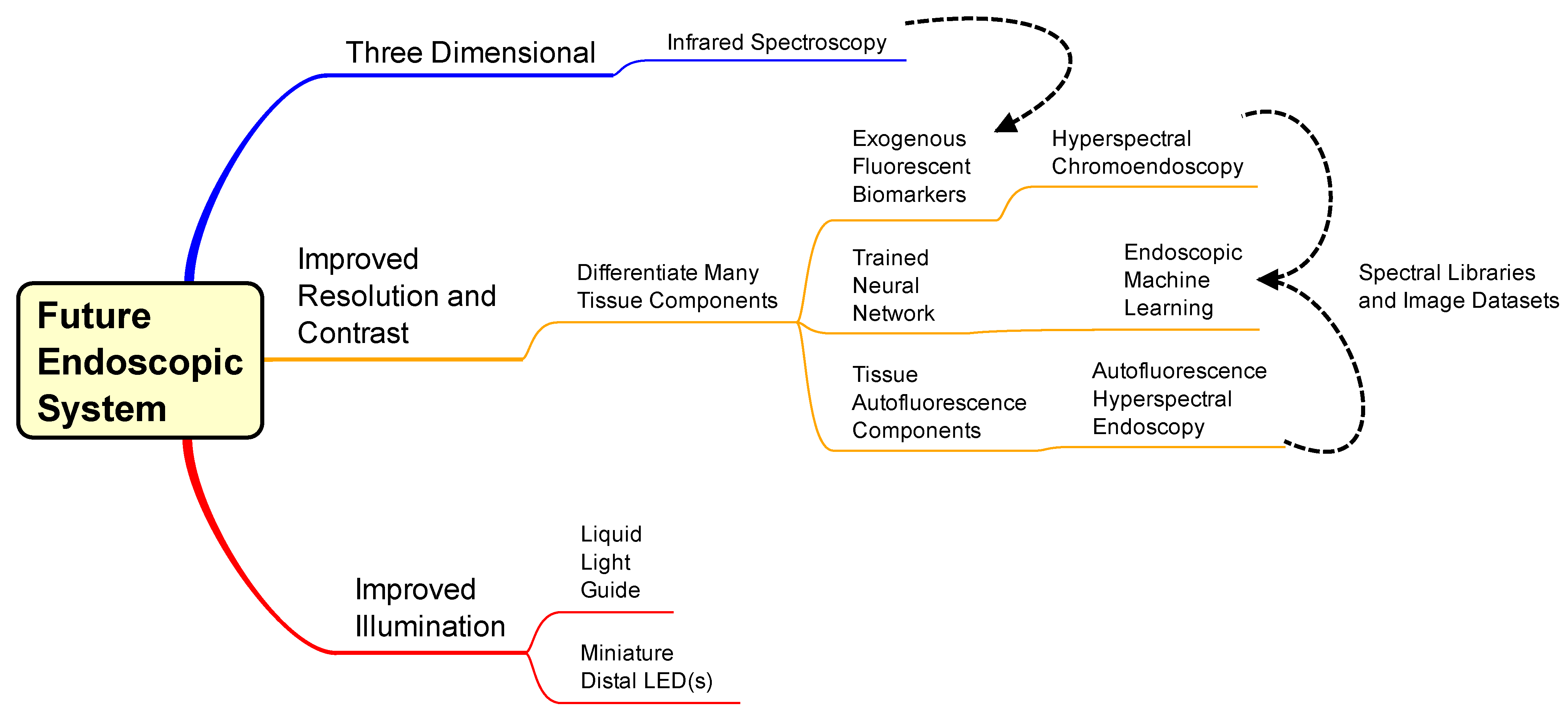
Figure 4. Mind map for design of a next-generation endoscopic system. The middle and top pathways are considerations of techniques to implement, and the bottom pathway is component upgrades of the current endoscope system. Dashed lines convey interconnectivity between topics: Infrared analysis could implement with exogenous fluorescent biomarkers and hyperspectral chromoendoscopy and autofluorescence hyperspectral endoscopy could be analyzed and displayed via machine learning algorithms.
The mind map has two parts: technology for implementation (top two pathways) and components to improve current systems (bottom pathway). Components for increased illumination throughput include miniature LEDs at the distal end of the scope and/or a liquid light guide instead of fiber bundles. When digital camera sensors became small enough, they were integrated at the distal end of endoscopes, reducing transmission losses by eliminating the secondary (imaging) light guide. If white light LEDs or three respective RGB LEDs were small enough to replace the illumination fiber optic area of the distal tip, then transmission losses would be further reduced. A major factor in creating a new hot illumination is the potential for heat dissipation and mitigating damage to the patient cavity. Another option is exchanging illumination fiber bundles for liquid light guides (LLG) to increase the throughput of the endoscope. An LLG has no void fraction, a higher numerical aperture, and a higher acceptance angle compared to fiber optic bundles accepting more illumination proximally, minimal internal refraction, and a diffuse illumination at the distal end (potentially illuminating a larger area). A limitation to using LLGs would be a slightly smaller spectral transmission range (normally 200–600 or 400–2000 nm) than that of a fiber optic bundle with broader transmittance (200–2000 nm).
Another option is infrared spectroscopy for additional information to WLE. Infrared wavelengths are longer and penetrate deeper than visible wavelengths. Therefore, there is potential for image information within deeper layers of tissue beyond the mucosa. A benefit of extended optical penetration depth could be identifying density variations in the tissue beyond the mucosal wall [38][39][40]. Similar to the vasculature, higher density could correlate to lesion growth, and this could enable early detection. Infrared illumination and imaging also has the potential to discriminate neoplasia from inflammation [41]. The limitation here is the need for a separate detector and illumination to outfit the infrared technology necessary to produce visible images for the user.
The mind map topics which combine new and old technologies to enhance contrast the images produced are detailed below. Here, they are labeled as: endoscopic machine learning, autofluorescence hyperspectral endoscopy, and hyperspectral chromoendoscopy. Machine learning has become an integral part of many fields, especially in applications that produce large datasets. Machine learning (ML), if implemented on a computational platform capable of real-time operation, could provide automated cues to clinicians that would aid in identifying potential abnormalities. ML outputs could be false-colored or overlayed in some other form with traditional WLE, NBI, AFI, or CE image data such that the cues are visible during a standard endoscopic technique. The requirements for ML in endoscopy need to focus on computation functioning in real-time. Hence, computational capabilities would have to be sufficiently developed to allow real-time classification and false coloring or superposition of classification results with traditional endoscopic procedures in order for this approach to be viable.
The specificity of AFI endoscopy techniques could be improved through enhanced contrast created by several endogenous fluorophores (native autofluorescence) in human tissue [42][43][44]. An optimal way to excite these autofluorescent biomarkers is hyperspectral imaging (particularly spectral scanning). Hyperspectral imaging generates complex image data, hypercubes, in which two dimensions represent spatial data and the third dimension represents spectroscopic data. Spectral hypercubes can be analyzed to estimate the contributions of different molecules, such as autofluorescent molecules, and these signatures can be false-colored and overlayed to generate added contrast in endoscopy images. However, autofluorescence is an inherently low signal, so the spectral illumination has to be powerful enough to provide sufficient excitation and emission signals for the detector. Current autofluorescence imaging in endoscopy typically highlights one or two endogenous molecules with one or two excitation sources [45][46][47][48][49]. Minimal excitation sources allow for longer acquisition and higher signal while maintaining video rates. For hyperspectral autofluorescence imaging of 5 or more endogenous molecules (assuming notable unique molecular and spectral contributions), shorter acquisition is required for the video rate, lowering the excitation signal. However, the excitation overlap could compound the excitation signal for each fluorescent biomarker. Most hyperspectral setups of this nature come with a trade-off between spatial resolution, acquisition rates, and spectral sampling. Researchers would expect that future endoscopic system will mitigate this trade-off in order to maintain the requirements of high definition and video rate imaging, while providing hyperspectral imaging capabilities.
The requirements discussed above for autofluorescence hyperspectral endoscopy would also apply for hyperspectral chromoendoscopy to be a viable addition to the endoscopic system. In this case, exogenous fluorophores (fluorescent dyes and stains) could be used to identify certain components, tissue types, or proteins, creating a list of biomarkers to image. This fluorescence mixture could be administered during bowel preparation or during the procedure, as is performed in traditional chromoendoscopy. Hyperspectral imaging, as described above, could then be performed to allow the identification of each of the many fluorescent labels. An additional benefit to exogenous fluorophores is that they produce greater emission signaling than autofluorescence. There are also exogenous fluorophores in the near-infrared range to expand components stained and increase the contrast [50][51]. Importantly, both of these hyperspectral techniques can provide new or complimentary data for machine learning scenarios to automatically identify and flag suspicious regions for further investigation.
To understand the potential technologies that could be incorporated into a next-generation endoscope, a Pugh matrix (Table 1) was constructed. The Pugh matrix is a system engineering tool that can be used to evaluate the importance and potential impact of each endoscope technology when considering a range of parameters. Modern endoscopic procedures are included as well for reference. Scoring parameters for the Pugh matrix were determined by first considering the patient (safety, invasiveness, and comfort), then prioritizing clinician training and technology implementation (i.e., operational training and implementation cost), and finally considering the additional image data and information that could be produced (new image data and contrast). Scoring (described below) was conducted by a panel of six gastroenterologists from the University of South Alabama Division of Gastroenterology. Each category (column) within the technology row was averaged among the n = 6 responses and the standard deviation was calculated. The matrix did not include any weighted metrics and was primarily used to compare new or potential technologies to the gold standard of WLE (labeled as Current Endoscopy) and to consider which technology could represent a future next-generation endoscope. The metrics were graded as follows: Safety—How safe would this procedure be? 5 = most, 1 = least. Invasive—How invasive would this procedure be? 5 = highly, 1 = minimally. Patient Comfort 5 = comfort, 1 = discomfort. Operational Training—How much operational training would be required? 5 = extensive, 1 = minimal. Example Image Training—How much training with example images is needed? 5 = extensive, 1 = minimal. Implementation—How easy would the technology be implemented? 5 = challenging, 1 = easy. Cost to Implement—How much would this technology cost to integrate? High = $$$ = 5, Low = $ = 1. Additional Image Data—How much additional image information would be produced? 5 = substantial, 1 = minimal. High Contrast—Would this technique produce a higher contrast than WLE? 5 = substantial, 1 = minimal. The final column of the matrix totals the scores for comparison (the values are in bold to highlight the overall results of the table). For this work, Invasive, Operational Training, Training Image Data, Implementation, and Cost were all subtracted from four, so the total was the summation of the positive connotations for each technology (i.e., the “inverse” of the aforementioned metrics were considered for the total—Invasive: five translated to a one for noninvasive).
Table 1. Pugh matrix ranking alternative technologies compared to the gold standard (WLE). Metrics for scoring include safety, training, implementation, and added information. The scores are totaled for comparison.
| Safety | Invasive | Patient Comfort | Operational Training |
Example Image Training | Implementation * | Cost to Implement | Additional Image Data |
Higher Contrast | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current Endoscopy | 4.3 ± 0.7 | 3.0 ± 1.6 | 4.0 ± 1.2 | 4.2 ± 0.7 | 3.7 ± 0.7 | 3.3 ± 1.4 | 2.8 ± 1.5 | 3.5 ± 1.4 | 3.0 ± 1.3 | 17.8 |
| Virtual Endoscopy | 4.7 ± 0.5 | 2.5 ± 1.5 | 4.5 ± 0.5 | 4.0 ± 0.8 | 4.2 ± 0.7 | 3.5 ± 0.8 | 3.5 ± 1.4 | 3.3 ± 1.1 | 3.0 ± 1.5 | 17.8 |
| Capsule Endoscopy | 4.2 ± 0.7 | 2.8 ± 1.2 | 4.0 ± 0.6 | 3.8 ± 0.7 | 4.2 ± 0.7 | 3.2 ± 1.3 | 3.5 ± 0.8 | 3.3 ± 1.1 | 2.8 ± 1.3 | 16.8 |
| Infrared Imaging Endoscopy |
3.7 ± 0.9 | 3.3 ± 1.2 | 4.0 ± 0.8 | 4.2 ± 0.4 | 4.2 ± 0.7 | 4.0 ± 0.8 | 3.8 ± 0.7 | 3.8 ± 0.7 | 3.2 ± 1.2 | 15.2 |
| Autofluorescence Hyperspectral Endoscopy |
4.0 ± 0.8 | 3.3 ± 1.2 | 4.0 ± 0.8 | 4.3 ± 0.7 | 4.7 ± 0.5 | 4.5 ± 0.8 | 3.7 ± 1.5 | 4.2 ± 0.9 | 4.0 ± 1.4 | 15.7 |
| Hyperspectral Chromoendoscopy |
3.8 ± 0.7 | 3.3 ± 1.2 | 4.2 ± 0.9 | 4.5 ± 0.8 | 4.7 ± 0.5 | 4.3 ± 0.7 | 3.8 ± 1.5 | 4.5 ± 0.8 | 4.3 ± 1.1 | 16.2 |
| Neural Network Endoscopy |
4.2 ± 0.9 | 3.5 ± 1.4 | 4.3 ± 0.9 | 4.7 ± 0.5 | 4.2 ± 0.9 | 4.2 ± 1.1 | 3.7 ± 1.9 | 4.2 ± 1.2 | 4.2 ± 1.2 | 16.7 |
References
- Boehm, B.W. A Spiral Model of Software Development and Enhancement. Computer 1988, 21, 61–72.
- Hossain, N.U.I.; Dayarathna, V.L.; Nagahi, M.; Jaradat, R. Systems Thinking: A Review and Bibliometric Analysis. Systems 2020, 8, 23.
- Lieberman, D.A.; Garewal, H. Use of Colonoscopy to Screen Asymptomatic Adults for Colorectal Cancer. N. Engl. J. Med. 2000, 343, 162–168.
- Kaltenbach, T.; Friedland, S.; Soetikno, R. A Randomised Tandem Colonoscopy Trial of Narrow Band Imaging versus White Light Examination to Compare Neoplasia Miss Rates. Gut 2008, 57, 1406.
- Levin, B.; Murphy, G.P. Revision in American Cancer Society Recommendations for the Earlydetection of Colorectal Cancer. CA Cancer J. Clin. 1992, 42, 296–299.
- Chiu, H.-M.; Chang, C.-Y.; Chen, C.-C.; Lee, Y.-C.; Wu, M.-S.; Lin, J.-T.; Shun, C.-T.; Wang, H.-P. A Prospective Comparative Study of Narrow-Band Imaging, Chromoendoscopy, and Conventional Colonoscopy in the Diagnosis of Colorectal Neoplasia. Gut 2007, 56, 373–379.
- Cho, J.-H. Advanced Imaging Technology Other than Narrow Band Imaging. Clin. Endosc. 2015, 48, 503.
- Negreanu, L.; Preda, C.; Ionescu, D.; Ferechide, D. Progress in Digestive Endoscopy: Flexible Spectral Imaging Colour Enhancement (FICE)-Technical Review. J. Med. Life 2015, 8, 416–422.
- Yung, D.E.; Carvalho, P.B.; Giannakou, A.; Kopylov, U.; Rosa, B.; Rondonotti, E.; Toth, E.; Plevris, J.N.; Koulaouzidis, A. Clinical Validity of Flexible Spectral Imaging Color Enhancement (FICE) in Small-Bowel Capsule Endoscopy: A Systematic Review and Meta-Analysis. Endoscopy 2017, 49, 258–269.
- Blachar, A.; Sosna, J. CT Colonography (Virtual Colonoscopy): Technique, Indications and Performance. Digestion 2007, 76, 34–41.
- Bressler, B.; Paszat, L.F.; Chen, Z.; Rothwell, D.M.; Vinden, C.; Rabeneck, L. Rates of New or Missed Colorectal Cancers After Colonoscopy and Their Risk Factors: A Population-Based Analysis. Gastroenterology 2007, 132, 96–102.
- Chung, S.J.; Kim, D.; Song, J.H.; Kang, H.Y.; Chung, G.E.; Choi, J.; Kim, Y.S.; Park, M.J.; Kim, J.S. Comparison of Detection and Miss Rates of Narrow Band Imaging, Flexible Spectral Imaging Chromoendoscopy and White Light at Screening Colonoscopy: A Randomised Controlled Back-to-Back Study. Gut 2014, 63, 785–791.
- Kim, N.H.; Jung, Y.S.; Jeong, W.S.; Yang, H.-J.; Park, S.-K.; Choi, K.; Park, D.I. Miss Rate of Colorectal Neoplastic Polyps and Risk Factors for Missed Polyps in Consecutive Colonoscopies. Intest. Res. 2017, 15, 411–418.
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal Cancer Statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34.
- Bolin, S.; Nilsson, E.; Sjödahl, R. Carcinoma of the Colon and Rectum--Growth Rate. Ann. Surg. 1983, 198, 151–158.
- Burke, J.R.; Brown, P.; Quyn, A.; Lambie, H.; Tolan, D.; Sagar, P. Tumour Growth Rate of Carcinoma of the Colon and Rectum: Retrospective Cohort Study. BJS Open 2020, 4, 1200–1207.
- Oto, A. Virtual Endoscopy. Eur. J. Radiol. 2002, 42, 231–239.
- Adler, S.N.; Metzger, Y.C. PillCam COLON Capsule Endoscopy: Recent Advances and New Insights. Ther. Adv. Gastroenterol. 2011, 4, 265–268.
- Gossum, A.V.; Fernandez-Urien, I.; Delvaux, M.; Neuhaus, H.; Riccioni, M.E.; Fraser, C.; Hagenmuller, F.; Devière, J. Capsule Endoscopy versus Colonoscopy for the Detection of Polyps and Cancer. N. Engl. J. Med. 2009, 361, 264–270.
- Azevedo, I.L.; Morgan, M.G.; Morgan, F. The Transition to Solid-State Lighting. Proc. IEEE 2009, 97, 481–510.
- Smith, S. The Scientist & Engineer’s Guide to Digital Signal Processing; California Technical Pub: Los Angeles, CA, USA, 1997.
- Tsai, T.-H.; Fujimoto, J.G.; Mashimo, H. Endoscopic Optical Coherence Tomography for Clinical Gastroenterology. Diagnostics 2014, 4, 57–93.
- Gaab, M.R. Instrumentation: Endoscopes and Equipment. World Neurosurg. 2013, 79, S14.e11–S14.e21.
- Swain, P. Role of Video Endoscopy in Managing Small Bowel Disease. Gut 2004, 53, 1866–1875.
- Iakovidis, D.K. Software Engineering Applications in Gastroenterology. Glob. J. Gastroenterol. Hepatol. 2014, 2, 11–18.
- van der Sommen, F.; de Groof, J.; Struyvenberg, M.; van der Putten, J.; Boers, T.; Fockens, K.; Schoon, E.J.; Curvers, W.; de With, P.; Mori, Y.; et al. Machine Learning in GI Endoscopy: Practical Guidance in How to Interpret a Novel Field. Gut 2020, 69, 2035–2045.
- Berci, G.; Forde, K. History of Endoscopy. Surg. Endosc. 2000, 14, 5–15.
- Mann, G.; Bozzini, P.; Bozzini, D. Der Frankfurter Lichtleiter: Neues Über Philipp Bozzini Und Sein Endoskop. Medizinhist. J. 1973, 8, 105–130.
- Rehnberg, V.; Walters, E. The Life and Work of Adolph Kussmaul 1822–1902: ‘Sword Swallowers in Modern Medicine. J. Intensive Care Soc. 2017, 18, 71–72.
- Hounnou, G.; Destrieux, C.; Desme, J.; Bertrand, P.; Velut, S. Anatomical Study of the Length of the Human Intestine. Surg. Radiol. Anat. 2002, 24, 290–294.
- Berci, G.; Davids, J. Endoscopy and Television. Br. Med. J. 1962, 1, 1610–1613.
- Soulas, A.; Dubois De Montreynaud, J.M.; Edwards, R.J.; Gladu, A.J. Bronchoscopy and Television. Dis. Chest 1957, 31, 580–584.
- Berci, G.; Shulman, A.; Morgenstern, L.; Paz-Partlow, M.; Cuschierei, A.; Wood, R. Television Choledochoscopy. Surg. Gynecol. Obstet. 1985, 160, 176–177.
- Baillie, J. The Endoscope. Gastrointest. Endosc. 2007, 65, 886–893.
- Jacobson, M.C.; deVere White, R.W.; Demos, S.G. In Vivo Testing of a Prototype System Providing Simultaneous White Light and near Infrared Autofluorescence Image Acquisition for Detection of Bladder Cancer. J. Biomed. Opt. 2012, 17, 036011.
- McWade, M.A.; Paras, C.; White, L.M.; Phay, J.E.; Solórzano, C.C.; Broome, J.T.; Mahadevan-Jansen, A. Label-Free Intraoperative Parathyroid Localization With Near-Infrared Autofluorescence Imaging. J. Clin. Endocrinol. Metab. 2014, 99, 4574–4580.
- Iseki, K.; Tatsuta, M.; Iishi, H.; Sakai, N.; Yano, H.; Ishiguro, S. Effectiveness of the Near-Infrared Electronic Endoscope for Diagnosis of the Depth of Involvement of Gastric Cancers. Gastrointest. Endosc. 2000, 52, 755–762.
- Ortiz-Fernandez-Sordo, J.; Sami, S.S.; Mansilla-Vivar, R.; Subramanian, V.; Mannath, J.; Telakis, E.; Ragunath, K. Evaluation of a Novel Infra-Red Endoscopy System in the Assessment of Early Neoplasia in Barretts Esophagus: Pilot Study from a Single Center. Dis. Esophagus 2018, 31, dox137.
- Betz, C.S.; Stepp, H.; Janda, P.; Arbogast, S.; Grevers, G.; Baumgartner, R.; Leunig, A. A Comparative Study of Normal Inspection, Autofluorescence and 5-ALA-Induced PPIX Fluorescence for Oral Cancer Diagnosis. Int. J. Cancer 2002, 97, 245–252.
- Zhao, H.L.; Zhang, C.P.; Zhu, H.; Jiang, Y.F.; Fu, X.B. Autofluorescence of Collagen Fibres in Scar. Skin Res. Technol. 2017, 23, 588–592.
- Deal, J.; Mayes, S.; Browning, C.; Hill, S.; Rider, P.; Boudreaux, C.; Rich, T.C.; Leavesley, S.J. Identifying Molecular Contributors to Autofluorescence of Neoplastic and Normal Colon Sections Using Excitation-Scanning Hyperspectral Imaging. J. Biomed. Opt. 2018, 24, 021207.
- Takeuchi, Y.; Hanaoka, N.; Hanafusa, M.; Ishihara, R.; Higashino, K.; Iishi, H.; Uedo, N. Autofluorescence Imaging of Early Colorectal Cancer. J. Biophotonics 2011, 4, 490–497.
- Bae, S.-J.; Lee, D.-S.; Berezin, V.; Kang, U.; Lee, K.-H. Multispectral Autofluorescence Imaging for Detection of Cervical Lesions: A Preclinical Study. J. Obstet. Gynaecol. Res. 2016, 42, 1846–1853.
- Chen, W.; Gao, X.; Tian, Q.; Chen, L. A Comparison of Autofluorescence Bronchoscopy and White Light Bronchoscopy in Detection of Lung Cancer and Preneoplastic Lesions: A Meta-Analysis. Lung Cancer 2011, 73, 183–188.
- Ignjatovic, A.; East, J.; Guenther, T.; Hoare, J.; Morris, J.; Ragunath, K.; Shonde, A.; Simmons, J.; Suzuki, N.; Thomas-Gibson, S. What Is the Most Reliable Imaging Modality for Small Colonic Polyp Characterization? Study of White-Light, Autofluorescence, and Narrow-Band Imaging. Endoscopy 2011, 43, 94–99.
- Falk, G.W. Autofluorescence Endoscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 209–220.
- Becker, A.; Hessenius, C.; Licha, K.; Ebert, B.; Sukowski, U.; Semmler, W.; Wiedenmann, B.; Grötzinger, C. Receptor-Targeted Optical Imaging of Tumors with near-Infrared Fluorescent Ligands. Nat. Biotechnol. 2001, 19, 327–331.
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2014, 43, 16–29.
More
Information
Subjects:
Engineering, Biomedical; Gastroenterology & Hepatology; Imaging Science & Photographic Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
25 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




