
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luminita Marin | -- | 5507 | 2022-10-18 15:53:39 | | | |
| 2 | Camila Xu | Meta information modification | 5507 | 2022-10-19 03:19:58 | | |
Video Upload Options
Erythromycin (ERY) is a macrolide compound with a broad antimicrobial spectrum which is currently being used to treat a large number of bacterial infections affecting the skin, respiratory tract, intestines, bones and other systems, proving great value from a clinical point of view. Despite this major advantage, ERY has low water solubility and is not stable under acidic conditions which leads to a limited efficacy and bioavailability. Apart from this, higher doses promote drug resistance and undesirable effects. In order to overcome these disadvantages, during the past decades, a large variety of ERY formulations, including nanoparticles, have emerged. This work presents the preparation and performances reported for ERY vesicles, such as liposomes, ethosomes, niosomes, micelles, cubosomes and solid lipid nano(micro) particles.
1. Introduction
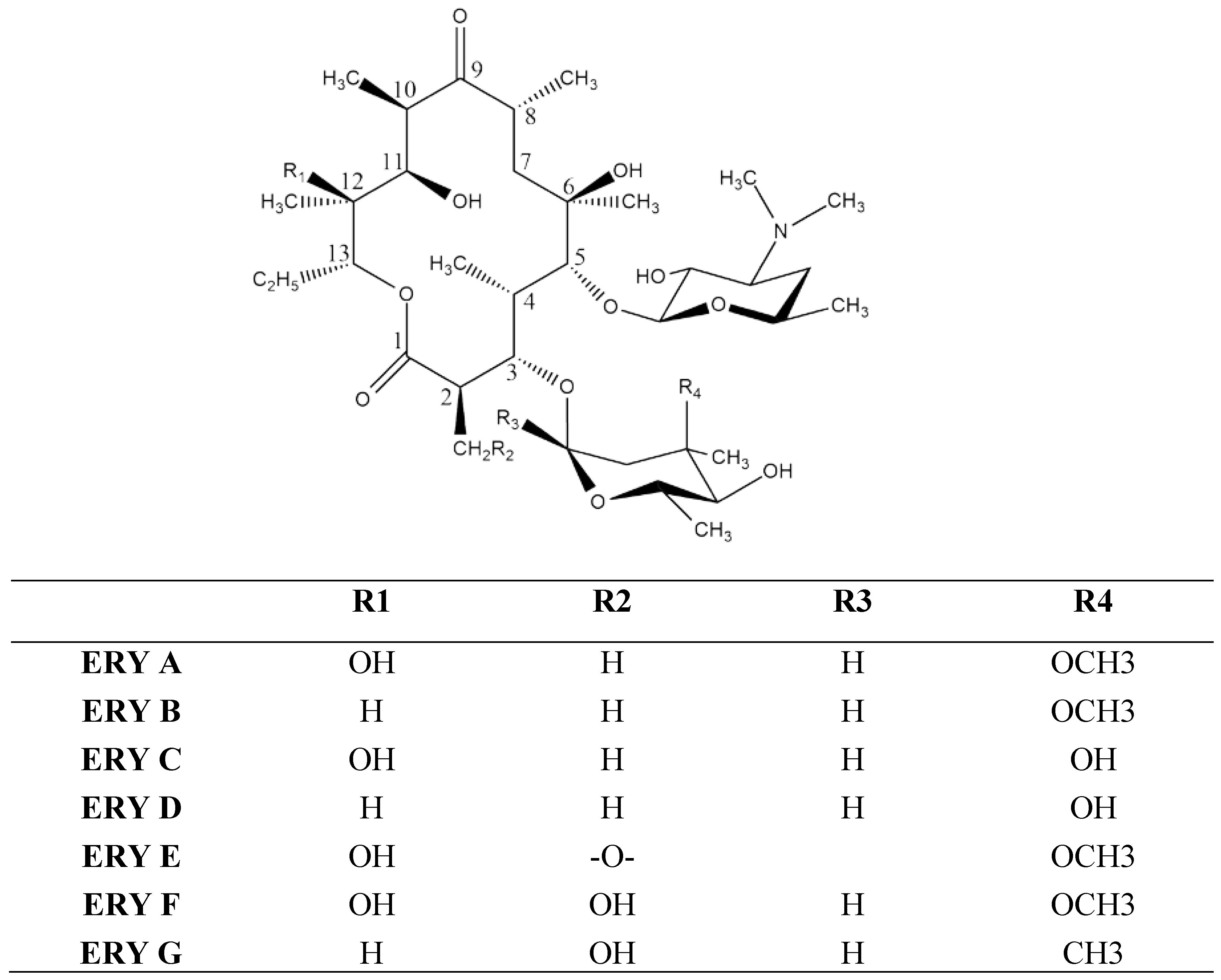
2. Vesicles
2.1. Liposomes
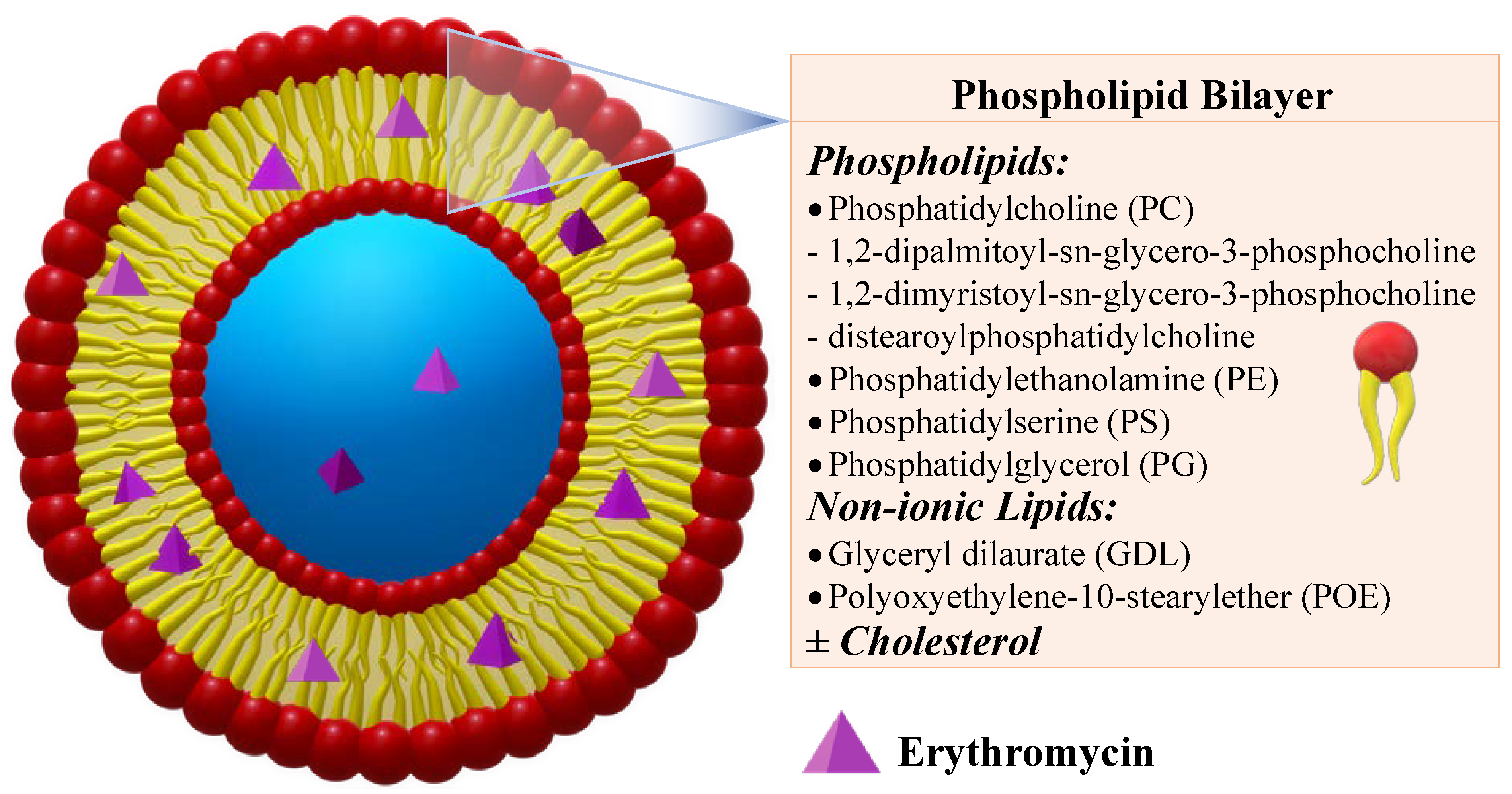
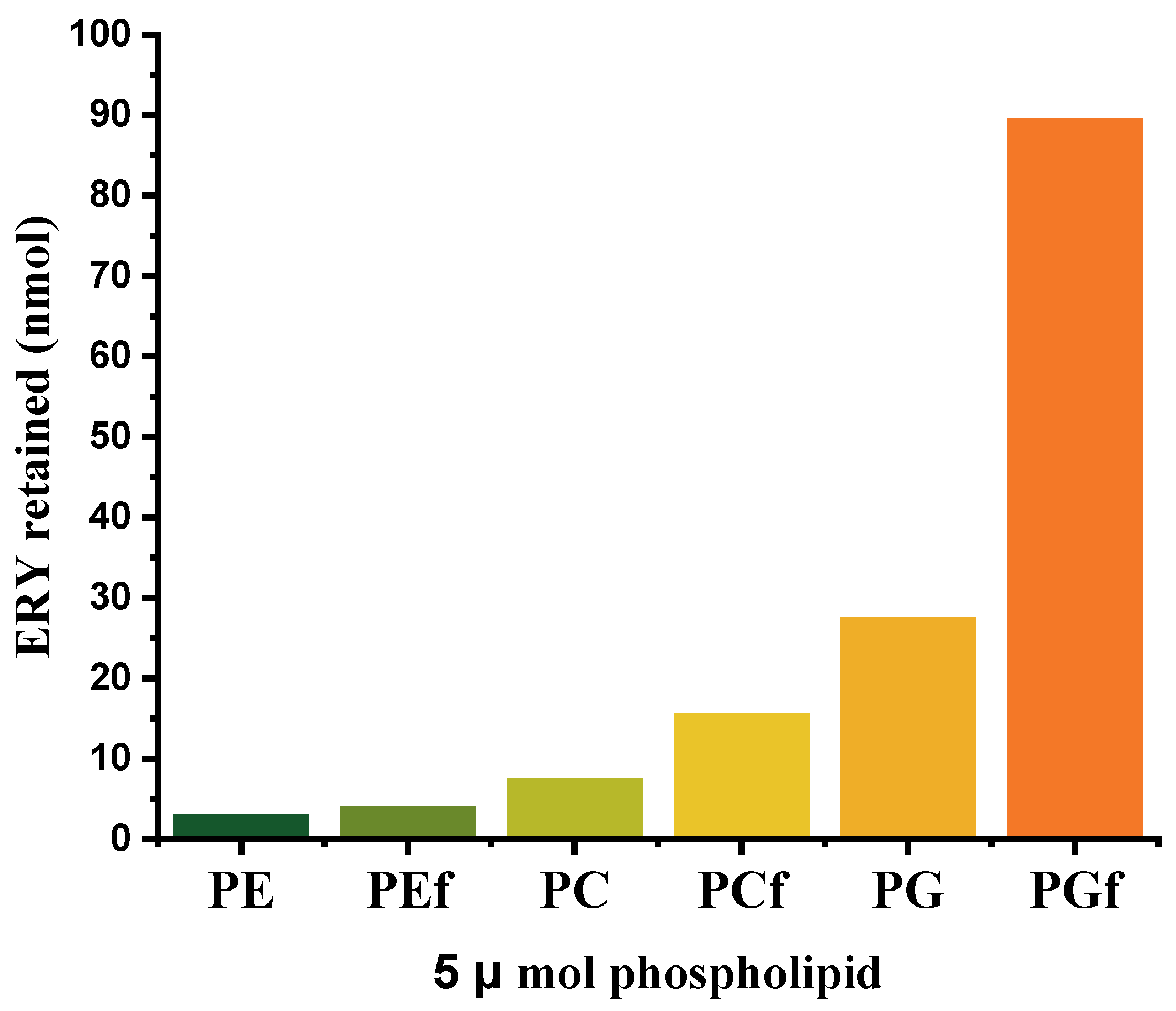
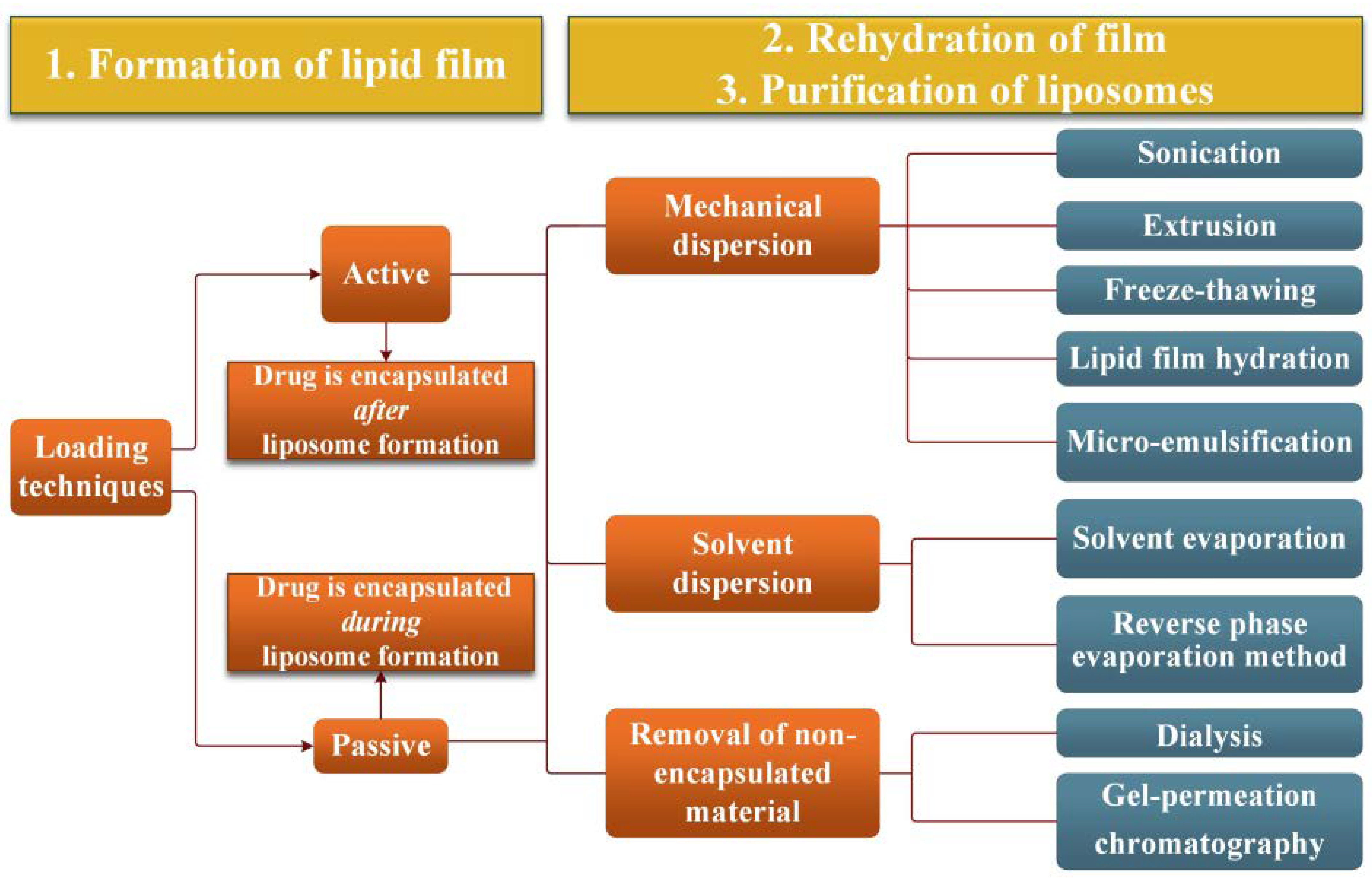
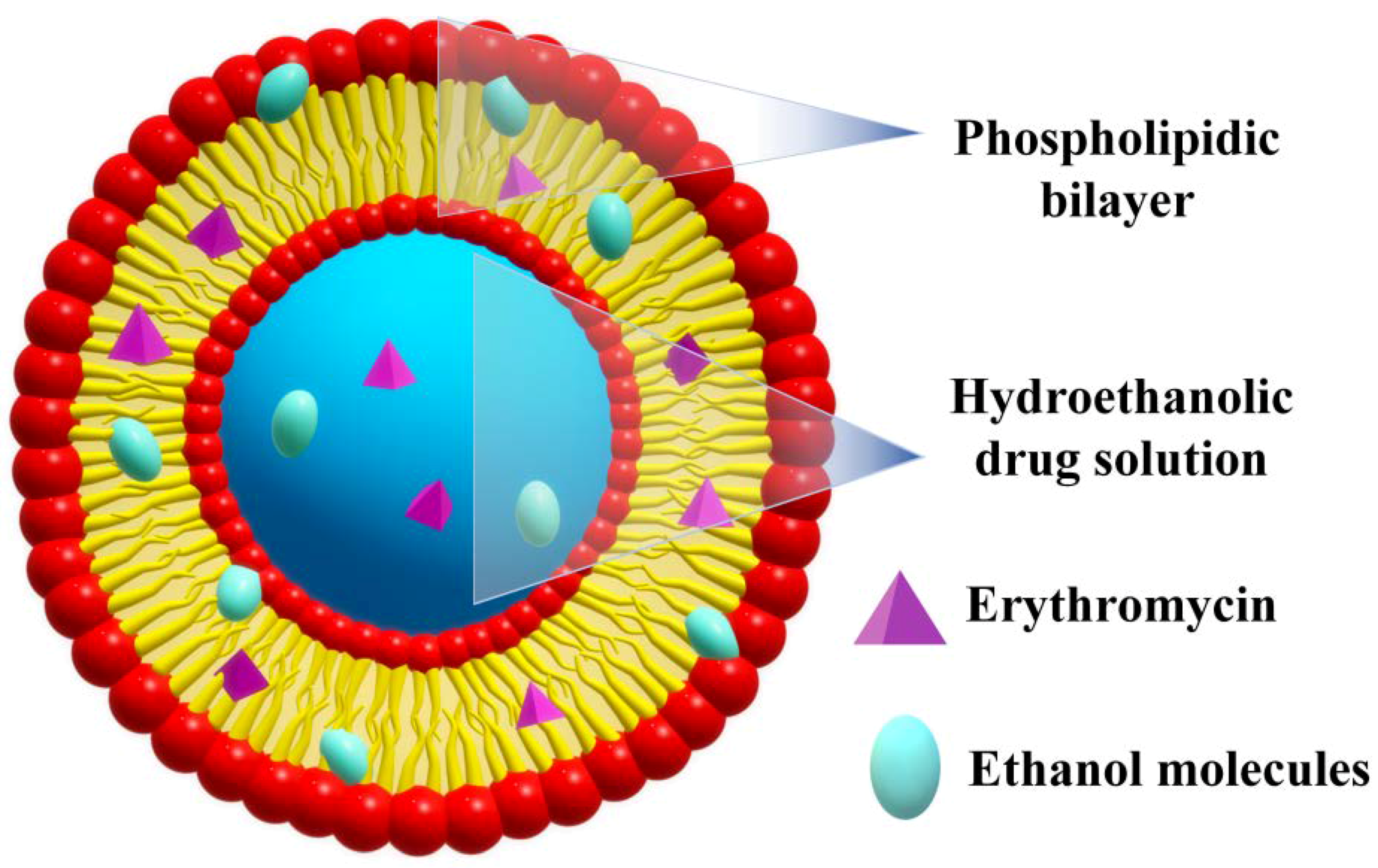
2.2. Ethosomes
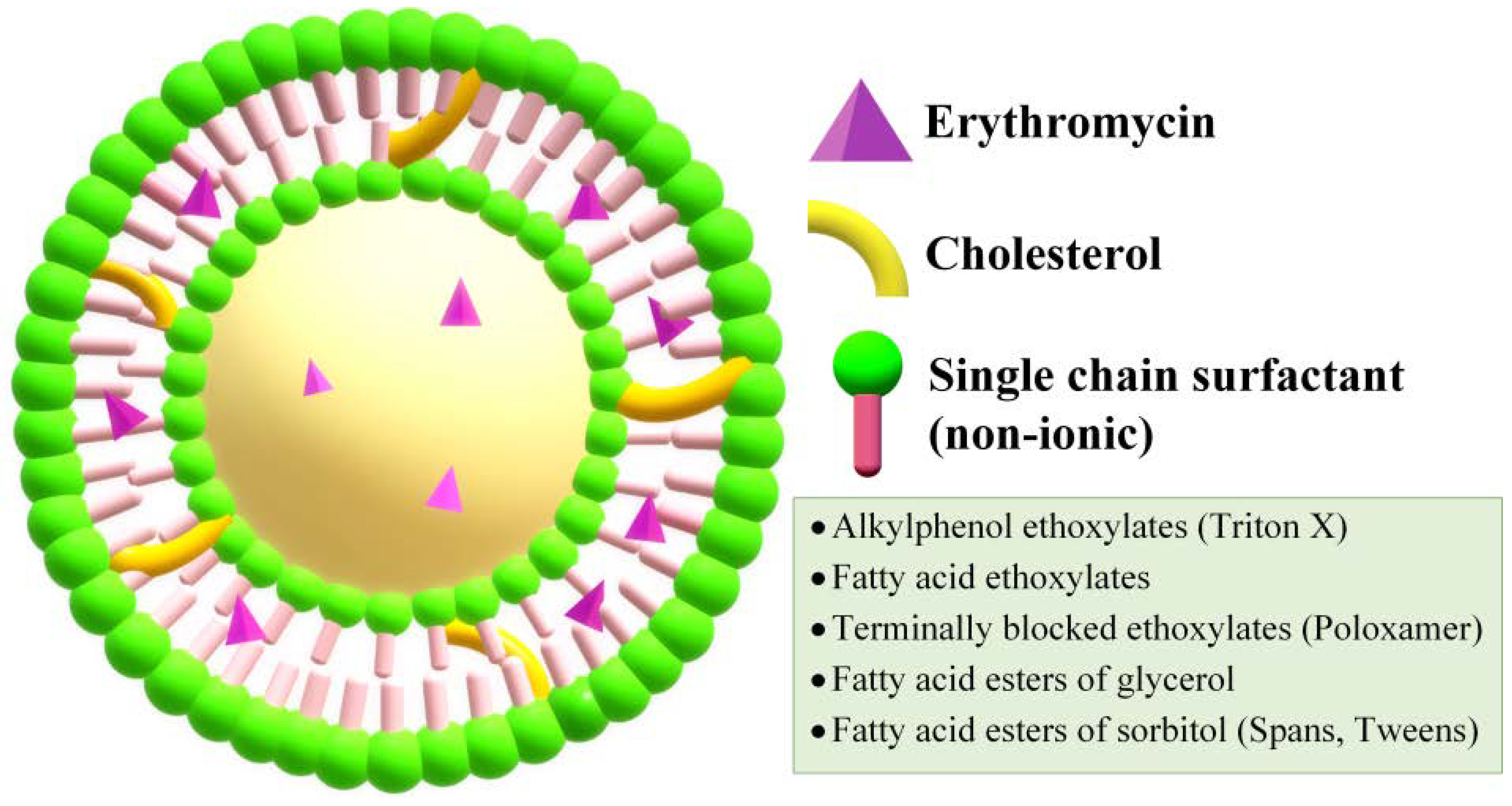
2.3. Niosomes
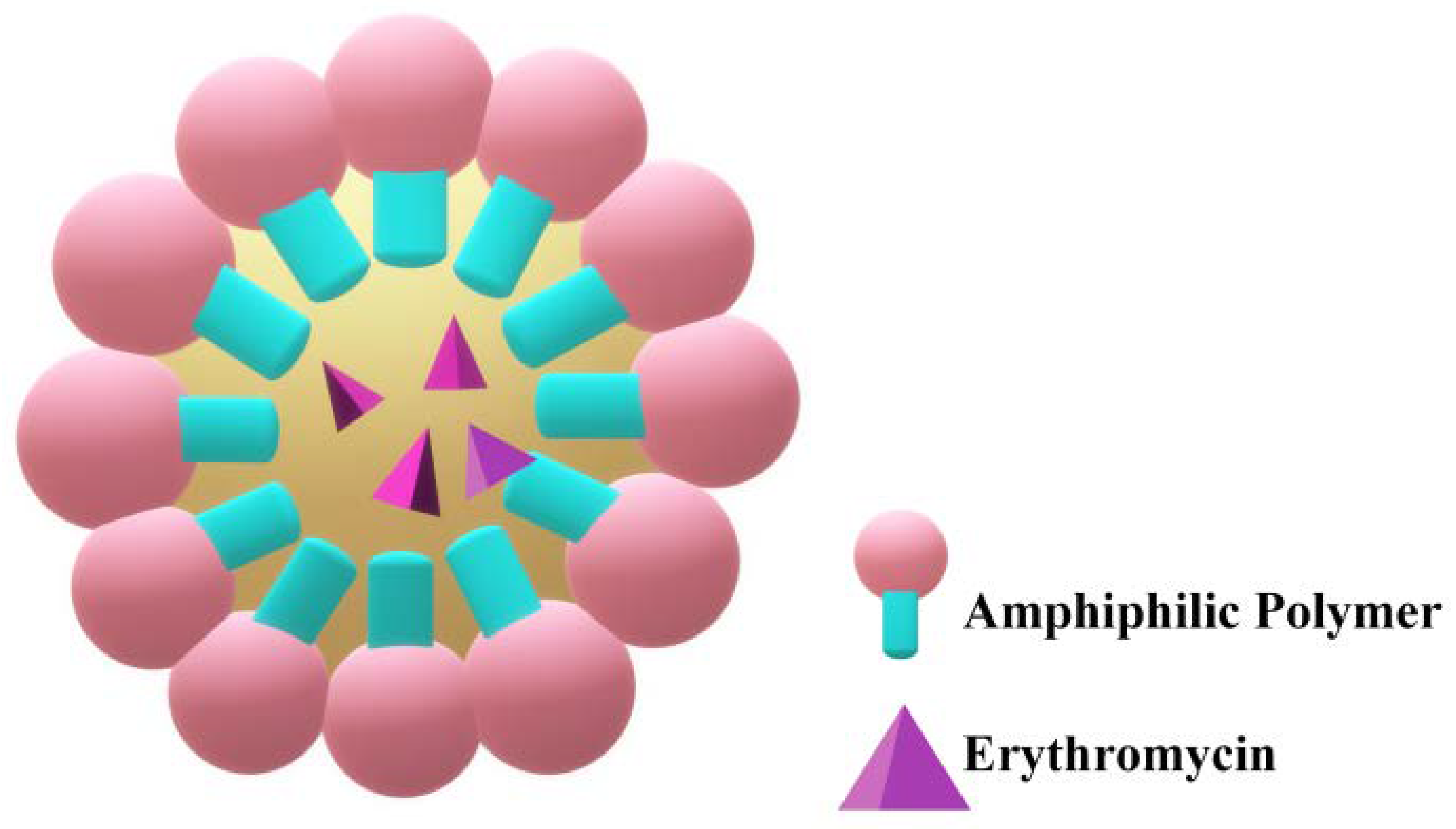
2.4. Micelles
2.5. Cubosomes
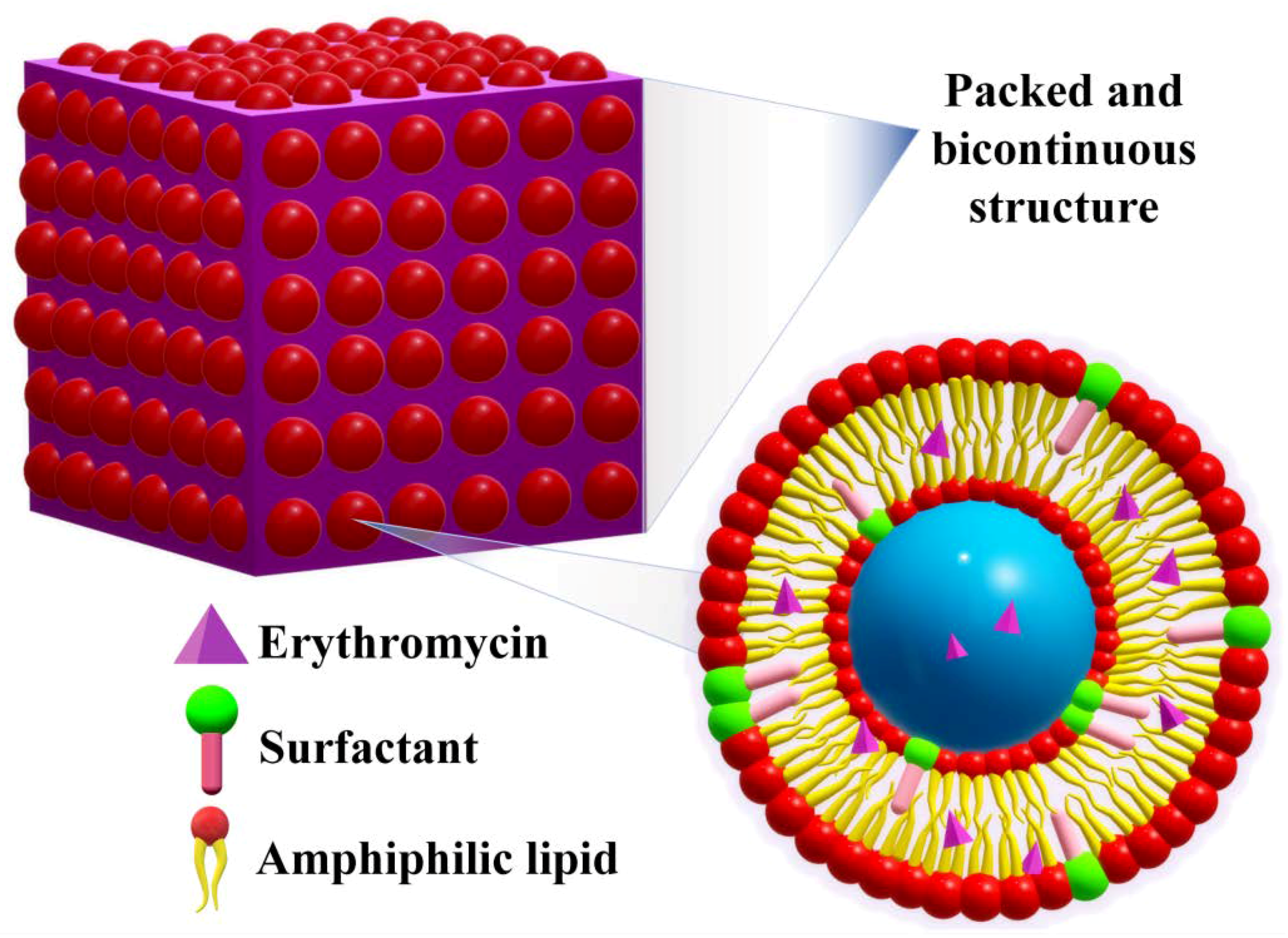
2.6. Solid Lipid Nano(Micro)Particles
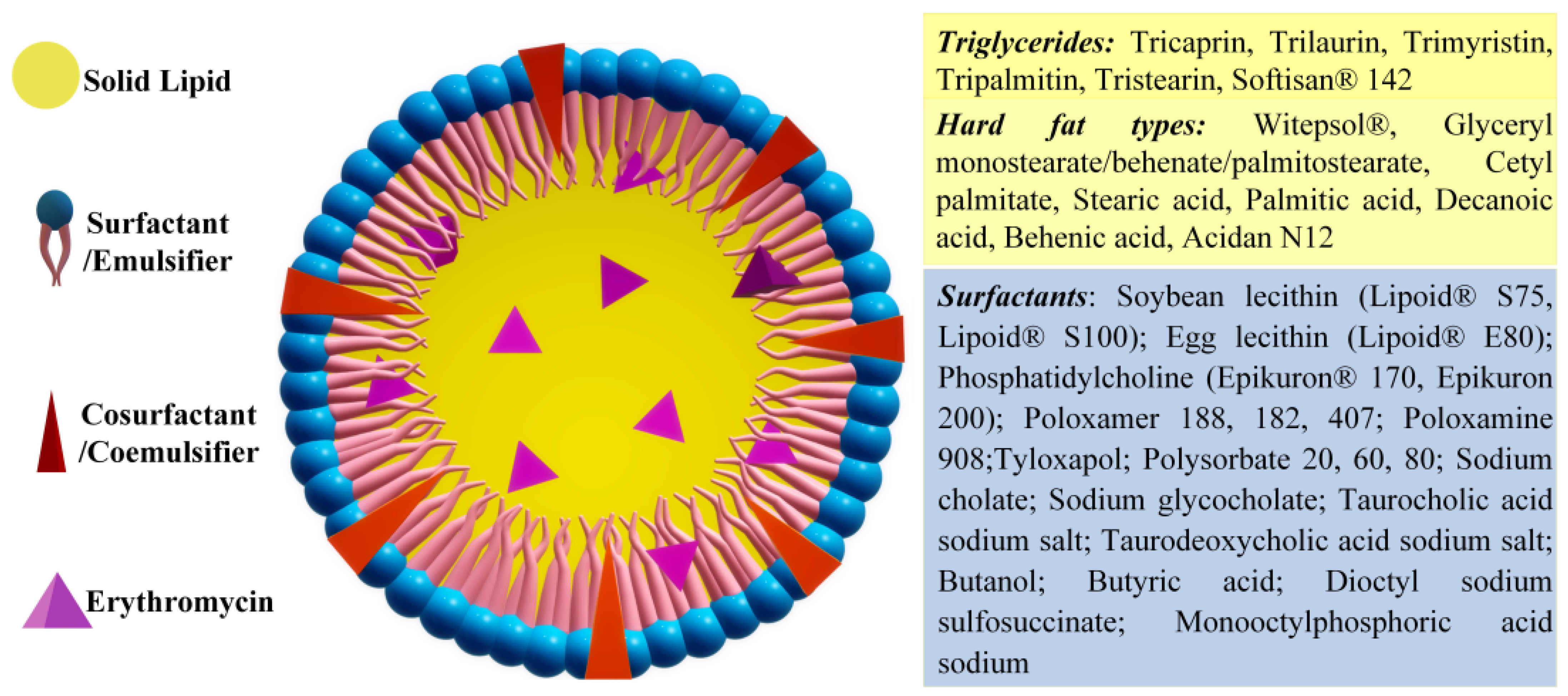
2.6.1. Solid Lipid Nanoparticles
2.6.2. Solid Lipid Microparticles
References
- Bunch, R.L.; Mcguire, J.M. Erythromycin, Its Salts, and Method of Preparation. U.S. Patent 2,653,899, 29 September 1952.
- P. Dehouck; Y. Vander Heyden; J. Smeyers-Verbeke; D.L. Massart; R.D. Marini; P. Chiap; Ph. Hubert; J. Crommen; W. Van de Wauw; J. De Beer; et al.R. CoxG. MathieuJ.C. ReepmeyerB. VoigtO. EstevenonA. NicolasA. Van SchepdaelE. AdamsJ. Hoogmartens Interlaboratory study of a liquid chromatography method for erythromycin: determination of uncertainty. Journal of Chromatography A 2003, 1010, 63-74, 10.1016/s0021-9673(03)01023-9.
- P. Dehouck; Y. Vander Heyden; J. Smeyers-Verbeke; D.L. Massart; R.D. Marini; P. Chiap; Ph. Hubert; J. Crommen; W. Van de Wauw; J. De Beer; et al.R. CoxG. MathieuJ.C. ReepmeyerB. VoigtO. EstevenonA. NicolasA. Van SchepdaelE. AdamsJ. Hoogmartens Interlaboratory study of a liquid chromatography method for erythromycin: determination of uncertainty. Journal of Chromatography A 2003, 1010, 63-74, 10.1016/s0021-9673(03)01023-9.
- F R Heilman; W E Herrell; W E Wellman; J E Geraci; Some laboratory and clinical observations on a new antibiotic, erythromycin (ilotycin).. Proceedings of the staff meetings. Mayo Clinic 1952, 27, 285–304.
- F R Heilman; W E Herrell; W E Wellman; J E Geraci; Some laboratory and clinical observations on a new antibiotic, erythromycin (ilotycin).. Proceedings of the staff meetings. Mayo Clinic 1952, 27, 285-304.
- Jeffrey C. Hoyt; Macrolide antibiotics and pulmonary inflammation. FEMS Microbiology Letters 2001, 205, 1-7, 10.1016/s0378-1097(01)00443-8.
- Stan Block; James Hedrick; Margaret R. Hammerschlag; Gail H. Cassell; J. Carl Craft; Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia. Pediatric Infectious Disease Journal 1995, 14, 471-477, 10.1097/00006454-199506000-00002.
- Horwitz, M.A.; Silverstein, S.C. Intracellular Multiplication of Legionnaires’ Disease Bacteria (Legionella Pneumophila) in Human Monocytes Is Reversibly Inhibited by Erythromycin and Rifampin. J. Clin. Investig. 1983, 71, 15–26.
- Rachel Kneen; Pham Ngoc Giao; Tom Solomon; Tran Thi My Van; Nguyen Thi Tuyet Hoa; Tran Buu Long; John Wain; Nicholas P. J. Day; Tran Tinh Hien; Christopher M. Parry; et al.Nicholas J. White Penicillin vs. Erythromycin in the Treatment of Diphtheria. Clinical Infectious Diseases 1998, 27, 845-850, 10.1086/514959.
- J. M. Rolain; P. Brouqui; J. E. Koehler; C. Maguina; M. J. Dolan; D. Raoult; Recommendations for Treatment of Human Infections Caused by Bartonella Species. Antimicrobial Agents and Chemotherapy 2004, 48, 1921-1933, 10.1128/aac.48.6.1921-1933.2004.
- Eduardo Salazar-Lindo; R. Bradley Sack; Elsa Chea-Woo; Bradford A. Kay; Zolia A. Piscoya; Raul Leon-Barua; August Yi; Early treatment with erythromycin of Campylobacter jejuni-associated dysentery in children. The Journal of Pediatrics 1986, 109, 355-360, 10.1016/s0022-3476(86)80404-8.
- Leslie Slaney; Hilary Chubb; Allan Ronald; Robert Brunham; In-vitro activity of azithromycin, erythromycin, ciprofloxacin and norfloxacin against Neisseria gonorrhoeae, Haemophilus ducreyi, and Chlamydia trachomatis. Journal of Antimicrobial Chemotherapy 1990, 25, 1-5, 10.1093/jac/25.suppl_a.1.
- Zheng Xu; Zengguo Wang; Yang Luan; Yarong Li; Xiaoguai Liu; Xiaokang Peng; Sophie Octavia; Michael Payne; Ruiting Lan; Genomic epidemiology of erythromycin-resistant Bordetella pertussis in China. Emerging Microbes & Infections 2019, 8, 461-470, 10.1080/22221751.2019.1587315.
- G.F. Webster; K.J. McGINLEY; J.J. Leyden; Inhibition of lipase production in Propionibacterium acnes by sub-minimal-inhibitory concentrations of tetracycline and erythromycin. British Journal of Dermatology 1981, 104, 453-457, 10.1111/j.1365-2133.1981.tb15317.x.
- Hikaru Tamura; Tomoki Maekawa; Hisanori Domon; Takumi Hiyoshi; Satoru Hirayama; Toshihito Isono; Karin Sasagawa; Daisuke Yonezawa; Naoki Takahashi; Masataka Oda; et al.Takeyasu MaedaKoichi TabetaYutaka Terao Effects of Erythromycin on Osteoclasts and Bone Resorption via DEL-1 Induction in Mice. Antibiotics 2021, 10, 312, 10.3390/antibiotics10030312.
- Samit Kumar Nandi; Prasenjit Mukherjee; Subhasis Roy; Biswanath Kundu; Dipak Kumar De; Debabrata Basu; Local antibiotic delivery systems for the treatment of osteomyelitis – A review. Materials Science and Engineering: C 2009, 29, 2478-2485, 10.1016/j.msec.2009.07.014.
- F H Weber; R D Richards; R W McCallum; Erythromycin: a motilin agonist and gastrointestinal prokinetic agent.. American Journal of Gastroenterology 1993, 88, 485–490.
- G J Sanger; Y Wang; A Hobson; J Broad; Motilin: towards a new understanding of the gastrointestinal neuropharmacology and therapeutic use of motilin receptor agonists. British Journal of Pharmacology 2013, 170, 1323-1332, 10.1111/bph.12075.
- Yang Fan; Wu Shan-Guang; Pan Yu-Fang; Song Feng-Lan; Li Tao; Preparation and characteristics of erythromycin microspheres for lung targeting. Drug Development and Industrial Pharmacy 2009, 35, 639-645, 10.1080/03639040802512243.
- Zhanzhong Wang; Jingkang Wang; And Meijing Zhang; Leping Dang; Solubility of Erythromycin A Dihydrate in Different Pure Solvents and Acetone + Water Binary Mixtures between 293 K and 323 K. Journal of Chemical & Engineering Data 2006, 51, 1062-1065, 10.1021/je0505265.
- Barry L. Carter; Jerold C. Woodhead; Katherine J. Cole; Gary Milavetz; Gastrointestinal Side Effects with Erythromycin Preparations. Drug Intelligence & Clinical Pharmacy 1987, 21, 734-738, 10.1177/106002808702100914.
- Dave Baguley; Emma Lim; Amanda Bevan; Ann Pallet; Saul Faust; Prescribing for children – taste and palatability affect adherence to antibiotics: a review. Archives of Disease in Childhood 2011, 97, 293-297, 10.1136/archdischild-2011-300909.
- Isadore Kanfer; Michael F Skinner; Roderick B Walker; Analysis of macrolide antibiotics. Journal of Chromatography A 1998, 812, 255-286, 10.1016/s0021-9673(98)00276-3.
- Jadhav, S.M.; Morey, P.; Karpe, M.M.; Kadam, V. Novel Vesicular System: An Overview. J. Appl. Pharm. Sci. 2012, 2, 193–202.
- Roxana-Maria Amarandi; Alina Ibanescu; Eugen Carasevici; Luminita Marin; Brindusa Dragoi; Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue. Pharmaceutics 2022, 14, 308, 10.3390/pharmaceutics14020308.
- Maria-Lucia Briuglia; Carlo Maria Rotella; Amber McFarlane; Dimitrios A. Lamprou; Influence of cholesterol on liposome stability and on in vitro drug release. Drug Delivery and Translational Research 2015, 5, 231-242, 10.1007/s13346-015-0220-8.
- W.W. Sułkowski; Danuta Pentak; K. Nowak; A. Sułkowska; The influence of temperature, cholesterol content and pH on liposome stability. Journal of Molecular Structure 2005, 744-747, 737-747, 10.1016/j.molstruc.2004.11.075.
- Sriram Vemuri; C.T Rhodes; Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharmaceutica Acta Helvetiae 1995, 70, 95-111, 10.1016/0031-6865(95)00010-7.
- Theresa M. Allen; Pieter R. Cullis; Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews 2013, 65, 36-48, 10.1016/j.addr.2012.09.037.
- A.D. Bangham; M.M. Standish; J.C. Watkins; Diffusion of univalent ions across the lamellae of swollen phospholipids. Journal of Molecular Biology 1965, 13, 238-252, 10.1016/s0022-2836(65)80093-6.
- Omid Rajabi; Pouran Layegh; Sara Hashemzadeh; Mohsen Khoddami; Topical liposomal azithromycin in the treatment of acute cutaneous leishmaniasis. Dermatologic Therapy 2016, 29, 358-363, 10.1111/dth.12357.
- Bahareh Abtahi-Naeini; Sajjad Hadian; Fatemeh Sokhanvari; Amirali Hariri; Jaleh Varshosaz; Zabihollah Shahmoradi; Awat Feizi; Farzin Khorvash; Atousa Hakamifard; Effect of Adjunctive Topical Liposomal Azithromycin on Systemic Azithromycin on Old World Cutaneous Leishmaniasis: A Pilot Clinical Study. null 2021, 20, 383-389, 10.22037/IJPR.2020.113710.14445.
- L. Stuhne-Sekalec; N. Z. Stanacev; S. Djokic; Liposomes as carriers of macrolides: Preferential association of erythromycin A and azithromycin with liposomes of phosphatidylglycerol containing unsaturated fatty acid(s). Journal of Microencapsulation 1991, 8, 171-183, 10.3109/02652049109071486.
- Clement Mugabe; Ali O. Azghani; Abdelwahab Omri; Preparation and characterization of dehydration–rehydration vesicles loaded with aminoglycoside and macrolide antibiotics. International Journal of Pharmaceutics 2006, 307, 244-250, 10.1016/j.ijpharm.2005.10.005.
- Clement Mugabe; Ali O. Azghani; Abdelwahab Omri; Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 2005, 55, 269-271, 10.1093/jac/dkh518.
- Shrenik P. Shah; Ambikanandan Misra; Liposomal amikacin dry powder inhaler: Effect of fines on in vitro performance. AAPS PharmSciTech 2004, 5, 107-113, 10.1208/pt050465.
- Douweh Gbian; Abdelwahab Omri; The Impact of an Efflux Pump Inhibitor on the Activity of Free and Liposomal Antibiotics against Pseudomonas aeruginosa. Pharmaceutics 2021, 13, 577, 10.3390/pharmaceutics13040577.
- Frédérick de Meyer; Berend Smit; Effect of cholesterol on the structure of a phospholipid bilayer. Proceedings of the National Academy of Sciences 2009, 106, 3654-3658, 10.1073/pnas.0809959106.
- Huangliang Zheng; Hai Tao; Jinzhao Wan; Kei Yan Lee; Zhanying Zheng; Sharon Shui Yee Leung; Preparation of Drug-Loaded Liposomes with Multi-Inlet Vortex Mixers. Pharmaceutics 2022, 14, 1223, 10.3390/pharmaceutics14061223.
- Abolfazl Akbarzadeh; Rogaie Rezaei-Sadabady; Soodabeh Davaran; Sang Woo Joo; Nosratollah Zarghami; Younes Hanifehpour; Mohammad Samiei; Mohammad Kouhi; Kazem Nejati-Koshki; Liposome: classification, preparation, and applications. Nanoscale Research Letters 2013, 8, 102-102, 10.1186/1556-276x-8-102.
- Shyamala C. Jayaraman; C. Ramachandran; Norman Weiner; Topical Delivery of Erythromycin from Various Formulations: An in Vivo Hairless Mouse Study. Journal of Pharmaceutical Sciences 1996, 85, 1082-1084, 10.1021/js960040u.
- T J. Majack; M B. Rust; K C. Massee; . G. W. Kissil; R W. Hardy; M E. Peterson; Bioencapsulation of erythromycin using adult brine shrimp, Artemia franciscana (Latreille). Journal of Fish Diseases 2000, 23, 71-76, 10.1046/j.1365-2761.2000.00210.x.
- Loredana Nicoleta Hilițanu; Liliana Mititelu-Tarțău; Grațiela Eliza Popa; Beatrice Rozalina Buca; Liliana Lăcrămioara Pavel; Ana-Maria Pelin; Andreea-Daniela Meca; Maria Bogdan; Daniela Angelica Pricop; The Analysis of Chitosan-Coated Nanovesicles Containing Erythromycin—Characterization and Biocompatibility in Mice. Antibiotics 2021, 10, 1471, 10.3390/antibiotics10121471.
- Kulkarni, P.; Yadav, J.; Vaidya, K. Liposomes: A Novel Drug Delivery System. International Journal of Current Pharmaceutical Research. 2011, 3, 10–18.
- Nina Filipczak; Jiayi Pan; Satya Siva Kishan Yalamarty; Vladimir P. Torchilin; Recent advancements in liposome technology. Advanced Drug Delivery Reviews 2020, 156, 4-22, 10.1016/j.addr.2020.06.022.
- Songhee Jeong; Jonghwan Lee; Byeong Nam Im; Hyung Park; Kun Na; Combined photodynamic and antibiotic therapy for skin disorder via lipase-sensitive liposomes with enhanced antimicrobial performance. Biomaterials 2017, 141, 243-250, 10.1016/j.biomaterials.2017.07.009.
- Hyejin Park; Hyung Park; Kun Na; Dual Propionibacterium acnes therapy using skin penetration-enhanced liposomes loaded with a photosensitizer and an antibiotic. Journal of Porphyrins and Phthalocyanines 2015, 19, 956-966, 10.1142/s1088424615500686.
- Abolfazl Akbarzadeh; Mohammad Samiei; Soodabeh Davaran; Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Research Letters 2012, 7, 144-144, 10.1186/1556-276x-7-144.
- Bassant M. Salah; Mai Rady; Mohammad Abdel-Halim; Heba M. Fahmy; Nermeen S. El-Din; Mohamed H. Gaber; Alternating Magnetic Field Induced Membrane Permeability in Erythromycin Magneto-Liposomes A Potential Solution to Antibiotic Resistance. Biophysics 2021, 66, 264-272, 10.1134/s0006350921020196.
- Joan Estelrich; Elvira Escribano; Josep Queralt; Maria Antònia Busquets; Iron Oxide Nanoparticles for Magnetically-Guided and Magnetically-Responsive Drug Delivery. International Journal of Molecular Sciences 2015, 16, 8070-8101, 10.3390/ijms16048070.
- Nicusor Fifere; Anton Airinei; Daniel Timpu; Aurelian Rotaru; Liviu Sacarescu; Laura Ursu; New insights into structural and magnetic properties of Ce doped ZnO nanoparticles. Journal of Alloys and Compounds 2018, 757, 60-69, 10.1016/j.jallcom.2018.05.031.
- J.-P. Montenez; Françoise Van Bambeke; J. Piret; R. Brasseur; P.M. Tulkens; M.-P. Mingeot-Leclercq; Interactions of Macrolide Antibiotics (Erythromycin A, Roxithromycin, Erythromycylamine [Dirithromycin], and Azithromycin) with Phospholipids: Computer-Aided Conformational Analysis and Studies on Acellular and Cell Culture Models. Toxicology and Applied Pharmacology 1999, 156, 129-140, 10.1006/taap.1999.8632.
- Ibrahim M. Abdulbaqi; Yusrida Darwis; Nurzalina Abdul Karim Khan; Reem Abou Assi; Arshad Ali Khan; Ethosomal nanocarriers: the impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. International Journal of Nanomedicine 2016, ume 11, 2279-2304, 10.2147/ijn.s105016.
- Elka Touitou; Hiba Natsheh; Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129, 10.3390/pharmaceutics13122129.
- Hiba Natsheh; Elka Touitou; Phospholipid Vesicles for Dermal/Transdermal and Nasal Administration of Active Molecules: The Effect of Surfactants and Alcohols on the Fluidity of Their Lipid Bilayers and Penetration Enhancement Properties. Molecules 2020, 25, 2959, 10.3390/molecules25132959.
- Biana Godin; Elka Touitou; Erythromycin Ethosomal Systems: Physicochemical Characterization and Enhanced Antibacterial Activity. Current Drug Delivery 2005, 2, 269-275, 10.2174/1567201054367931.
- B. Godin; E. Touitou; E. Rubinstein; A. Athamna; M. Athamna; A new approach for treatment of deep skin infections by an ethosomal antibiotic preparation: an in vivo study. Journal of Antimicrobial Chemotherapy 2005, 55, 989-994, 10.1093/jac/dki125.
- Ketousetuo Kuotsu; Kazi Masud Karim; Asim Sattwa Mandal; Nikhil Biswas; Arijit Guha; Sugata Chatterjee; Mamata Behera; Niosome: A future of targeted drug delivery systems. Journal of Advanced Pharmaceutical Technology & Research 2010, 1, 374-80, 10.4103/0110-5558.76435.
- Vyas, J.; Puja, V.; Sawant, K. Formulation and Evaluation of Topical Niosomal Gel of Erythromycin. International Journal of Pharmacy and Pharmaceutical Sciences 2011, 3, 123–126.
- Vyas, J.; Vishal, G.; Tejas, G.; Vishal, C.; Umesh, U. Formulation and Characterization of Topical Gel of Erythromycin Entrapped into Niosomes. International Journal of PharmTech Research 2011, 3, 1714–1718.
- Mohammadi, S.; Farajzadeh, S.; Pardakhty, A.; Khalili Meybodi, M.; Mohebbi, A.; Yousefian, M.R.; Aflatoonian, M. A Survey to Compare the Efficacy of Niosomal Erythromycin Alone versus Combination of Erythromycin and Zinc Acetate in the Treatment of Acne Vulgaris. Journal of Kerman University of Medical Sciences 2017, 24, 420–430.
- Tao Liu; Ting Wu; Hongxi Liu; Bo Ke; Hongxing Huang; Zhenyou Jiang; Mingqiang Xie; Ultraviolet-crosslinked hydrogel sustained-release hydrophobic antibiotics with long-term antibacterial activity and limited cytotoxicity. Journal of Applied Polymer Science 2014, 131, 40438, 10.1002/app.40438.
- Rajib Basak; Ranjini Bandyopadhyay; Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350-4356, 10.1021/la304836e.
- Y Huang; Y Sun; Q Wang; Encapsulation and in vitro release of erythromycin using biopolymer micelle.. Cellular and molecular biology 2015, 61, 60-64.
- Huiling Song; Yu Yin; Jiahui Peng; Zixiu Du; Wei Bao; Preparation, Characteristics, and Controlled Release Efficiency of the Novel PCL-PEG/EM Rod Micelles. Journal of Nanomaterials 2021, 2021, 1-10, 10.1155/2021/8132868.
- Hanna Maria Gabriella Barriga; Margaret Nancy Holme; Molly M. Stevens; Cubosomes: The Next Generation of Smart Lipid Nanoparticles?. Angewandte Chemie International Edition 2019, 58, 2958-2978, 10.1002/anie.201804067.
- Sana Khan; Poorva Jain; Sourabh Jain; Richa Jain; Saurabh Bhargava; Aakanchha Jain; Topical Delivery of Erythromycin Through Cubosomes for Acne. Pharmaceutical Nanotechnology 2018, 6, 38-47, 10.2174/2211738506666180209100222.
- Séverine Jaspart; Géraldine Piel; Luc Delattre; Brigitte Evrard; Solid lipid microparticles: formulation, preparation, characterisation, drug release and applications. Expert Opinion on Drug Delivery 2005, 2, 75-87, 10.1517/17425247.2.1.75.
- Christian Joachim; To be nano or not to be nano?. Nature Materials 2005, 4, 107-109, 10.1038/nmat1319.
- Nicolae Strambeanu; Laurentiu Demetrovici; Dan Dragos; Mihai Lungu; Nanoparticles: Definition, Classification and General Physical Properties. Nanoparticles’ Promises and Risks: Characterization, Manipulation, and Potential Hazards to Humanity and the Environment 2014, null, 3-8, 10.1007/978-3-319-11728-7_1.
- Preshita P. Desai; Abhijit A. Date; Vandana B. Patravale; Overcoming poor oral bioavailability using nanoparticle formulations – opportunities and limitations. Drug Discovery Today: Technologies 2012, 9, e87-e95, 10.1016/j.ddtec.2011.12.001.
- Wolfgang Mehnert; Solid lipid nanoparticles Production, characterization and applications. Advanced Drug Delivery Reviews 2001, 47, 165-196, 10.1016/s0169-409x(01)00105-3.
- Sebastián Scioli Montoto; Giuliana Muraca; María Esperanza Ruiz; Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Frontiers in Molecular Biosciences 2020, 7, 587997, 10.3389/fmolb.2020.587997.
- Anil Kumar Sahu; Tekeshwar Kumar; Vishal Jain; Formulation Optimization of Erythromycin Solid Lipid Nanocarrier Using Response Surface Methodology. BioMed Research International 2014, 2014, 1-8, 10.1155/2014/689391.
- Pallavi Dhillon; Mohd. Aamir Mirza; Khalid Anwer; Abdullah Saud Alshetaili; Saad Maria Alshahrani; Zeenat Iqbal; Development and optimization of erythromycin-loaded lipid-based gel by Taguchi design: In vitro characterization and antimicrobial evaluation. Brazilian Journal of Pharmaceutical Sciences 2019, 55, e17395, 10.1590/s2175-97902019000217395.
- Pignatello, R.; Fuochi, V.; Petronio Petronio, G.; Greco, A.; Furneri, P.M. Formulation and Characterization of Erythromycin–Loaded Solid Lipid Nanoparticles. Biointerface Research in Applied Chemistry 2017, 7, 2145–2150
- Mumuni A. Momoh; Emmanuel C. Ossai; Omeje E. Chidozie; Omenigbo O. Precscila; Franklin C. Kenechukwu; Kenneth O. Ofokansi; Anthony A. Attama; Kunle O. Olobayo; A new lipid-based oral delivery system of erythromycin for prolong sustain release activity. Materials Science and Engineering: C 2018, 97, 245-253, 10.1016/j.msec.2018.12.041.
- Ifeanyi T. Nzekwe; Anselm C. Okere; Ifeanyi E. Okoye; Kokonne E. Ekere; Adaobi A. Ezenwa; Chukwuma O. Agubata; Bioassay-guided optimization of lipid-based erythromycin microparticles. Tropical Journal of Pharmaceutical Research 2020, 19, 1351-1358, 10.4314/tjpr.v19i7.2.
- Salvador Alvarez-Elcoro; Mark J. Enzler; The Macrolides: Erythromycin, Clarithromycin, and Azithromycin. Mayo Clinic Proceedings 1999, 74, 613-634, 10.4065/74.6.613.




