Fluorescently labelled heterocyclic compounds are useful in bioanalytical applications, including in vivo imaging, high throughput screening, diagnostics, and light-emitting diodes. These compounds have various therapeutic properties, including antifungal, antitumor, antimalarial, anti-inflammatory, and analgesic activities. Different neutral fluorescent markers containing nitrogen heterocycles (quinolones, azafluoranthenes, pyrazoloquinolines, etc.) have several electrochemical, biological, and nonlinear optic applications. Photodynamic therapy (PDT), which destroys tumors and keeps normal tissues safe, works in the presence of molecular oxygen with light and a photosensitizing drugs (dye) to obtain a therapeutic effect.
1. Introduction
Molecular luminescence approaches include phosphorescence and fluorescence. A photon is absorbed by an analyte molecule, which stimulates a species. The emission spectrum can be used for quantitative and qualitative studies
[1][2]. Because of their potential various functional applications, luminous materials have received significant attention lately
[3]. They have been widely employed, including in the food, pharmaceutical, optical, and textile sectors
[4][5]. Conventionally, inorganic emitting materials were commonly used; however, organic luminescent materials with brilliant emission have largely replaced them due to their wide range of uses, including emergency lighting, low cost, environmental friendliness, long-term solutions, anti-counterfeiting displays, and in food, cosmetics, polymers, bioactive molecules, and biochemistry
[6][7]. Furthermore, in today’s research, the design of novel luminous hybrid organic–nonorganic materials is critical
[8][9]. The combination of phosphorescent dye as a sensitizer co-doped with a fluorescence emitter has made progress in developing luminescent materials for organic light-emitting diodes (OLEDs) in recent years
[10][11].
Fluorescence quenching or fluorescence enhancement is employed as an analytical technique
[12][13]. The fluorescent labelling of the host molecule complex provides a useful tool for detecting the analyte’s attachment to the host molecule
[14][15][16]. For example, protein labelling using small molecule-based fluorescent probes is used in various biological experiments and is a valuable technique for determining the expression level and localization of a protein of interest in living cells
[17].
Due to particular biological activity, crown ether’s derivate and
N-containing heterocyclic chemicals and their derivatives have been widely employed in agronomy and medicine
[18]. Similar organic chemicals are of interest in pharmacology as effective tissue oxygenators and antidepressants, as well as in biotechnology; these compounds are employed for macromolecule binding
[19][20][21][22][23].
N-containing heterocyclic compounds and macrocycle derivative crown ethers have remarkable photochemical, catalytic, and luminescent capabilities, indicating that they might be used to diagnose and cure various ailments. A few of their applications include photodynamic treatment and antimicrobial/antiparasitic activities against human pathogens and malarial parasites. Employment of fluorophores, including organic chromophores and crown ethers, with high selectivity, sensitivity, and stability constants while detecting tumor cells opens up new avenues for cancer research
[24][25].
Macrocyclic molecules, for example, crown ether, have been used in a wide range of chemical processes, including selective metal complexing agents and photo-induced electron transfer bio-mimetic research
[26][27][28][29]. In contrast to the extensive coordination chemistry, little is known about crown ether coordination compounds’ photoluminescence (PL). Crown ethers substituted with particular fluorescent dyes were the most commonly reported for PL. The use of such dye-substituted systems in sensing and analytical chemistry to detect the presence of particular metal cations was intensively investigated
[30][31][32].
A smart fluorescent probe with a crown ether moiety might be constructed as a sensor for metal anions, ions, and other biomolecules and then used to monitor biological processes in vivo
[33]. The solvent effects of a crown ether complex containing a fluorescent anthracene unit are exceptional
[34].
Supramolecular chemistry, inspired by nature’s vast array of assemblies, has garnered significant attention in recent decades due to its diverse supra-structures, which consist of micelles, vesicles, and fibers, as well as its wide-ranging applications in sensors, drug delivery, luminescent materials, and bioimaging
[35][36][37][38].
Fluorescence characteristics of
N-containing heterocyclic compounds have recently received considerable interest. For example, fluorescent compounds known as quinolines have attracted the attention of scientists because of their use in high-tech applications
[39]. Similarly, derivatives of the pyrazoloquinoline (PQ) family and quinoline are an example of fluorescent substances that may be of interest for several applications, including their use as oxidant scavengers and growth promoters
[40][41]. These have also been found naturally in a wide range of foods and appear to be easily absorbed. More recently, heterocyclic azo compounds such as benzothiazole, pyrazole, and thiazole have been employed for electrochemical, biological, and nonlinear optics applications and structure–activity relationships for drug designing [SAR]
[42][43][44]. Thiophene and thienopyrimidine derivatives have fluorescence features and are more efficient than other aromatic chemicals for anti-avian influenza virus (H5N1) action. Porphyrins are N-heterocyclic chemicals present in a wide variety of biological systems. Metalloporphyrins contain solely -pyrrolic substituents in biological systems and appear attached to proteins, creating supramolecular structures such as haemoglobin, myoglobin, cytochromes, catalases, and peroxidases, as well as chlorophylls and bacteriochlorophylls in reduced forms
[45].
2. Applications of Heterocyclic Compounds
2.1. Anti-Mycobacterial Activity
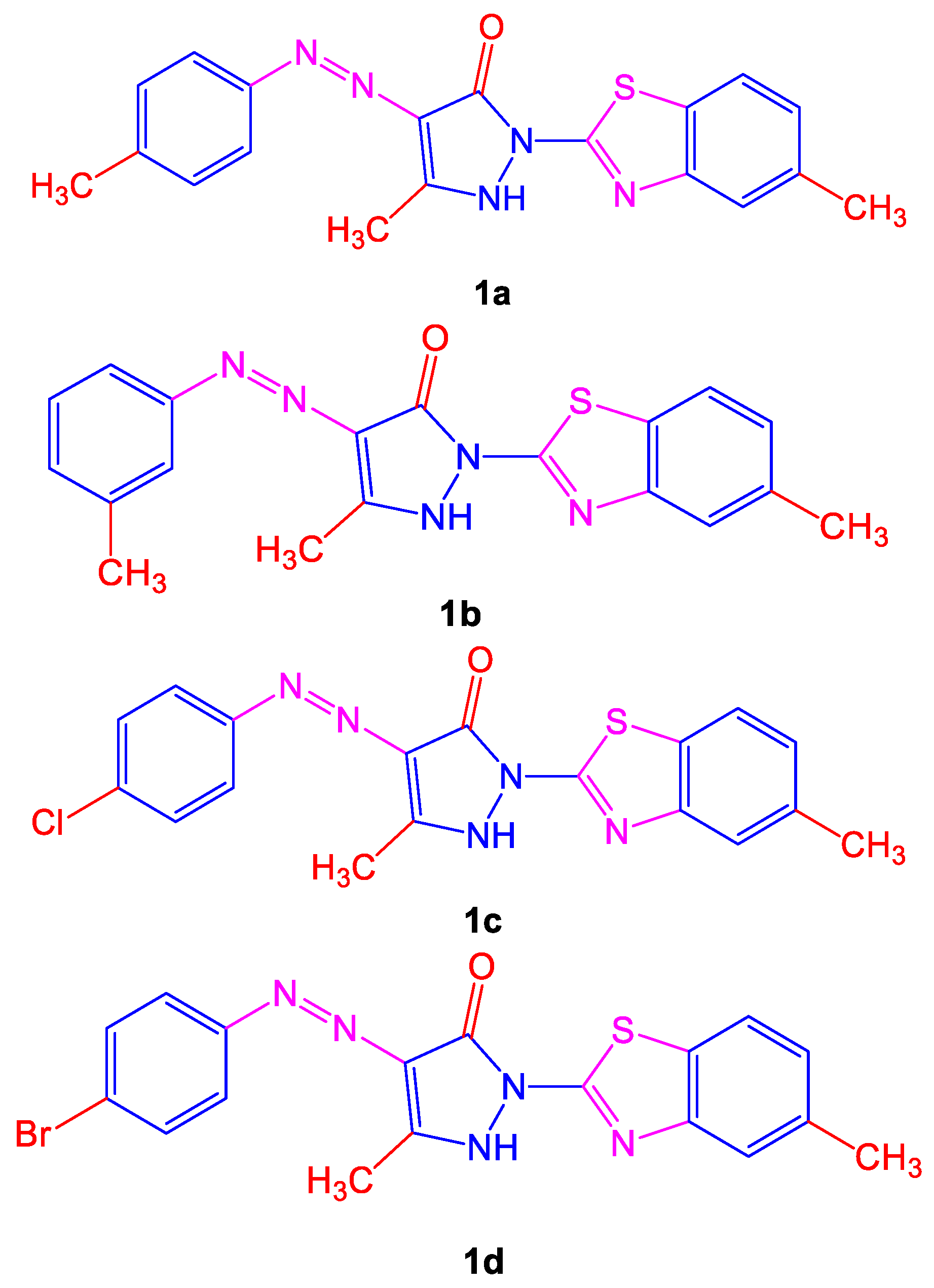
Different symptoms such as respiratory issues, long-term coughs, and tuberculosis are treated by various plants in African and Asian countries. Many anti-tubercular drugs, with toxicity and side effects, are still used to treat tuberculosis. For treating
M. tuberculosis, the synthesis of azo compounds was monitored and showed anti-tubercular activity. Maximum activity was shown by compounds 5-methyl-2-(5-methylbenzo[
d]thiazol-2-yl)-4-(
p-tolyldiazenyl)-1
H-pyrazol-3(2
H)-one
(1a) and 5-methyl-2-(5-methylbenzo[
d]thiazol-2-yl)-4-(
m-tolyldiazenyl)-1
H-pyrazol-3(2
H)-one
(1b) when compared to the copounds 4-((4-chlorophenyl)diazenyl)-5-methyl-2-(5-methylbenzo[
d]thiazol-2-yl)-1
H-pyrazol-3(2
H)-one
(1c) and 4-((4-bromophenyl)diazenyl)-5-methyl-2-(5-methylbenzo[
d]-thiazol-2-yl)-1
H-pyrazol-3(2
H)-one
(1d) shown below in
Figure 1, correspondingly. A previous study shows that the presence of a side chain to an azo dye along with a phenyl group substituent and a significantly enhanced electron-donating group ultimately decreased the growth of bacteria
[5].
Figure 1. Structures of azo dye compounds (1a–1d) showing anti-mycobacterial activity.
2.2. Anticancer Activity
The photochemistry and the anti-tuberculosis activity of the in vitro azo compounds discussed above yielded good results, so their anticancer activity was also studied. An MTT test was performed for cell proliferation, and for this reason, different human cancer cell lines were used, such as chronic myeloid leukaemia (K562), lung carcinoma (A549), colon (HCT116), and T-lymphocyte (Jurkat) cell lines.
Table 1 shows their anticancer activity results. Data revealed that K562, Jurkat, and A549cell lines containing various synthesized azo compounds displayed fair in vitro results (IC
50 > 50). However, on the other hand, in comparison with other human cell lines, the HCT116 cell line showed relatively good activity in the presence of various compounds
[46].
Table 1. Anticancer activities of azo compounds (1a–d).
2.3. Therapeutic and Biological Applications
Various applications, such as anti-inflammatory, antibacterial, analgesic, antiviral, antipyretic, and anti-convulsant activities, belonged to 3-aminopyrroles derivatives, which are considered an essential family of compounds
[47]. Thiophene compounds also play a significant role as agrochemicals
[48][49], anti-avian influenza virus (H5N1), anti-tubercular, anti-breast cancer agents, AMPK activators, HIV, and multi-target kinase inhibitors
[50].
The majority of roles, including serving as precursors for different biological molecules or connecting to various sulphur and nitrogen heterocycles, are imparted by some structural units combined to form a 2-aminothiophene product. Apart from this, UV-visible absorption and fluorescence of these compounds make them important for biological purposes. Thiophene derivatives can be used explicitly as valuable fluorescent dyes in confocal microscopy for bio-imaging
[51].
2.4. Antiparasitic Activity of Metalloporphyrins and Their Role as Potentiometric Biosensors
Metalloporphyrins, known for their β-pyrrolic substitution, are important in forming useful supramolecules such as cytochromes, haemoglobin, peroxidases, myoglobin, and catalases
[52][53]. The main reason porphyrins are gaining importance in the biological world day by day is their diverse functionality along with their remarkable structural features and positive properties in the field of photochemistry and spectroscopy. The use of metalloporphyrins as potentiometric sensors is common among all other functions—for example, Mn(III)-porphyrin derivatives are being used in the chloride ion measurement in samples of human serum
[54].
The increase in antiparasitic activity of porphyrins is related to the presence of electrically charged substituents on these compounds. An ultimate decrease in the oxidative damage to the mosquitoes’ larvae of genera
Culex, Aedes [55][56], and
Anopheles [57], while of adult flies of
Ceratitis capitates,
Bactrocera oleae species, and
Stomoxys calcitrans [54][58] can be observed by porphyrin-based drugs. Photosensitization makes hematoporphyrin IX a powerful eco-friendly drug.
2.5. Antioxidant Activity
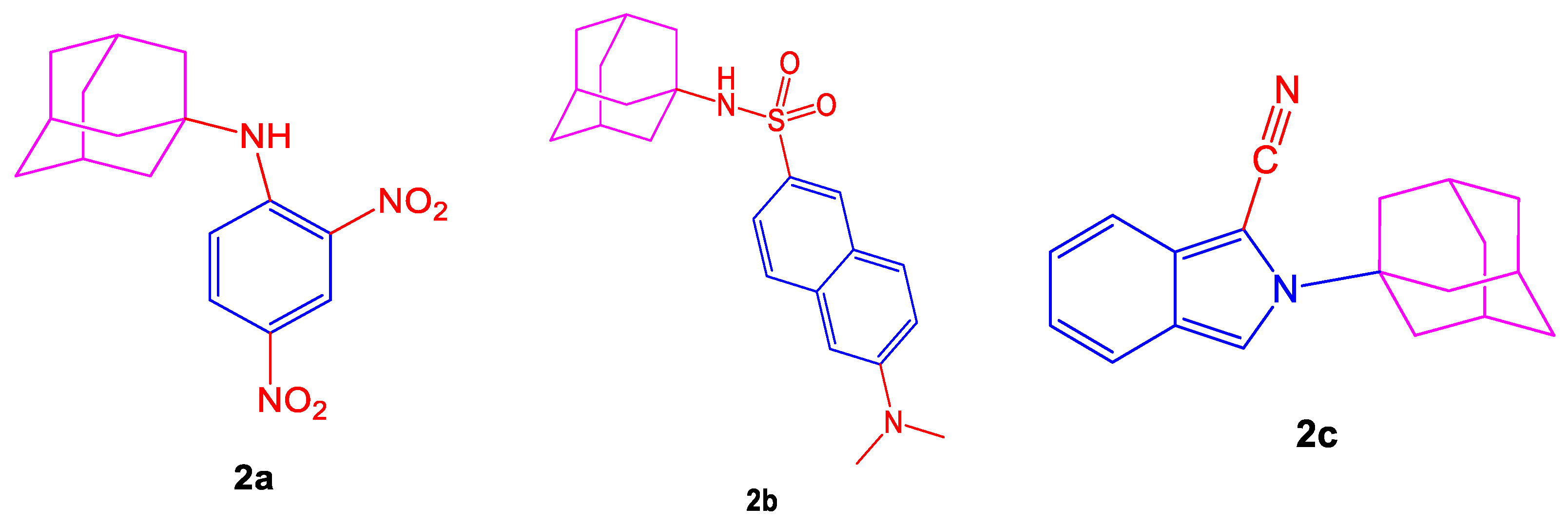
Disordered physiological processes such as neurodegenerative disorders are studied by reactive nitrogen and oxygen species or heterocyclic compounds
[59]. Neuroprotection involves an option of antioxidant therapy, so antioxidants can be described as compounds capable of searching for free radicals. Dicsussing specific fluorescent heterocycles shown in
Figure 2 such as (3s,5s,7s)-
N-(2,4-dinitrophenyl)adamantan-1-amine (
2a),
N-((3s,5s,7s)-adamantan-1-yl)-6-(dimethylamino)-naphthalene-2-sulfonamide (
2b), 2-(adamantan-1-yl)-2
H-isoindole-1-carbonitrile (
2c), provide a guide to the pharmacological industry as they are of great interest as antioxidant agents
[60].
Figure 2. Structures of compounds (2a–2c) having antioxidant activities.