Fluorescently labelled heterocyclic compounds are useful in bioanalytical applications, including in vivo imaging, high throughput screening, diagnostics, and light-emitting diodes. These compounds have various therapeutic properties, including antifungal, antitumor, antimalarial, anti-inflammatory, and analgesic activities. Different neutral fluorescent markers containing nitrogen heterocycles (quinolones, azafluoranthenes, pyrazoloquinolines, etc.) have several electrochemical, biological, and nonlinear optic applications. Photodynamic therapy (PDT), which destroys tumors and keeps normal tissues safe, works in the presence of molecular oxygen with light and a photosensitizing drugs (dye) to obtain a therapeutic effect.
- fluorescence
- heterocyclic compounds
- antitumor
1. Introduction
2. Applications of Heterocyclic Compounds
2.1. Anti-Mycobacterial Activity
Different symptoms such as respiratory issues, long-term coughs, and tuberculosis are treated by various plants in African and Asian countries. Many anti-tubercular drugs, with toxicity and side effects, are still used to treat tuberculosis. For treating M. tuberculosis, the synthesis of azo compounds was monitored and showed anti-tubercular activity. Maximum activity was shown by compounds 5-methyl-2-(5-methylbenzo[d]thiazol-2-yl)-4-(p-tolyldiazenyl)-1H-pyrazol-3(2H)-one (1a) and 5-methyl-2-(5-methylbenzo[d]thiazol-2-yl)-4-(m-tolyldiazenyl)-1H-pyrazol-3(2H)-one (1b) when compared to the copounds 4-((4-chlorophenyl)diazenyl)-5-methyl-2-(5-methylbenzo[d]thiazol-2-yl)-1H-pyrazol-3(2H)-one (1c) and 4-((4-bromophenyl)diazenyl)-5-methyl-2-(5-methylbenzo[d]-thiazol-2-yl)-1H-pyrazol-3(2H)-one (1d) shown below in Figure 1, correspondingly. A previous study shows that the presence of a side chain to an azo dye along with a phenyl group substituent and a significantly enhanced electron-donating group ultimately decreased the growth of bacteria [5].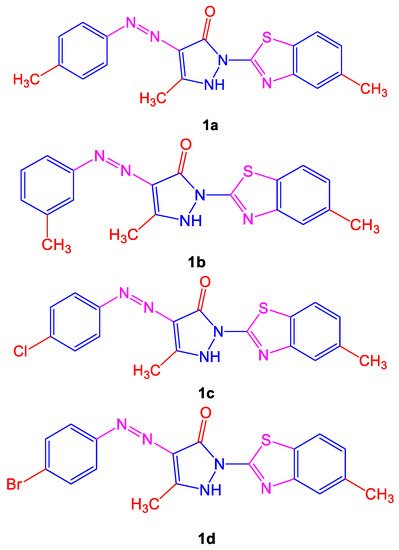
2.2. Anticancer Activity
The photochemistry and the anti-tuberculosis activity of the in vitro azo compounds discussed above yielded good results, so their anticancer activity was also studied. An MTT test was performed for cell proliferation, and for this reason, different human cancer cell lines were used, such as chronic myeloid leukaemia (K562), lung carcinoma (A549), colon (HCT116), and T-lymphocyte (Jurkat) cell lines. Table 1 shows their anticancer activity results. Data revealed that K562, Jurkat, and A549cell lines containing various synthesized azo compounds displayed fair in vitro results (IC50 > 50). However, on the other hand, in comparison with other human cell lines, the HCT116 cell line showed relatively good activity in the presence of various compounds [46].| IC50 (μM) | ||||
|---|---|---|---|---|
| Compounds | HCT116 | A549 | Jurkat | K562 |
| 1a | 34.65 ± 0.35 | ˃50 | ˃50 | ˃50 |
| 1b | ˃50 | ˃50 | ˃50 | ˃50 |
| 1c | 43.33 ± 0.14 | ˃50 | ˃50 | ˃50 |
| 1d | 48.19 ± 0.31 | ˃50 | ˃50 | ˃50 |
2.3. Therapeutic and Biological Applications
Various applications, such as anti-inflammatory, antibacterial, analgesic, antiviral, antipyretic, and anti-convulsant activities, belonged to 3-aminopyrroles derivatives, which are considered an essential family of compounds [47]. Thiophene compounds also play a significant role as agrochemicals [48][49][48,49], anti-avian influenza virus (H5N1), anti-tubercular, anti-breast cancer agents, AMPK activators, HIV, and multi-target kinase inhibitors [50]. The majority of roles, including serving as precursors for different biological molecules or connecting to various sulphur and nitrogen heterocycles, are imparted by some structural units combined to form a 2-aminothiophene product. Apart from this, UV-visible absorption and fluorescence of these compounds make them important for biological purposes. Thiophene derivatives can be used explicitly as valuable fluorescent dyes in confocal microscopy for bio-imaging [51].2.4. Antiparasitic Activity of Metalloporphyrins and Their Role as Potentiometric Biosensors
Metalloporphyrins, known for their β-pyrrolic substitution, are important in forming useful supramolecules such as cytochromes, haemoglobin, peroxidases, myoglobin, and catalases [52][53][52,53]. The main reason porphyrins are gaining importance in the biological world day by day is their diverse functionality along with their remarkable structural features and positive properties in the field of photochemistry and spectroscopy. The use of metalloporphyrins as potentiometric sensors is common among all other functions—for example, Mn(III)-porphyrin derivatives are being used in the chloride ion measurement in samples of human serum [54]. The increase in antiparasitic activity of porphyrins is related to the presence of electrically charged substituents on these compounds. An ultimate decrease in the oxidative damage to the mosquitoes’ larvae of genera Culex, Aedes [55][56][55,56], and Anopheles [57], while of adult flies of Ceratitis capitates, Bactrocera oleae species, and Stomoxys calcitrans [54][58][54,58] can be observed by porphyrin-based drugs. Photosensitization makes hematoporphyrin IX a powerful eco-friendly drug.2.5. Antioxidant Activity
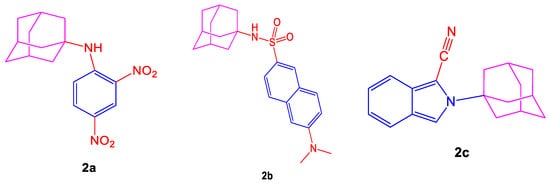
Disordered physiological processes such as neurodegenerative disorders are studied by reactive nitrogen and oxygen species or heterocyclic compounds [59]. Neuroprotection involves an option of antioxidant therapy, so antioxidants can be described as compounds capable of searching for free radicals. Dicsussing specific fluorescent heterocycles shown in Figure 2 such as (3s,5s,7s)-N-(2,4-dinitrophenyl)adamantan-1-amine (2a), N-((3s,5s,7s)-adamantan-1-yl)-6-(dimethylamino)-naphthalene-2-sulfonamide (2b), 2-(adamantan-1-yl)-2H-isoindole-1-carbonitrile (2c), provide us a guide to the pharmacological industry as they are of great interest as antioxidant agents [60].