
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wang Fenghuan | -- | 2706 | 2022-10-17 13:27:06 | | | |
| 2 | Beatrix Zheng | + 12 word(s) | 2718 | 2022-10-18 07:47:43 | | |
Video Upload Options
It is well known that long-term intake of selenium in excess amounts can have adverse physiological effects on humans. Long-term intake of selenium in excess amounts leads to rapid development of severe gastrointestinal and neurological symptoms, followed by acute respiratory failure, myocardial infarction, and renal failure, and may increase the risk of cancer. The Food and Nutrition Board set a tolerable upper Se intake level (UL) for adults at 400 μg/day. Toxicity testing is an important concern in the improvement in selenium-containing anticancer drugs. Various forms of selenium could serve as pro-oxidant toxic agents and promote DNA strand disruption and necrosis of cancer cells. Among the many types of nanoparticles, SeNPs have selective anticancer activity on cancer cells and low toxicity to normal cells. They exhibit low toxicity, better bioavailability and higher activity as compared to organic and inorganic selenium compounds. In the peritoneal cavity, the clearance rate of SeNPs was slower than in plasma, so they may activate an enhanced drug concentration near the cancer-related peritoneal cavity. Therefore, by maintaining a low total drug level, the use of active doses in the cancer model was expected to be less cause of suspected adverse reactions.
1. Presumed Anticancer Mechanism
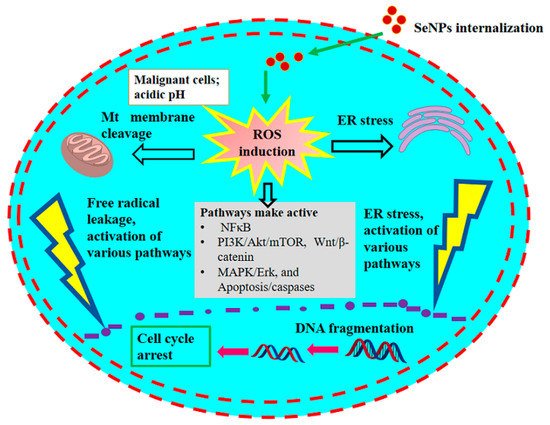
2. Effective against Breast Cancer
3. Effective against Prostate Cancer
4. Effective against Lung Cancer
5. Effective against Hepatic Carcinoma
6. Effective against Colon Cancer
7. Effective as Antioxidants
References
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium nanoparticles: A potent chemotherapeutic agent and an elucidation of its mechanism. Colloids and Surf. B Biointerfaces 2018, 170, 280–292.
- Spyridopoulou, K.; Tryfonopoulou, E.; Aindelis, G.; Ypsilantis, P.; Sarafidis, C.; Kalogirou, O.; Chlichlia, K. Biogenic selenium nanoparticles produced by Lactobacillus casei ATCC 393 inhibit colon cancer cell growth in vitro and in vivo. Nanoscale Adv. 2021, 3, 2516–2528.
- Liu, T.; Zeng, L.; Jiang, W.; Fu, Y.; Zheng, W.; Chen, T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 947–958.
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812.
- Echeverría, S.E.; Borrell, L.N.; Brown, D.; Rhoads, G. A local area analysis of racial, ethnic, and neighborhood disparities in breast cancer staging. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3024–3029.
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249.
- Nigam, J.S.; Yadav, P.; Sood, N. A retrospective study of clinico-pathological spectrum of carcinoma breast in a West Delhi, India. South Asian J. Cancer 2014, 3, 179.
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27.
- Yaqoob, I.; Saeed, M.; Azhar, A. MMP-2 levels evaluation and their relationship with breast cancer progression. Prof. Med. J. 2020, 27, 424–430.
- Vasaturo, F.; Solai, F.; Malacrino, C.; Nardo, T.; Vincenzi, B.; Modesti, M.; Scarpa, S. Plasma levels of matrix metalloproteinases 2 and 9 correlate with histological grade in breast cancer patients. Oncol. Lett. 2013, 5, 316–320.
- Mohammad, M.A.; Ismael, N.R.; Shaarawy, S.M.; El-Merzabani, M.M. Prognostic value of membrane type 1 and 2 matrix metalloproteinase expression and gelatinase A activity in bladder cancer. Int. J. Biol. Markers 2010, 25, 69–74.
- Shakibaie, M.; Khorramizadeh, M.R.; Faramarzi, M.A.; Sabzevari, O.; Shahverdi, A.R. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinaseor expression. Biotechnol. Appl. Biochem. 2010, 56, 7–15.
- Lipari, L.; Mauro, A.; Tortorici, S.; Burruano, F.; Leone, A.; Spatola, G.F.; Tetè, S. Immunohistochemical and transcriptional expression of the matrix metalloproteinases MMP-2 and MMP-9 in normal and pathological human oral mucosa. J. Biol. Regul. Homeost. Agents 2009, 23, 259–267.
- Jeffery, N.; McLean, M.H.; Elffery, E.M.; Murray, G.I. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology 2009, 54, 820–828.
- Wang, K.; Sun, X.J.; Li, S.G.; Shang, W.H.; Jia, P.B.; Feng, H.A. Expressions of matrix metalloproteinase 2 and carbohydrate antigen 50 in colorectal carcinoma, transitional mucosa and normal colorectal mucosa and its clinical significance. Chin. J. Bases Clin. Gen. Surg. 2006, 13, 417–420.
- Yamamura, T.; Nakanishi, K.; Hiroi, S.; Kumaki, F.; Sato, H.; Aida, S.; Kawai, T. Expression of membrane-type-1-matrix metalloproteinase and metalloproteinase-2 in nonsmall cell lung carcinomas. Lung Cancer 2002, 35, 249–255.
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F.; Têtu, B. Membrane-type-1 matrix metalloproteinase, matrix metalloproteinase 2, and tissue inhibitor of matrix proteinase 2 in prostate cancer: Identification of patients with poor prognosis by immunohistochemistry. Hum. Pathol. 2008, 39, 731–739.
- Feng, G.; Tan, Y. Expression and significance of MMP2 and type IV collagen in gastric cancer. Chin. J. Surg. 2000, 38, 775–777.
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193.
- Shoeibi, S.; Mashreghi, M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elements Med. Biol. 2017, 39, 135–139.
- Wadhwani, S.A.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; Chopade, B.A. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: Optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed. 2017, 12, 6841.
- Sonkusre, P. Specificity of biogenic selenium nanoparticles for prostate cancer therapy with reduced risk of toxicity: An in vitro and in vivo study. Front. Oncol. 2020, 9, 1541.
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittelforschung 2012, 62, 525–531.
- Yazdi, M.H.; Mahdavi, M.; Varastehmoradi, B.; Faramarzi, M.A.; Shahverdi, A.R. The immunostimulatory effect of biogenic selenium nanoparticles on the 4T1 breast cancer model: An in vivo study. Biol. Trace Elem. Res. 2012, 149, 22–28.
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33.
- Yazdi, M.H.; Mahdavi, M.; Faghfuri, E.; Faramarzi, M.A.; Sepehrizadeh, Z.; Hassan, Z.M.; Gholami, M.; Shahverdi, A.R. Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: Preliminary vaccine model. Iran. J. Biotechnol. 2015, 13, 1–9.
- Yazdi, M.H.; Varastehmoradi, B.; Faghfuri, E.; Mavandadnejad, F.; Mahdavi, M.; Shahverdi, A.R. Adjuvant effect of biogenic selenium nanoparticles improves the immune responses and survival of mice receiving 4T1 cell antigens as vaccine in breast cancer murine model. J. Nanosci. Nanotechnol. 2015, 15, 10165–10172.
- Faghfuri, E.; Yazdi, M.H.; Mahdavi, M.; Sepehrizadeh, Z.; Faramarzi, M.A.; Mavandadnejad, F.; Shahverdi, A.R. Dose-response relationship study of selenium nanoparticles as an immunostimulatory agent in cancer-bearing mice. Arch. Med. Res. 2015, 46, 31–37.
- Chen, P.; Wang, L.; Li, N.; Liu, Q.; Ni, J. Comparative proteomics analysis of sodium selenite-induced apoptosis in human prostate cancer cells. Metallomics 2013, 5, 541–550.
- Xiang, N.; Zhao, R.; Zhong, W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 351–362.
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403.
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43.
- Sonkusre, P.; Nanduri, R.; Gupta, P.; Cameotra, S.S. Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J. Nanomed. Nanotechnol. 2014, 5, 1.
- Bao, P.; Xiao, K.Q.; Wang, H.J.; Xu, H.; Xu, P.P.; Jia, Y.; Häggblom, M.M.; Zhu, Y.G. Characterization and Potential Applications of a Selenium Nanoparticle Producing and Nitrate Reducing Bacterium Bacillus oryziterrae sp. nov. Sci. Rep. 2016, 6, 34054.
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075.
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90.
- Ali, E.N.; El-Sonbaty, S.M.; Salem, F.M. Evaluation of selenium nanoparticles as a potential chemopreventive agent against lung carcinoma. Int. J. Pharm. Biol. Sci. 2013, 2, 38–46.
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2013, 63, 11–30.
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585.
- Cheng, Y.; Xiao, X.; Li, X.; Song, D.; Lu, Z.; Wang, F.; Wang, Y. Characterization, antioxidant property and cytoprotection of exopolysaccharide-capped elemental selenium particles synthesized by Bacillus paralicheniformis SR14. Carbohydr. Polym. 2017, 178, 18–26.
- American Cancer Society. Cancer Facts and Figures. 2022. Exit Disclaimer. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 12 January 2022).
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnol. 2017, 15, 1–12.
- Spyridopoulou, K.; Aindelis, G.; Pappa, A.; Chlichlia, K. Anticancer Activity of Biogenic Selenium Nanoparticles: Apoptotic and Immunogenic Cell Death Markers in Colon Cancer Cells. Cancers 2021, 13, 5335.
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144.
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-nanoparticles-loaded chitosan/chitooligosaccharide microparticles and their antioxidant potential: A chemical and in vivo investigation. Pharmaceutics 2020, 12, 43.
- Zhang, W.; Zhang, J.; Ding, D.; Zhang, L.; Muehlmann, L.A.; Deng, S.E.; Wang, X.; Li, W.; Zhang, W. Synthesis and antioxidant properties of Lycium barbarum polysaccharides capped selenium nanoparticles using tea extract. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1463–1470.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197.
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778.
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390.
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462.
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Perez-Lebena, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Bains, M.; Hall, E.D. Antioxidant therapies in traumatic brain and spinal cord injury. Biochim. Biophys. Acta-Mol. Basis Dis. 2012, 1822, 675–684.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26.
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95.
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-containing proteins/peptides from plants: A review on the structures and functions. J. Agric. Food Chem. 2020, 68, 15061–15073.
- Ullah, A.; Sun, B.; Wang, F.; Yin, X.; Xu, B.; Ali, N.; Mirani, Z.A.; Mehmood, A.; Naveed, M. Isolation of selenium, B.; et al. Hrichment conditions for selected probiotic Bacillus subtilis (BSN313). J. Food Biochem. 2020, 44, e13227.
- Xu, C.; Qiao, L.; Ma, L.; Yan, S.; Guo, Y.; Dou, X.; Zhang, B.; Roman, A. Biosynthesis of polysaccharides-capped selenium nanoparticles using Lactococcus lactis NZ9000 and their antioxidant and anti-inflammatory activities. Front. Microbiol. 2019, 10, 1632.
- Qiao, L.; Dou, X.; Yan, S.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020, 11, 3020–3031.




