
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joydip Sengupta | -- | 2032 | 2022-10-17 11:26:08 | | | |
| 2 | Lindsay Dong | -39 word(s) | 1993 | 2022-10-18 04:08:27 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1993 | 2022-10-18 04:09:42 | | |
Video Upload Options
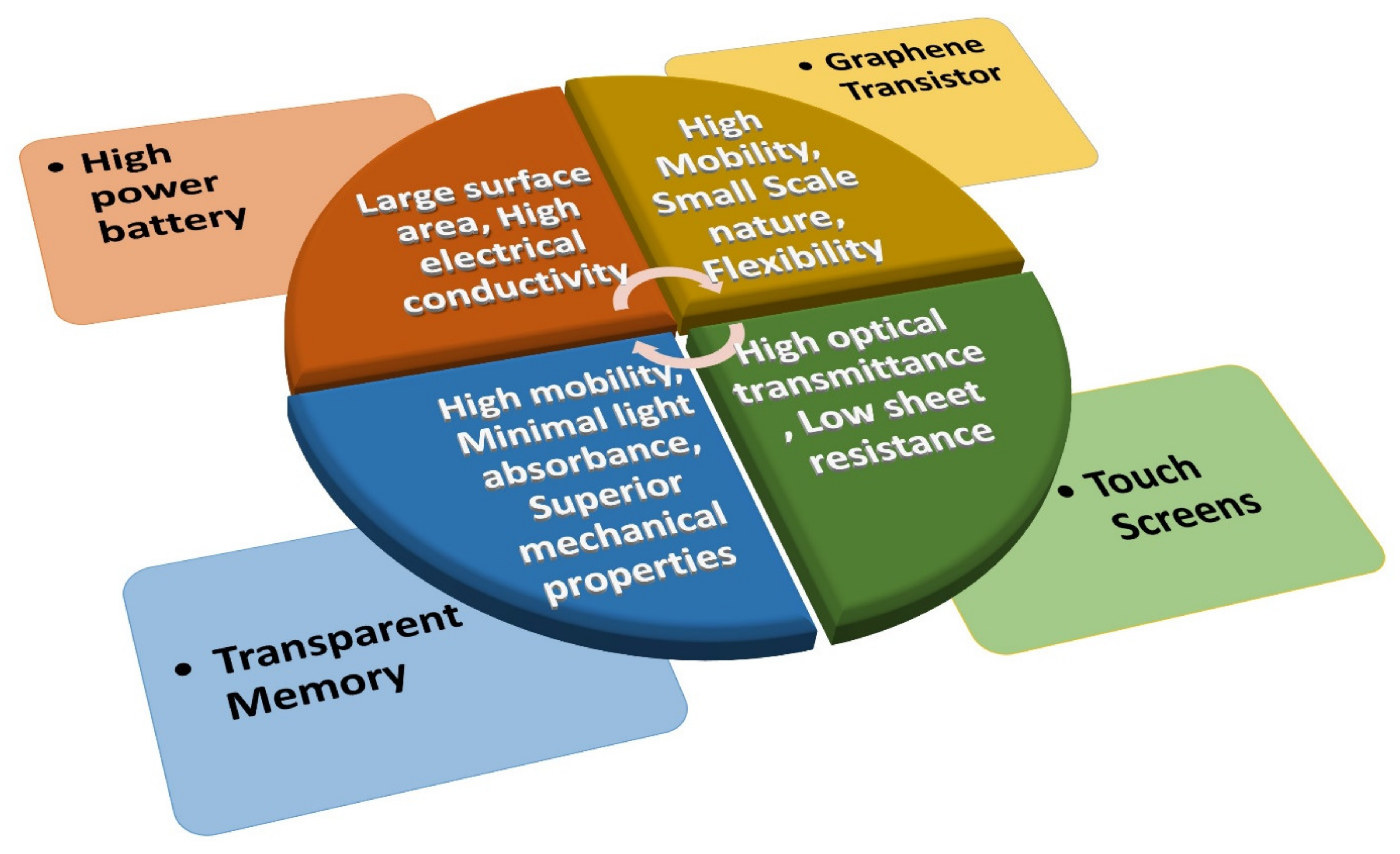
Graphene achieved a peerless level among nanomaterials in terms of its application in electronic devices, owing to its fascinating and novel properties. Its large surface area and high electrical conductivity combine to create high-power batteries. In addition, because of its high optical transmittance, low sheet resistance, and the possibility of transferring it onto plastic substrates, graphene is also employed as a replacement for indium tin oxide (ITO) in making electrodes for touch screens. Moreover, it was observed that graphene enhances the performance of transparent flexible electronic modules due to its higher mobility, minimal light absorbance, and superior mechanical properties. Graphene is even considered a potential substitute for the post-Si electronics era, where a high-performance graphene-based field-effect transistor (GFET) can be fabricated to detect the lethal SARS-CoV-2. Hence, graphene incorporation in electronic devices can facilitate immense device structure/performance advancements.
1. Introduction
2. Graphene Structure and Properties
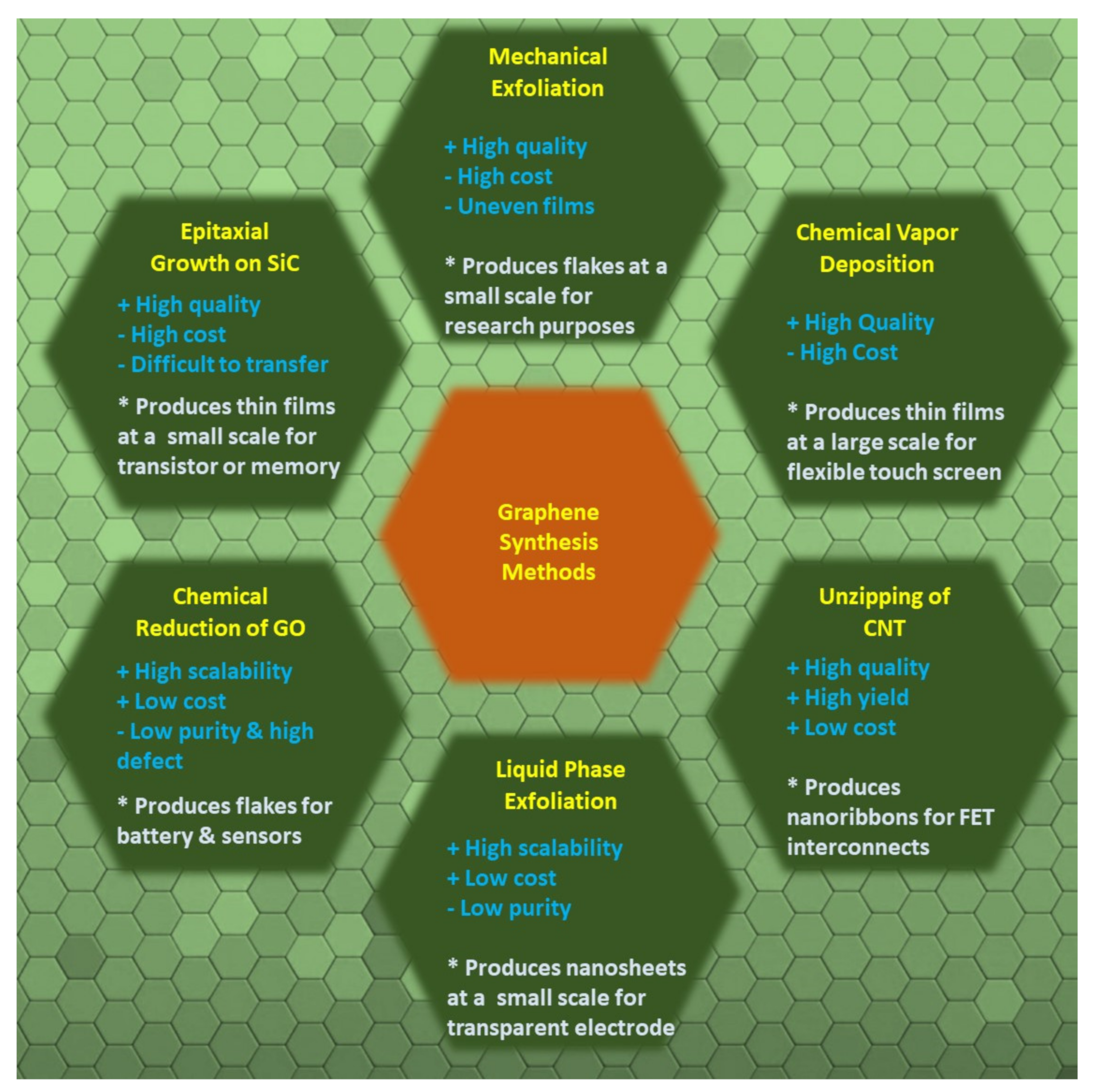
Graphene is an atomically thin two-dimensional allotrope of carbon, comprising a single sheet of sp2-hybridized carbon atoms, where one atom forms each vertex. Here, the σ-bond is formed by the combination of S, Px, and the Py orbital, while the Pz orbital creates the π-bond. The π bond is mostly responsible for the large electrical conductivity of graphene [17].
3. Application of Graphene in Electronic Devices
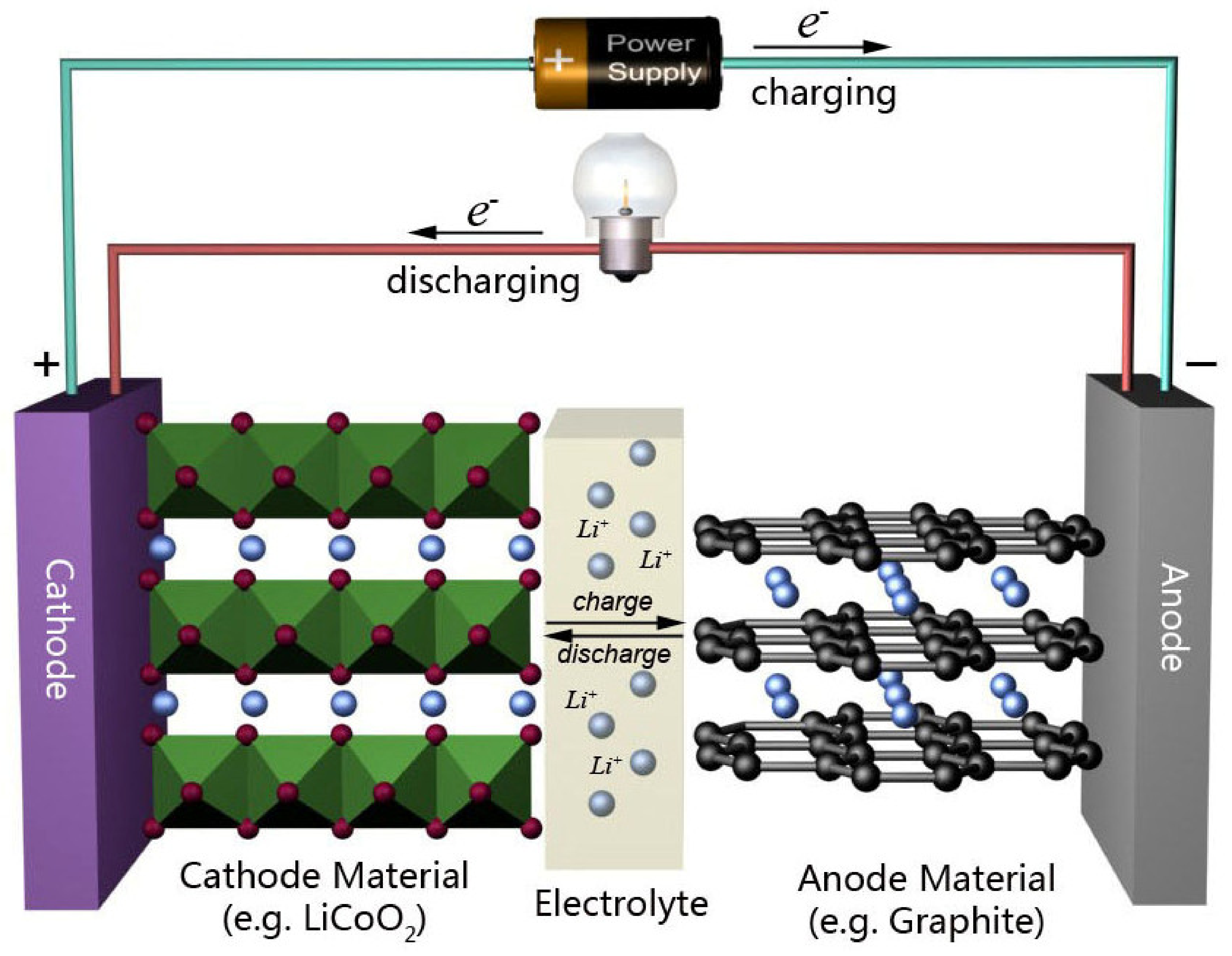
3.1. Graphene in Batteries
3.2. Graphene Electrodes for Touch Screens
3.3. Transparent Memory with Graphene
3.4. Integrated Circuits with Graphene Transistors
References
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634.
- Boehm, H.P.; Clauss, A.; Fischer, G.O.; Hofmann, U. Dünnste Kohlenstoff-Folien. Z. Nat. B 1962, 17, 150–153.
- Boehm, H.P.; Setton, R.; Stumpp, E. Nomenclature and Terminology of Graphite Intercalation Compounds (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 1893–1901.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Li, Q.; Guo, B.D.; Yu, J.G.; Ran, J.R.; Zhang, B.H.; Yan, H.J.; Gong, J.R. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS-Cluster-Decorated Graphene Nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884.
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191.
- Lee, G.-H.; Cooper, R.C.; An, S.J.; Lee, S.; van der Zande, A.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor-Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076.
- Vekeman, J.; Sánchez-Marín, J.; Sánchez de Merás, A.; Garcia Cuesta, I.; Faginas-Lago, N. Flexibility in the Graphene Sheet: The Influence on Gas Adsorption from Molecular Dynamics Studies. J. Phys. Chem. C 2019, 123, 28035–28047.
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388.
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely High Thermal Conductivity of Graphene: Prospects for Thermal Management Applications in Nanoelectronic Circuits. Appl. Phys. Lett. 2008, 92, 151911.
- Pop, E.; Varshney, V.; Roy, A.K. Thermal Properties of Graphene: Fundamentals and Applications. MRS Bull. 2012, 37, 1273–1281.
- Stoller, M.D.; Park, S.; Yanwu, Z.; An, J.; Ruoff, R.S.; Zhu, Y.; An, J.; Ruoff, R.S.; Yanwu, Z.; An, J.; et al. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502.
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308.
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh Electron Mobility in Suspended Graphene. Solid State Commun. 2008, 146, 351–355.
- Choi, J.S.; Choi, H.; Kim, K.C.; Jeong, H.Y.; Yu, Y.J.; Kim, J.T.; Kim, J.S.; Shin, J.W.; Cho, H.; Choi, C.G. Facile Fabrication of Properties-Controllable Graphene Sheet. Sci. Rep. 2016, 6, 24525.
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-Roll Production of 30-Inch Graphene Films for Transparent Electrodes. Nat. Nanotechnol. 2010, 5, 574–578.
- Littlejohn, S.D. Electrical Properties of Graphite Nanoparticles in Silicone; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-319-00740-3.
- Martin, I.; Blanter, Y.M. Transport in Disordered Graphene Nanoribbons. Phys. Rev. B 2009, 79, 235132.
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97, 216803.
- Ni, Z.H.; Wang, H.M.; Kasim, J.; Fan, H.M.; Yu, T.; Wu, Y.H.; Feng, Y.P.; Shen, Z.X.; About, M.; Article, T. Reflection and Contrast Spectroscopy Graphene Thickness Determination Using Reflection and Contrast Spectroscopy. Nano Lett. 2007, 7, 2758–2763.
- Vinal, G.W. Storage Batteries. J. Opt. Soc. Am. 1925, 11, 263–274.
- Lithium-Ion Batteries Need to Be Greener and More Ethical. Nature 2021, 595, 7.
- Hein, S.; Danner, T.; Westhoff, D.; Prifling, B.; Scurtu, R.; Kremer, L.; Hoffmann, A.; Hilger, A.; Osenberg, M.; Manke, I.; et al. Influence of Conductive Additives and Binder on the Impedance of Lithium-Ion Battery Electrodes: Effect of Morphology. J. Electrochem. Soc. 2020, 167, 013546.
- Liu, R.; Duay, J.; Lee, S.B. Heterogeneous Nanostructured Electrode Materials for Electrochemical Energy Storage. Chem Commun. 2011, 47, 1384–1404.
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416.
- Takahashi, Y.; Tode, S.; Kinoshita, A.; Fujimoto, H.; Nakane, I.; Fujitani, S. Development of Lithium-Ion Batteries with a LiCoO Cathode Toward High Capacity by Elevating Charging Potential. J. Electrochem. Soc. 2008, 155, A537–A541.
- Lee, M.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High Performance LiMn2O4 Cathode Materials Grown with Epitaxial Layered Nanostructure for Li-Ion Batteries. Nano Lett. 2014, 14, 993–999.
- Zhang, Y.; Wang, C.Y.; Tang, X. Cycling Degradation of an Automotive LiFePO4 lithium-Ion Battery. J. Power Sources 2011, 196, 1513–1520.
- Gaubicher, J.; Wurm, C.; Goward, G.; Masquelier, C.; Nazar, L. Rhombohedral Form of Li3V2(PO4)3 as a Cathode in Li-Ion Batteries. Chem. Mater. 2000, 12, 3240–3242.
- Wang, S.; Lu, Z.; Wang, D.; Li, C.; Chen, C.; Yin, Y. Porous Monodisperse V2O5 Microspheres as Cathode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 6365–6369.
- Deng, L.-F.; Yu, K.-M. Influence of Graphene on the Electrochemical Performance of LiCoO2 Cathode Materials for Lithium-Ion Batteries. J. Funct. Mater. 2014, 45, 84–88.
- Pyun, M.H.; Park, Y.J. Graphene/LiMn2O4 Nanocomposites for Enhanced Lithium Ion Batteries with High Rate Capability. J. Alloys Comp. 2015, 643, S90–S94.
- Lung-Hao Hu, B.; Wu, F.Y.; Lin, C.T.; Khlobystov, A.N.; Li, L.J. Graphene-Modified LiFePO4 Cathode for Lithium Ion Battery beyond Theoretical Capacity. Nat. Commun. 2013, 4, 1687.
- Johnson, E.A. Touch Display—A Novel Input/Output Device for Computers. Electron. Lett. 1965, 1, 219–220.
- Yu, X.; Marks, T.J.; Facchetti, A. Metal Oxides for Optoelectronic Applications. Nat. Mater. 2016, 15, 383–396.
- Hong, C.-H.; Shin, J.-H.; Ju, B.-K.; Kim, K.-H.; Park, N.-M.; Kim, B.-S.; Cheong, W.-S. Index-Matched Indium Tin Oxide Electrodes for Capacitive Touch Screen Panel Applications. J. Nanosci. Nanotechnol. 2013, 13, 7756–7759.
- Her, S.-C.; Chang, C.-F. Fabrication and Characterization of Indium Tin Oxide Films. J. Appl. Biomater. Funct. Mater. 2017, 15, 170–175.
- Geiger, S.; Kasian, O.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. Stability Limits of Tin-Based Electrocatalyst Supports. Sci. Rep. 2017, 7, 4595.
- Layani, M.; Kamyshny, A.; Magdassi, S. Transparent Conductors Composed of Nanomaterials. Nanoscale 2014, 6, 5581–5591.
- Arvidsson, R.; Kushnir, D.; Molander, S.; Sandén, B.A. Energy and Resource Use Assessment of Graphene as a Substitute for Indium Tin Oxide in Transparent Electrodes. J. Clean. Prod. 2016, 132, 289–297.
- Das, T.; Sharma, B.K.; Katiyar, A.K.; Ahn, J.-H. Graphene-Based Flexible and Wearable Electronics. J. Semicond. 2018, 39, 011007.
- Ryu, J.; Kim, Y.; Won, D.; Kim, N.; Park, J.S.; Lee, E. Fast Synthesis of High-Performance Graphene by Rapid Thermal Chemical Vapor Deposition. ACS Nano 2014, 8, 950–956.
- Wang, J.; Liang, M.; Fang, Y.; Qiu, T.; Zhang, J.; Zhi, L. Rod-Coating: Towards Large-Area Fabrication of Uniform Reduced Graphene Oxide Films for Flexible Touch Screens. Adv. Mater. 2012, 24, 2874–2878.
- Dong, T.; Sun, Y.; Zhu, Z.; Wu, X.; Wang, J.; Shi, Y.; Xu, J.; Chen, K.; Yu, L. Monolithic Integration of Silicon Nanowire Networks as a Soft Wafer for Highly Stretchable and Transparent Electronics. Nano Lett. 2019, 19, 6235–6243.
- Yoon, J.-S.; Lee, S.; Lee, J.; Jeong, J.; Yun, H.; Kang, B.; Baek, R.-H. Source/Drain Patterning FinFETs as Solution for Physical Area Scaling Toward 5-Nm Node. IEEE Access 2019, 7, 172290–172295.
- Lu, H.-W.; Kane, A.A.; Parkinson, J.; Gao, Y.; Hajian, R.; Heltzen, M.; Goldsmith, B.; Aran, K. The Promise of Graphene-Based Transistors for Democratizing Multiomics Studies. Biosens. Bioelectron. 2022, 195, 113605.
- Lemme, M.C.; Member, S.; Echtermeyer, T.J.; Baus, M.; Kurz, H. A Graphene Field-Effect Device. IEEE Electron. Dev. Lett. 2007, 28, 282–284.
- Sun, Y.-L.; Xie, D.; Xu, J.-L.; Li, X.-M.; Zhang, C.; Dai, R.-X.; Li, X.; Meng, X.-J.; Zhu, H.-W. Tunable Transport Characteristics of Double-Gated Graphene Field-Effect Transistors Using P(VDF-TrFE) Ferroelectric Gating. Carbon 2016, 96, 695–700.





