
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sulaiman Al-Zuhair | -- | 3325 | 2022-10-17 08:23:00 | | | |
| 2 | Jessie Wu | + 59 word(s) | 3384 | 2022-10-18 07:10:02 | | | | |
| 3 | Jessie Wu | Meta information modification | 3384 | 2022-10-18 07:11:58 | | |
Video Upload Options
Biomass feedstock is a material of biological origin that can be converted to various bio-based products, such as ethanol, in a biorefinery. Lignocellulosic biomass is considered an environmentally friendly, sustainable energy production resource. The various lignocellulosic biomass sources include industrial and agricultural waste, as well as forestry lignocellulosic biomass. The depletion of fossil fuel resources and the negative impact of their use on the climate have resulted in the need for alternative sources of clean, sustainable energy. One available alternative, bioethanol, is a potential substitute for, or additive to, petroleum-derived gasoline. In the lignocellulose-to-bioethanol process, the cellulose hydrolysis step represents a major hurdle that hinders commercialization.
1. Introduction
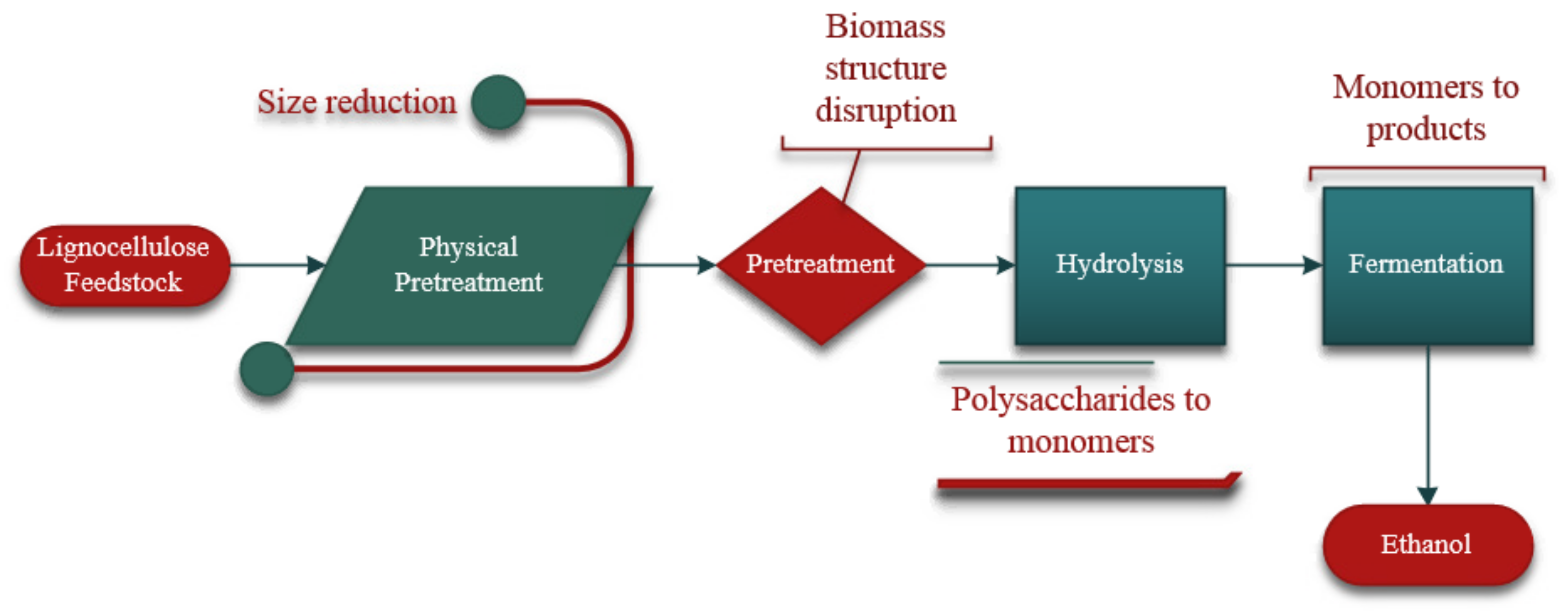
2. Pretreatment
2.1. Physical Pretreatment
2.2. Chemical Pretreatment
Acidic Pretreatment
Alkaline Pretreatment
Oxidative Pretreatment
2.3. Physicochemical Pretreatment
Solvent Fractionation
Steam Explosion
Hydrothermal Pretreatment
2.4. Biological Pretreatment
3. Hydrolysis
3.1. Chemical Hydrolysis
3.2. Enzymatic Hydrolysis
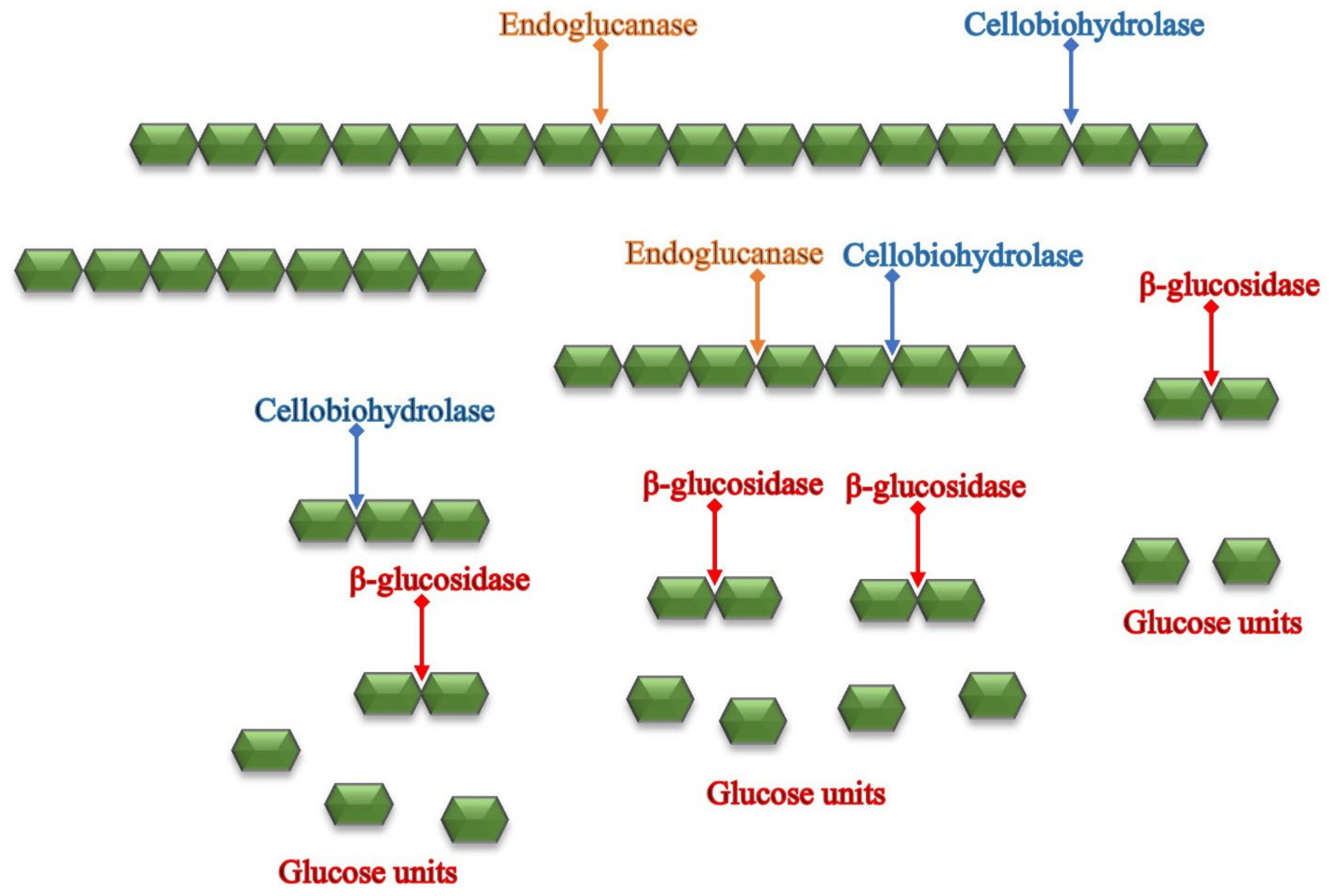
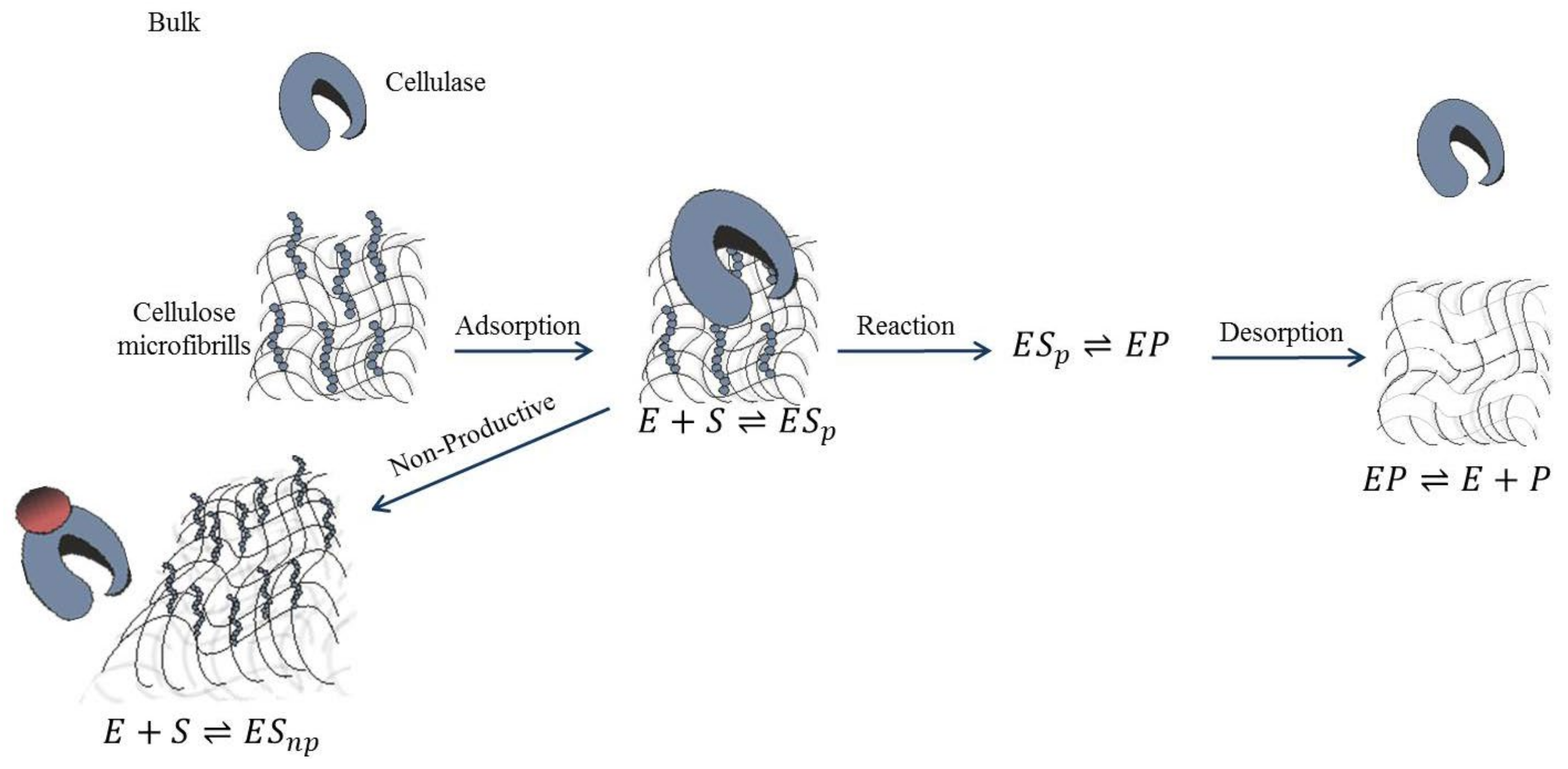
4. Enzyme Kinetics and Modeling
References
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685.
- da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, B.E. ‘Cradle-to-grave’assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347.
- Gaikwad, A. Effect of Particle Size on the Kinetics of Enzymatic Hydrolysis of Microcrystalline Cotton Cellulose: A Modeling and Simulation Study. Appl. Biochem. Biotechnol. 2019, 187, 800–816.
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309.
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58.
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977.
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Saddler, J.N. Substrate Pretreatment: The Key to Effective Enzymatic Hydrolysis of Lignocellulosics. In Biofuels; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 67–93.
- Hsu, T.-C.; Guo, G.-L.; Chen, W.-H.; Hwang, W.-S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913.
- Song, Y.; Zhang, J.; Zhang, X.; Tan, T. The correlation between cellulose allomorphs (I and II) and conversion after removal of hemicellulose and lignin of lignocellulose. Bioresour. Technol. 2015, 193, 164–170.
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48.
- Wu, H.; Dai, X.; Zhou, S.-L.; Gan, Y.-Y.; Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.; Yang, L.; Wu, Z.-K.; Wang, T.-L. Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour. Technol. 2017, 241, 70–74.
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617.
- Raj, K.; Krishnan, C. High sugar yields from sugarcane (Saccharum officinarum) bagasse using low-temperature aqueous ammonia pretreatment and laccase-mediator assisted enzymatic hydrolysis. Ind. Crops Prod. 2018, 111, 673–683.
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front Chem. 2018, 6, 141.
- An, S.; Li, W.; Liu, Q.; Xia, Y.; Zhang, T.; Huang, F.; Lin, Q.; Chen, L. Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour. Technol. 2019, 271, 283–288.
- Johansson, A.; Aaltonen, O.; Ylinen, P. Organosolv pulping—Methods and pulp properties. Biomass 1987, 13, 45–65.
- Sun, F.; Chen, H. Organosolv pretreatment by crude glycerol from oleochemicals industry for enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2008, 99, 5474–5479.
- Kim, S.J.; Um, B.H.; Im, D.J.; Lee, J.H.; Oh, K.K. Combined Ball Milling and Ethanol Organosolv Pretreatment to Improve the Enzymatic Digestibility of Three Types of Herbaceous Biomass. Energies 2018, 11, 2457.
- Tang, C.; Shan, J.; Chen, Y.; Zhong, L.; Shen, T.; Zhu, C.; Ying, H. Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour. Technol. 2017, 232, 222–228.
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16.
- Zhu, Z.; Sathitsuksanoh, N.; Vinzant, T.; Schell, D.J.; McMillan, J.D.; Zhang, Y.-H.P. Comparative study of corn stover pretreated by dilute acid and cellulose solvent-based lignocellulose fractionation: Enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnol. Bioeng. 2009, 103, 715–724.
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.-H.P. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30.
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532.
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714.
- Grewal, J.; Ahmad, R.; Khare, S.K. Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour. Technol. 2017, 242, 236–243.
- Husson, E.; Auxenfans, T.; Herbaut, M.; Baralle, M.; Lambertyn, V.; Rakotoarivonina, H.; Rémond, C.; Sarazin, C. Sequential and simultaneous strategies for biorefining of wheat straw using room temperature ionic liquids, xylanases and cellulases. Bioresour. Technol. 2018, 251, 280–287.
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372.
- Kataria, R.; Mol, A.; Schulten, E.; Happel, A.; Mussatto, S.I. Bench scale steam explosion pretreatment of acid impregnated elephant grass biomass and its impacts on biomass composition, structure and hydrolysis. Ind. Crops Prod. 2017, 106, 48–58.
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15.
- Scholl, J.L.; Menegol, D.; Pitarelo, A.P.; Fontana, R.C.; Filho, A.Z.; Ramos, L.P.; Dillon, A.J.P.; Camassola, M. Ethanol production from sugars obtained during enzymatic hydrolysis of elephant grass (Pennisetum purpureum, Schum.) pretreated by steam explosion. Bioresour. Technol. 2015, 192, 228–237.
- Stanley, H.; Ezeife, C.; Onwukwe, C. Bioethanol Production from Elephant Grass (Pennisetum purpureum). Niger. J. Biotechnol. 2017, 32, 1–6.
- Montipó, S.; Ballesteros, I.; Fontana, R.C.; Liu, S.; Martins, A.F.; Ballesteros, M.; Camassola, M. Integrated production of second generation ethanol and lactic acid from steam-exploded elephant grass. Bioresour. Technol. 2018, 249, 1017–1024.
- Zheng, R.; Zhang, H.; Zhao, J.; Lei, M.; Huang, H. Direct and simultaneous determination of representative byproducts in a lignocellulosic hydrolysate of corn stover via gas chromatography–mass spectrometry with a Deans switch. J. Chromatogr. A 2011, 1218, 5319–5327.
- Du, J.; Li, Y.; Zhang, H.; Zheng, H.; Huang, H. Factors to decrease the cellulose conversion of enzymatic hydrolysis of lignocellulose at high solid concentrations. Cellulose 2014, 21, 2409–2417.
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 39354.
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor. 2019, 10, 235–251.
- Maibam, P.D.; Maiti, S.K. A Strategy for Simultaneous Xylose Utilization and Enhancement of Cellulase Enzyme Production by Trichoderma reesei Cultivated on Liquid Hydrolysate Followed by Induction with Feeding of Solid Sugarcane Bagasse. Waste Biomass Valor. 2020, 11, 3151–3160.
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Chapter 7—Biological pretreatment of lignocellulosic biomass—Current trends and future perspectives. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212.
- Machado, A.d.S.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22.
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7.
- Wu, Y.; Ge, S.; Xia, C.; Mei, C.; Kim, K.-H.; Cai, L.; Smith, L.M.; Lee, J.; Shi, S.Q. Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew. Sustain. Energy Rev. 2021, 136, 110442.
- Fei, X.; Jia, W.; Wang, J.; Chen, T.; Ling, Y. Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation. Int. J. Biol. Macromol. 2020, 145, 733–739.
- Gu, H.; An, R.; Bao, J. Pretreatment refining leads to constant particle size distribution of lignocellulose biomass in enzymatic hydrolysis. Chem. Eng. J. 2018, 352, 198–205.
- Manmai, N.; Unpaprom, Y.; Ponnusamy, V.K.; Ramaraj, R. Bioethanol production from the comparison between optimization of sorghum stalk and sugarcane leaf for sugar production by chemical pretreatment and enzymatic degradation. Fuel 2020, 278, 118262.
- Sołowski, G.; Konkol, I.; Cenian, A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. J. Clean. Prod. 2020, 267, 121721.
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119.
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefining 2007, 1, 119–134.
- Srivastava, N.; Srivastava, M.; Mishra, P.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P. Applications of fungal cellulases in biofuel production: Advances and limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386.
- Tiwari, R.; Nain, L.; Labrou, N.E.; Shukla, P. Bioprospecting of functional cellulases from metagenome for second generation biofuel production: A review. Crit. Rev. Microbiol. 2018, 44, 244–257.
- Rosgaard, L.; Pedersen, S.; Cherry, J.R.; Harris, P.; Meyer, A.S. Efficiency of new fungal cellulase systems in boosting enzymatic degradation of barley straw lignocellulose. Biotechnol. Prog. 2006, 22, 493–498.
- Barcelos, C.A.; Rocha, V.A.; Groposo, C.; de Castro, A.M.; Pereira, N.P., Jr. Enzymes and accessory proteins involved in the hydrolysis of lignocellulosic biomass for bioethanol production. Mycol. Curr. Future Dev. 2016, 1, 23–56.
- Jeoh, T.; Cardona, M.J.; Karuna, N.; Mudinoor, A.R.; Nill, J. Mechanistic kinetic models of enzymatic cellulose hydrolysis—A review. Biotechnol. Bioeng. 2017, 114, 1369–1385.
- Al-Zuhair, S. The effect of crystallinity of cellulose on the rate of reducing sugars production by heterogeneous enzymatic hydrolysis. Bioresour. Technol. 2008, 99, 4078–4085.
- Petrášek, Z.; Eibinger, M.; Nidetzky, B. Modeling the activity burst in the initial phase of cellulose hydrolysis by the processive cellobiohydrolase Cel7A. Biotechnol. Bioeng. 2019, 116, 515–525.
- Praestgaard, E.; Elmerdahl, J.; Murphy, L.; Nymand, S.; McFarland, K.C.; Borch, K.; Westh, P. A kinetic model for the burst phase of processive cellulases. FEBS J. 2011, 278, 1547–1560.
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848.
- Warden, A.C.; Little, B.A.; Haritos, V.S. A cellular automaton model of crystalline cellulose hydrolysis by cellulases. Biotechnol. Biofuels 2011, 4, 39.
- Yang, B.; Willies, D.M.; Wyman, C.E. Changes in the enzymatic hydrolysis rate of Avicel cellulose with conversion. Biotechnol. Bioeng. 2006, 94, 1122–1128.
- Huang, A.A. Kinetic studies on insoluble cellulose–cellulase system. Biotechnol. Bioeng. 1975, 17, 1421–1433.
- Peitersen, N.; Ross, E.W. Mathematical model for enzymatic hydrolysis and fermentation of cellulose by Trichoderma. Biotechnol. Bioeng. 1979, 21, 997–1017.
- Gan, Q.; Allen, S.; Taylor, G. Analysis of process integration and intensification of enzymatic cellulose hydrolysis in a membrane bioreactor. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 688–698.
- Gan, Q.; Allen, S.; Taylor, G. Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: An overview, an experimental study and mathematical modelling. Process Biochem. 2003, 38, 1003–1018.
- van Zyl, J.M.; van Rensburg, E.; van Zyl, W.H.; Harms, T.M.; Lynd, L.R. A Kinetic Model for Simultaneous Saccharification and Fermentation of Avicel With Saccharomyces cerevisiae. Biotechnol. Bioeng. 2011, 108, 924–933.
- Zhang, Y.-H.P.; Lynd, L.R. A functionally based model for hydrolysis of cellulose by fungal cellulase. Biotechnol. Bioeng. 2006, 94, 888–898.




