
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexia Madeleine DIEVART | -- | 2975 | 2022-10-14 14:36:47 | | | |
| 2 | Amina Yu | + 177 word(s) | 3152 | 2022-10-17 04:37:48 | | |
Video Upload Options
Photoautotrophic euendoliths, including cyanobacteria, and red and green microalgae, are part of the endolithic community. The term ‘endolith’ refers to a morphologically and physiologically heterogenous group of microorganisms living within a rock or other stony matter, such as coral skeletons or animal shells, and more specifically, to organisms that actively bore into relatively soluble substrates, such as phosphate and carbonate substrates. Euendoliths are ubiquitous, as they can be found in almost every environment, geographical location, or depth, where the appropriate substratum (e.g., relatively soluble carbonate and phosphate substrates) is available and the requirements for photosynthesis are met. The most diverse and abundant modern euendolithic communities can be found in the marine environment. Euendoliths, as microorganisms infesting inanimate substrates, were first thought to be ecologically irrelevant. Numerous studies have subsequently shown that euendoliths can colonize living marine calcifying organisms, such as coral skeletons and bivalve shells, causing both sub-lethal and lethal damage. Moreover, under suitable environmental conditions, their presence can have surprising benefits for the host. Thus, infestation by photoautotrophic euendoliths has significant consequences for calcifying organisms that are of particular importance in the case of ecosystems underpinned by calcifying ecosystem engineers.
1. What Are Euendoliths and How Are They Observed?
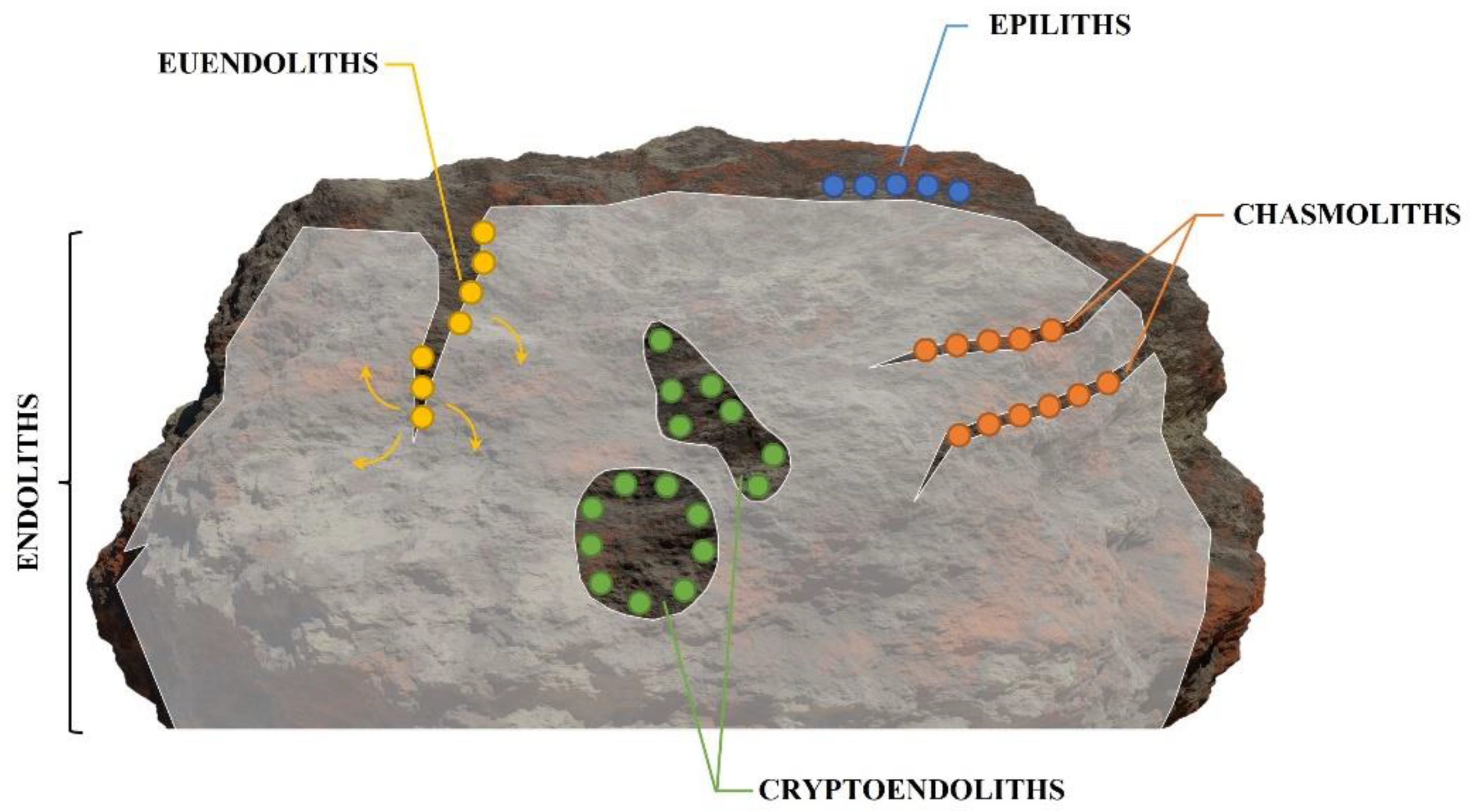
-
Epiliths that live on the surface of the substrate;
-
Chasmoliths (chasm = cleft) that adhere to the surface of fissures and cracks in the substrate;
-
Cryptoendoliths (crypto = hidden) that adhere to the surface of pre-existing cavities within porous rocks, including spaces produced and vacated by euendoliths, with no dissolution action;
-
Euendoliths (eu = true) that actively penetrate carbonate (and phosphate) substrates and reside partially or completely inside cavities of their own making.
2. Incidence of Photoautotrophic Euendoliths in Marine Ecosystems
2.1. Light Availability
2.2. Nature of the Substrate
2.3. Biotic and Abiotic Environmental Factors
-
Grazers are attracted to the substrate by the presence of photoautotrophic euendoliths, as these represent a renewable source of food [18][62][113]. The boring activity of euendoliths weakens the superficial layers of the substrate, which can facilitate the settlement of macroborers with their own bioerosive activity, as well as grazing;
-
On the one hand, macrograzers constantly remove the superficial layers of the substrate, thus extending the depth to which the light can penetrate and, therefore, the depth to which the endoliths can bore, increasing microboring rates [112][114]. Grazing also reduces the settlement and growth of epilithic organisms that compete with euendoliths for space and diminish light availability [115]. On the other hand, macroborers excrete different waste products within the infested substrate, such as ammonium, phosphates, or CO2. Such waste products act as fertilizer for euendolithic communities, which increase in abundance, biomass, and productivity in the vicinity of macroborers [111][114][116].
3. Euendolithic Infestation in Marine Bioengineered Ecosystems
While numerous studies have assessed euendolith-induced biodegradation of carbonate skeletal materials, until recently, severe harm to living host organisms was understood to be limited to the erosive activity of invertebrates or fungal borers [124]. Due to low light penetration within the substratum, photoautotrophic euendoliths were generally thought to be unable to inflict significant structural damage on live organisms, as they eroded only the uppermost layers of the carbonate substrate [124].
Over the last three decades, mounting evidence has shown that the eroding activity of photoautotrophic euendoliths can be the source of severe, often lethal, damage to living calcifying organisms [see Table 1 in Dievart et al. (2022)].
| Responses to Endolithic Infestation | Live Calcifying Hosts | References | |||
|---|---|---|---|---|---|
| Corals | Coralline Algae | Bivalves | Others | ||
| Physiological Parameters | |||||
| Growth | ↓ = | ↓ | ↓ | [105][125][126][127][128][129] | |
| General condition | = | ↓ | [52][67][125][127][130][131][132] | ||
| Reproduction | ↓ | = | [125][128][133][134] | ||
| Attachment strength | ↓ | [67][132][133] | |||
| General survival | ↑ = | ↓ ✞ | ↓ | [66][105][106][125][130][131][133][135][136][137] | |
| Individual survival to heat stress | ↑ (lim) | ↑ (lim) | [68][69][74][76][138][139][140][141] | ||
| Calcified structures | |||||
| Microbioerosion | ↑ | ↑↓ | ↑ | ↑ | [18][56][68][91][105][125][129][142] |
| Thickness | ↑ | ↓ ✞ | ↓ | [54][105][125][126] | |
| Strength | ↓ | ↓ ✞ | ↓ | [67][105][125][129][132][134][136][137][143] | |
| Porosity | ↑ | ↑ | ↑ | [18][56][68][126] | |
| Deformations | ↑ | ↑ ✞ | ↑ | [52][66][126][134][144][145][146] | |
| Maintenance costs | ↑ | ↑ | ↑ | [52][54][105][125][126][128][144] | |
| Mineralogy | ~ | ~ | [52][97] | ||
| Biological interactions | |||||
| Epibionts | ↑ | [132] | |||
| Predators | ↑ | ↑ | [67] | ||
| Grazers | ↑ | ↑ | ↑ | [113][137] | |
| Photoautotrophic euendoliths | ↔ | ↔ | ↔ | [18][68][106][126][130][131] | |
| Bioengineered ecosystems | |||||
| Architectural complexity | ↑↓ | ↓ | [91][97] | ||
| Coastal protection from waves and other stressors | ↓ | ↑↓ | ↓ | ↓ | [67][91][97][134][136][142] |
| Mitigation of environmental stressors for associated species | ↑ | [69][74][76] | |||
| Resistance to anthropogenic stressors | ↓ | [136][147] | |||
Symbols for effects: (=)—no effect; (↑)—positive effect, reinforcement; (↓)—negative effect, reduction; (↓↑)—variable responses depending on the host species and/or environmental conditions; (~)—alteration in the composition of the parameter; (✞)—mortality observed in the host species; (↔)—mutualistic relationship; (lim)—effect observed during unusual harsh environmental conditions (i.e., heatwaves). Please note that some effects presented in this table are based on observations of a single species or different life cycle stages of the host species.
However, the presence of euendoliths has also been observed to have beneficial effects. In corals, photoassimilates are translocated directly from the euendoliths to the host until symbiotic zooxanthellae recolonize the coral tissue [130][131][138][148]. However, this mutualistic relationship is limited in the case of rapid heat wave-induced bleaching events, as high light intensities coupled with high temperatures inhibit euendolithic photosynthetic activity [141]. In bivalves, photoautotrophic euendoliths indirectly enhance the albedo of the shell, thus reducing the overall body temperature and the mortality rates experienced by infested bivalves [68][76][139]. The beneficial effects of euendoliths can extend to neighboring mussels, further increasing the thermal buffering provided by mussel beds to associated species on rocky shores [74][76]. In CCA crusts, photoautotrophic euendoliths preferentially remove the highly dissoluble fraction of the carbonate skeleton, thus increasing its resistance to bioerosion, either due to OA or photoautotrophic euendoliths themselves [65][91][96][97].
4. Photoautotrophic Euendoliths and Marine Calcifiers in the Anthropocene
Marine calcifiers and their future relationship with photoautotrophic euendoliths will be influenced by global climate change (GCC) [89][149][150][151]. For marine calcifiers, rising sea surface temperatures (SST), ocean acidification, and the increase in solar radiation will negatively impact calcification, survival, growth, and reproduction, and diminish their resistance to other environmental stressors, such as pollution [152][153][154][155][156]. With decreasing calcification and a weakening of existing calcified structures due to passive dissolution in a more acidic ocean, marine calcifying organisms will become more susceptible to bioerosion [151]. Euendolithic infestation by photoautotrophs of carbonate substrates, especially those of live calcifying organisms, is expected to increase in prevalence with increased SST, solar radiation, and OA [69][76][91][142][157][158]. As the negative effects of euendolithic infestation on live calcifying organisms are expected to increase in intensity under future oceanic conditions, so might the beneficial effects. Photoautotrophic euendoliths can contribute to host survival under OA and heat waves. Both detrimental and beneficial effects of euendolithic infestation in live calcifying organisms are expected to increase in intensity with the ongoing GCC and OA. Nevertheless, in the long term, euendolithic infestation is detrimental to its calcifying hosts, ultimately leading to their death.
References
- Carpenter, W. On the Microscopic Structure of Shells. Rep. Br. Assoc. Adv. Sci. 1845, 14, 1–24.
- Bornet, M.E.; Flahault, C. Sur Quelques Plantes Vivant Dans Le Test Calcaire Des Mollusques. Bull. Soc. Bot. Fr. 1889, 36, CXLVII–CLXXVI.
- Kölliker, A.V. On the Frequent Occurrence of Vegetable Parasites in the Hard Structures of Animals. Proc. R. Soc. Lond. 1860, 10, 95–99.
- Wedl, C. On the Significance of the Canals Found in Many Mollusc and Gastropod Shells. Sitzungsberichte Kais. Akad. Wiss. 1859, 33, 451–472.
- Gary, M.; McAfee, R.; Wolf, C.L. Glossary of Geology; American Geological Institute: Washington, WA, USA, 1973.
- Golubic, S.; Friedmann, I.; Schneider, J. The Lithobiontic Ecological Niche, with Special Reference to Microorganisms. J. Sediment. Petrol. 1981, 51, 475–478.
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring Microorganisms and Microborings in Carbonate Substrates. In The Study of Trace Fossils; Frey, R.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 229–259. ISBN 978-3-642-65925-6.
- Couradeau, E.; Roush, D.; Guida, B.S.; Garcia-Pichel, F. Diversity and Mineral Substrate Preference in Endolithic Microbial Communities from Marine Intertidal Outcrops (Isla de Mona, Puerto Rico). Biogeosciences 2017, 14, 311–324.
- Amarelle, V.; Carrasco, V.; Fabiano, E. The Hidden Life of Antarctic Rocks. In The Ecological Role of Micro-organisms in the Antarctic Environment; Castro-Sowinski, S., Ed.; Springer Polar Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 221–237. ISBN 978-3-030-02785-8.
- Ercegović, A. Études Écologiques et Sociologiques Des Cyanophycées Lithophytes de La Côte Yougoslave de l’Adriatique. Bull. Int. Acad. Yougosl. Sci. B-Arts 1932, 26, 33–56.
- Wisshak, M. Microbioerosion. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 64, pp. 213–243. ISBN 978-0-444-53813-0.
- Schroeder, J.H. Calcified Filaments of an Endolithic Alga in Recent Bermuda Reefs. Neues Jahrb. Geol. Palaontol. Mon. 1972, 1972, 16–33.
- Drew, K.M. Studies in the Bangioideae. III. The Life-History of Porphyra Umbilicalis (L.) Kütz. Var. Laciniata (Lighf.) J. Ag. A. The Conchocelis-Phase in Culture. Ann. Bot. 1954, XVIII, 184–209.
- Drew, K.M. Studies in the Bangiophycidae. IV. The Conchocelis-Phase of Bangia Fuscopurpurea (Dillw.) Lyngbye in Culture. Publ. Sta. Zool. Napoli. 1958, 30, 358–372.
- Lagerheim, G. Note Sur Le Mastigocoleus, Nouveau Genre Des Algues Marines de l’Ordre Des Phycochromacées. Notarisia 1886, 1, 65–69.
- Golubic, S.; Schneider, J.; Le Campion-Alsumard, T.; Campbell, S.E.; Hook, J.E.; Radtke, G. Approaching Microbial Bioerosion. Facies 2019, 65, 25.
- Rooney, W.S.J.; Perkins, R.D. Distribution and Geologic Significance of Microboring Organisms within Sediments of the Arlington Reef Complex, Australia. Geol. Soc. Am. Bull. 1972, 83, 1139–1150.
- Tribollet, A.; Payri, C. Bioerosion of the Coralline Alga Hydrolithon Onkodes by Microborers in the Coral Reefs of Moorea, French Polynesia. Oceanol. Acta 2001, 24, 329–342.
- Al-Thukair, A.A.; Golubic, S.; Rosen, G. New Euendolithic Cyanobacteria from the Bahama Bank and the Arabian Gulf: Hyella Racemus Sp. Nov. 1. J. Phycol. 1994, 30, 764–769.
- Ndhlovu, A.; McQuaid, C.D.; Nicastro, K.R.; Zardi, G.I. Community Succession in Phototrophic Shell-Degrading Endoliths Attacking Intertidal Mussels. J. Molluscan Stud. 2021, 87, eyaa036.
- Golubic, S.; Brent, G.; Le Campion, T. Scanning Electron Microscopy of Endolithic Algae and Fungi Using a Multipurpose Casting-embedding Technique. Lethaia 1970, 3, 203–209.
- Chacón, E.; Berrendero, E.; Garcia-Pichel, F. Biogeological Signatures of Microboring Cyanobacterial Communities in Marine Carbonates from Cabo Rojo, Puerto Rico. Sediment. Geol. 2006, 185, 215–228.
- Al-Thukair, A.A. Calculating Boring Rate of Endolithic Cyanobacteria Hyella Immanis under Laboratory Conditions. Int. Biodeterior. Biodegrad. 2011, 65, 664–667.
- Pasella, M.M.; Lee, M.-F.E.; Marcelino, V.R.; Willis, A.; Verbruggen, H. Ten Ostreobium (Ulvophyceae) Strains from Great Barrier Reef Corals as a Resource for Algal Endolith Biology and Genomics. Phycologia 2022, 61, 452–458.
- Cunningham, J.A.; Rahman, I.A.; Lautenschlager, S.; Rayfield, E.J.; Donoghue, P.C.J. A Virtual World of Paleontology. Trends Ecol. Evol. 2014, 29, 347–357.
- Sutton, M.D. Tomographic Techniques for the Study of Exceptionally Preserved Fossils. Proc. R. Soc. B Biol. Sci. 2008, 275, 1587–1593.
- Silbiger, N.; Guadayol, Ò.; Thomas, F.; Donahue, M. Reefs Shift from Net Accretion to Net Erosion along a Natural Environmental Gradient. Mar. Ecol. Prog. Ser. 2014, 515, 33–44.
- Wisshak, M.; Titschack, J.; Kahl, W.-A.; Girod, P. Classical and New Bioerosion Trace Fossils in Cretaceous Belemnite Guards Characterised via Micro-CT. Foss. Rec. 2017, 20, 173–199.
- Golubic, S. Distribution, Taxonomy, and Boring Patterns of Marine Endolithic Algae. Am. Zool. 1969, 9, 747–751.
- Lukas, K.J. Two Species of the Chlorophyte Genus Ostreobium from Skeletons of Atlantic and Caribbean Reef Corals. J. Phycol. 1974, 10, 331–335.
- Verbruggen, H. Morphological Complexity, Plasticity, and Species Diagnosability in the Application of Old Species Names in DNA-Based Taxonomies. J. Phycol. 2014, 50, 26–31.
- Verbruggen, H.; Ashworth, M.; LoDuca, S.T.; Vlaeminck, C.; Cocquyt, E.; Sauvage, T.; Zechman, F.W.; Littler, D.S.; Littler, M.M.; Leliaert, F. A Multi-Locus Time-Calibrated Phylogeny of the Siphonous Green Algae. Mol. Phylogenet. Evol. 2009, 50, 642–653.
- Gutner-Hoch, E.; Fine, M. Genotypic Diversity and Distribution of Ostreobium Quekettii within Scleractinian Corals. Coral Reefs 2011, 30, 643–650.
- Marcelino, V.R.; Verbruggen, H. Multi-Marker Metabarcoding of Coral Skeletons Reveals a Rich Microbiome and Diverse Evolutionary Origins of Endolithic Algae. Sci. Rep. 2016, 6, 31508.
- Sauvage, T.; Schmidt, W.E.; Suda, S.; Fredericq, S. A Metabarcoding Framework for Facilitated Survey of Endolithic Phototrophs with TufA. BMC Ecol. 2016, 16, 8.
- Gonzalez-Zapata, F.L.; Gómez-Osorio, S.; Sánchez, J.A. Conspicuous Endolithic Algal Associations in a Mesophotic Reef-Building Coral. Coral Reefs 2018, 37, 705–709.
- Ricci, F.; Fordyce, A.; Leggat, W.; Blackall, L.L.; Ainsworth, T.; Verbruggen, H. Multiple Techniques Point to Oxygenic Phototrophs Dominating the Isopora Palifera Skeletal Microbiome. Coral Reefs 2021, 40, 275–282.
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards Next-Generation Biodiversity Assessment Using DNA Metabarcoding. Mol. Ecol. 2012, 21, 2045–2050.
- Yang, S.-H.; Tandon, K.; Lu, C.-Y.; Wada, N.; Shih, C.-J.; Hsiao, S.S.-Y.; Jane, W.-N.; Lee, T.-C.; Yang, C.-M.; Liu, C.-T.; et al. Metagenomic, Phylogenetic, and Functional Characterization of Predominant Endolithic Green Sulfur Bacteria in the Coral Isopora Palifera. Microbiome 2019, 7, 3.
- Roush, D.; Giraldo-Silva, A.; Garcia-Pichel, F. Cydrasil 3, a Curated 16S rRNA Gene Reference Package and Web App for Cyanobacterial Phylogenetic Placement. Sci. Data 2021, 8, 230.
- Tandon, K.; Pasella, M.M.; Iha, C.; Ricci, F.; Hu, J.; O’Kelly, C.J.; Medina, M.; Kühl, M.; Verbruggen, H. Every Refuge Has Its Price: Ostreobium as a Model for Understanding How Algae Can Live in Rock and Stay in Business. Semin. Cell Dev. Biol. 2022, S1084952122000775.
- Behrendt, L.; Larkum, A.W.; Norman, A.; Qvortrup, K.; Chen, M.; Ralph, P.; Sørensen, S.J.; Trampe, E.; Kühl, M. Endolithic Chlorophyll d-Containing Phototrophs. ISME J. 2011, 5, 1072–1076.
- Friedmann, I.E.; Hua, M.; Ocampo-Friedmann, R. Cryptoendolithic Lichen and Cyanobacterial Communities of the Ross Desert, Antarctica. Polarforschung 1988, 58, 251–259.
- Ascaso, C.; Wierzchosb, J.; Castelloa, R. Study of the Biogenic Weathering of Calcareous Litharenite Stones Caused by Lichen and Endolithic Microorganisms. Int. Biodeterior. Biodegrad. 1998, 42, 29–38.
- Gaylarde, P.M.; Jungblut, A.-D.; Gaylarde, C.C.; Neilan, B.A. Endolithic Phototrophs from an Active Geothermal Region in New Zealand. Geomicrobiol. J. 2006, 23, 579–587.
- Huber, J.; Jadin, F. Sur Une Algue Perforante d’Eau Douce. C R. Hebd. Séances L’Académie Sci. Paris 1892, 115, 262–264.
- Akpan, E.B. Bioerosion of Oyster Shells in Brackish Modern Mangrove Swamps, Nigeria. Ichnos 1990, 1, 125–132.
- Schneider, J.; Le Campion-Alsumard, T. Construction and Destruction of Carbonates by Marine and Freshwater Cyanobacteria. Eur. J. Phycol. 1999, 34, 417–426.
- Cerrano, C.; Bavestrello, G.; Calcinai, B.; Cattaneo-Vietti, R.; Chiantore, M.; Guidetti, M.; Sarà, A. Bioerosive Processes in Antarctic Seas. Polar Biol. 2001, 24, 790–792.
- Ghirardelli, L.A. Endolithic Microorganisms in Live and Dead Thalli of Coralline Red Algae (Corallinales, Rhodophyta) in the Northern Adriatic Sea. Acta Geol. Hisp. 2002, 37, 53–60.
- Pantazidou, A.; Louvrou, I.; Economou-Amilli, A. Euendolithic Shell-Boring Cyanobacteria and Chlorophytes from the Saline Lagoon Ahivadolimni on Milos Island, Greece. Eur. J. Phycol. 2006, 41, 189–200.
- Ćurin, M.; Peharda, M.; Calcinai, B.; Golubić, S. Incidence of Damaging Endolith Infestation of the Edible Mytilid Bivalve Modiolus Barbatus. Mar. Biol. Res. 2014, 10, 179–189.
- Akpan, E.B.; Farrow, G.E. Shell-Boring Algae on the Scottish Continental Shelf: Identification, Distribution, Bathymetric Zonation. Trans. R. Soc. Edinb. Earth Sci. 1984, 75, 1–12.
- Försterra, G.; Beuck, L.; Häussermann, V.; Freiwald, A. Shallow-Water Desmophyllum Dianthus (Scleractinia) from Chile: Characteristics of the Biocoenoses, the Bioeroding Community, Heterotrophic Interactions and (Paleo)-Bathymetric Implications. In Cold-Water Corals and Ecosystems; Freiwald, A., Roberts, J.M., Eds.; Erlangen Earth Conference Series; Springer-Verlag: Berlin/Heidelberg, Germany, 2005; pp. 937–977. ISBN 978-3-540-24136-2.
- Wilkinson, M.; Burrows, E.M. The Distribution of Marine Shell-Boring Green Algae. J. Mar. Biol. Assoc. UK 1972, 52, 59–65.
- Le Campion-Alsumard, T.; Golubic, S.; Hutchings, P. Microbial Endoliths in Skeletons of Live and Dead Corals: Porites Lobata (Moorea, French Polynesia). Mar. Ecol. Prog. Ser. 1995, 117, 149–157.
- Mao Che, L.; Le Campion-Alsumard, T.; Boury-Esnault, N.; Payri, C.; Golubic, S.; Bézac, C. Biodegradation of Shells of the Black Pearl Oyster, Pinctada Margaritifera Var. Cumingii, by Microborers and Sponges of French Polynesia. Mar. Biol. 1996, 126, 509–519.
- Perry, C.T. Grain Susceptibility to the Effects of Microboring: Implications for the Preservation of Skeletal Carbonates. Sedimentology 1998, 45, 39–51.
- Tribollet, A. Dissolution of Dead Corals by Euendolithic Microorganisms Across the Northern Great Barrier Reef (Australia). Microb. Ecol. 2008, 55, 569–580.
- Meyer, N.; Wisshak, M.; Freiwald, A. Ichnodiversity and Bathymetric Range of Microbioerosion Traces in Polar Barnacles of Svalbard. Polar Res. 2020, 39, 3766.
- Campbell, S.E. The Modern Distribution and Geological History of Calcium Carbonate Boring Microorganisms. In Biomineralization and Biological Metal Accumulation; Westbroek, P., de Jong, E.W., Eds.; Springer: Dordrecht, The Netherlands, 1983; pp. 99–104. ISBN 978-94-009-7946-8.
- Chazottes, V.; Le Campion-Alsumard, T.; Peyrot-Clausade, M. Bioerosion Rates on Coral Reefs: Interactions between Macroborers, Microborers and Grazers (Moorea, French Polynesia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 1995, 113, 189–198.
- Gektidis, M. Development of Microbial Euendolithic Communities: The Influence of Light and Time. Bull. Geol. Soc. Den. 1999, 45, 147–150.
- Tribollet, A.; Golubic, S.; Radtke, G.; Reitner, J. On Microbiocorrosion. In Advances in Stromatolite Geobiology; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2011; Volume 131, pp. 265–276. ISBN 978-3-642-10414-5.
- Ramírez-Reinat, E.L.; Garcia-Pichel, F. Characterization of a Marine Cyanobacterium That Bores into Carbonates and the Redescription of the Genus Mastigocoleus. J. Phycol. 2012, 48, 740–749.
- Kaehler, S. Incidence and Distribution of Phototrophic Shell-Degrading Endoliths of the Brown Mussel Perna Perna. Mar. Biol. 1999, 135, 505–514.
- Zardi, G.I.; Nicastro, K.R.; McQuaid, C.D.; Gektidis, M. Effects of Endolithic Parasitism on Invasive and Indigenous Mussels in a Variable Physical Environment. PLoS ONE 2009, 4, e6560.
- Gehman, A.M.; Harley, C.D.G. Symbiotic Endolithic Microbes Alter Host Morphology and Reduce Host Vulnerability to High Environmental Temperatures. Ecosphere 2019, 10, e02683.
- Monsinjon, J.R.; McQuaid, C.D.; Nicastro, K.R.; Seuront, L.; Oróstica, M.H.; Zardi, G.I. Weather and Topography Regulate the Benefit of a Conditionally Helpful Parasite. Funct. Ecol. 2021, 35, 2691–2706.
- Gektidis, M.; Dubinsky, Z.; Goffredo, S. Microendoliths of the Shallow Euphotic Zone in Open and Shaded Habitats at 30°N–Eilat, Israel – Paleoecological Implications. Facies 2007, 53, 43–55.
- Wisshak, M.; Tribollet, A.; Golubic, S.; Jakobsen, J.; Freiwald, A. Temperate Bioerosion: Ichnodiversity and Biodiversity from Intertidal to Bathyal Depths (Azores). Geobiology 2011, 9, 492–520.
- Le Bris, S.; Le Campion-Alsumard, T.; Romano, J.-C. Caractéristiques du feutrage algal des récifs coralliens de Polynésie française soumis à différentes intensités de bioérosion. Oceanol. Acta 1998, 21, 695–708.
- Tribollet, A.; Golubic, S. Cross-Shelf Differences in the Pattern and Pace of Bioerosion of Experimental Carbonate Substrates Exposed for 3 Years on the Northern Great Barrier Reef, Australia. Coral Reefs 2005, 24, 422–434.
- Lourenço, C.R.; Nicastro, K.R.; McQuaid, C.D.; Sabour, B.; Zardi, G.I. Latitudinal Incidence of Phototrophic Shell-Degrading Endoliths and Their Effects on Mussel Bed Microclimates. Mar. Biol. 2017, 164, 129–139.
- Wisshak, M.; Gektidis, M.; Freiwald, A.; Lundälv, T. Bioerosion along a Bathymetric Gradient in a Cold-Temperate Setting (Kosterfjord, SW Sweden): An Experimental Study. Facies 2005, 51, 93–117.
- Zardi, G.I.; Monsinjon, J.R.; McQuaid, C.D.; Seuront, L.; Orostica, M.; Want, A.; Firth, L.B.; Nicastro, K.R. Foul-weather Friends: Modelling Thermal Stress Mitigation by Symbiotic Endolithic Microbes in a Changing Environment. Glob. Change Biol. 2021, 27, 2549–2560.
- Meyer, N.; Wisshak, M.; Freiwald, A. Bioerosion Ichnodiversity in Barnacles from the Ross Sea, Antarctica. Polar Biol. 2021, 44, 667–682.
- Hutchings, P.A. Biological Destruction of Coral Reefs. Coral Reefs 1986, 4, 239–252.
- Chazottes, V.; Le Campion-Alsumard, T.; Peyrot-Clausade, M.; Cuet, P. The Effects of Eutrophication-Related Alterations to Coral Reef Communities on Agents and Rates of Bioerosion (Reunion Island, Indian Ocean). Coral Reefs 2002, 21, 375–390.
- Chazottes, V.; Cabioch, G.; Golubic, S.; Radtke, G. Bathymetric Zonation of Modern Microborers in Dead Coral Substrates from New Caledonia—Implications for Paleodepth Reconstructions in Holocene Corals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 280, 456–468.
- Bentis, C.; Kaufman, L.; Golubic, S. Endolithic Fungi in Reef-Building Corals (Order: Scleractinia) Are Common, Cosmopolitan, and Potentially Pathogenic. Biol. Bull. 2000, 198, 254–260.
- Vogel, K.; Gektidis, M.; Golubic, S.; Kiene, W.E.; Radtke, G. Experimental Studies on Microbial Bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Australia: Implications for Paleoecological Reconstructions. Lethaia 2000, 33, 190–204.
- Radtke, G.; Golubic, S. Microborings in Mollusk Shells, Bay of Safaga, Egypt: Morphometry and Ichnology. Facies 2005, 51, 118–134.
- Golubic, S.; Campbell, S.E.; Drobne, K.; Cameron, B.; Balsam, W.L.; Cimerman, F.; Duboiss, L. Microbial Endoliths: A Benthic Overprint in the Sedimentary Record, and a Paleobathymetric Cross-Reference with Foraminifera. J. Paleontol. 1984, 58, 12.
- Golubic, S.; Schneider, J. Microbial Endoliths as Internal Biofilms. In Fossil and Recent Biofilms: A Natural History of Life on Earth; Krumbein, W.E., Paterson, D.M., Zavarzin, G.A., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 249–263. ISBN 978-90-481-6412-7.
- Kiene, W.E.; Radtke, G.; Gektidis, M.; Golubic, S.; Vogel, K. Factors Controlling the Distribution of Microborers in Bahamian Reef Environments. Facies 1995, 32, 176–188.
- Le Campion-Alsumard, T.; Campbell, S.E.; Golubic, S. Endoliths and the Depth of the Photic Zone: Discussion. J. Sediment. Petrol. 1982, 52, 1333–13338.
- Lukas, K.J. Depth Distribution and Form among Common Microboring Algae from the Florida Continental Shelf. In Proceedings of the Abstract with Programs; Boulder: Toronto, ON, Canada, 1978; Volume 10, pp. 1–448.
- Tribollet, A. The Boring Microflora in Modern Coral Reef Ecosystems: A Review of Its Roles. In Current Developments in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 67–94. ISBN 978-3-540-77597-3.
- Försterra, G.; Häussermann, V. Unusual Symbiotic Relationships between Microendolithic Phototrophic Organisms and Azooxanthellate Cold-Water Corals from Chilean Fjords. Mar. Ecol. Prog. Ser. 2008, 370, 121–125.
- Reyes-Nivia, C.; Diaz-Pulido, G.; Dove, S. Relative Roles of Endolithic Algae and Carbonate Chemistry Variability in the Skeletal Dissolution of Crustose Coralline Algae. Biogeosciences Discuss. 2014, 11, 2993–3021.
- Tribollet, A.; Veinott, G.; Golubic, S.; Dart, R. Infestation of the North American Freshwater Mussel Elliptio Complanata (Head Lake, Canada) by the Euendolithic Cyanobacterium Plectonema Terebrans Bornet et Flahault. Algol. Stud. 2008, 128, 65–77.
- Gaspard, D. Endolithic Algae, Fungi and Bacterial Activity in Holocene and Cretaceous Brachiopod Shells—Diagenetic Consequences. Mem. Assoc. Australas. Palaeontol. 2011, 41, 327–337.
- Lukas, K.J. Taxonomy and Ecology of the Endolithic Microflora of Reef Corals with a Review of the Literature on Endolithic Microphytes. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 1973.
- Perkins, R.D.; Halsey, S.D. Geologic Significance of Microboring Fungi and Algae in Carolina Shelf Sediments. J. Sediment. Res. 1971, 41, 843–853.
- Nash, M.C.; Opdyke, B.N.; Troitzsch, U.; Russell, B.D.; Adey, W.H.; Kato, A.; Diaz-Pulido, G.; Brent, C.; Gardner, M.; Prichard, J.; et al. Dolomite-Rich Coralline Algae in Reefs Resist Dissolution in Acidified Conditions. Nat. Clim. Chang. 2013, 3, 268–272.
- Diaz-Pulido, G.; Nash, M.C.; Anthony, K.R.N.; Bender, D.; Opdyke, B.N.; Reyes-Nivia, C.; Troitzsch, U. Greenhouse Conditions Induce Mineralogical Changes and Dolomite Accumulation in Coralline Algae on Tropical Reefs. Nat. Commun. 2014, 5, 3310.
- Golubic, S.; Radtke, G.; Campion-Alsumard, T.L. Endolithic Fungi in Marine Ecosystems. Trends Microbiol. 2005, 13, 229–235.
- Gutiérrez-Isaza, N.; Espinoza-Avalos, J.; León-Tejera, H.P.; González-Solís, D. Endolithic Community Composition of Orbicella Faveolata (Scleractinia) underneath the Interface between Coral Tissue and Turf Algae. Coral Reefs 2015, 34, 625–630.
- Keats, D.W.; Groener, A.; Chamberlain, Y.M. Cell Sloughing in the Littoral Zone Coralline Alga, Spongites Yendoi (Foslie) Chamberlain (Corallinales, Rhodophyta). Phycologia 1993, 32, 143–150.
- Owen, G.; Williams, A. The Caecum of Articulate Brachiopoda. Proc. R. Soc. Lond. B Biol. Sci. 1969, 172, 187–201.
- Scardino, A.; De Nys, R.; Ison, O.; O’Connor, W.; Steinberg, P. Microtopography and Antifouling Properties of the Shell Surface of the Bivalve Molluscs Mytilus Galloprovincialis and Pinctada Imbricata. Biofouling 2003, 19, 221–230.
- Scardino, A.; de Nys, R. Fouling Deterrence on the Bivalve Shell Mytilus Galloprovincialis: A Physical Phenomenon? Biofouling 2004, 20, 249–257.
- Bers, A.V.; Díaz, E.R.; da Gama, B.A.P.; Vieira-Silva, F.; Dobretsov, S.; Valdivia, N.; Thiel, M.; Scardino, A.J.; McQuaid, C.D.; Sudgen, H.E.; et al. Relevance of Mytilid Shell Microtopographies for Fouling Defence—A Global Comparison. Biofouling 2010, 26, 367–377.
- Prusina, I.; Peharda, M.; Ezgeta-Balic, D.; Puljas, S.; Glamuzina, B.; Golubic, S. Life-History Trait of the Mediterranean Keystone Species Patella Rustica: Growth and Microbial Bioerosion. Mediterr. Mar. Sci. 2015, 16, 393.
- Odum, H.T.; Odum, E.P. Trophic Structure and Productivity of a Windward Coral Reef Community on Eniwetok Atoll. Ecol. Monogr. 1955, 25, 291–320.
- Fine, M.; Roff, G.; Ainsworth, T.D.; Hoegh-Guldberg, O. Phototrophic Microendoliths Bloom during Coral “White Syndrome”. Coral Reefs 2006, 25, 577–581.
- Grange, J.S.; Rybarczyk, H.; Tribollet, A. The Three Steps of the Carbonate Biogenic Dissolution Process by Microborers in Coral Reefs (New Caledonia). Environ. Sci. Pollut. Res. 2015, 22, 13625–13637.
- Le Campion-Alsumard, T. Les Cyanophycées endolithes marines. Systématique, ultrastructure, écologie et biodestruction. Oceanol. Acta 1979, 2, 143–156.
- Tribollet, A.; Golubic, S. Reef Bioerosion: Agents and Processes. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 435–450. ISBN 978-94-007-0113-7.
- Fordyce, A.J.; Ainsworth, T.D.; Leggat, W. Microalgae, a Boring Bivalve and a Coral—A Newly Described Association Between Two Coral Reef Bioeroders Within Their Coral Host. Integr. Org. Biol. 2020, 2, obaa035.
- Schneider, J.; Torunski, H. Biokarst on Limestone Coasts, Morphogenesis and Sediment Production. Mar. Ecol. 1983, 4, 45–63.
- Nicholson, G.M.; Clements, K.D. Resolving Resource Partitioning in Parrotfishes (Scarini) Using Microhistology of Feeding Substrata. Coral Reefs 2020, 39, 1313–1327.
- Zubia, M.; Peyrot-Clausade, M. Internal Bioerosion of Acropora Formosa in Réunion (Indian Ocean): Microborer and Macroborer Activities. Oceanol. Acta 2001, 24, 251–262.
- Golubic, S.; Schneider, J. Carbonate Dissolution. Stud. Environ. Sci. 1979, 3, 107–129.
- Rice, M.M.; Maher, R.L.; Correa, A.M.S.; Moeller, H.V.; Lemoine, N.P.; Shantz, A.A.; Burkepile, D.E.; Silbiger, N.J. Macroborer Presence on Corals Increases with Nutrient Input and Promotes Parrotfish Bioerosion. Coral Reefs 2020, 39, 409–418.
- Pari, N.; Peyrot-Clausade, M.; Le Campion-Alsumard, T.; Hutchings, P.; Chazottes, V.; Golubic, S.; Le Campion, J.; Fontaine, M. Bioerosion of Experimental Substrates on High Islands and on Atoll Lagoons (French Polynesia) after Two Years of Exposure. Mar. Ecol. Prog. Ser. 1998, 166, 119–130.
- Pari, N.; Peyrot-Clausade, M.; Hutchings, P.A. Bioerosion of Experimental Substrates on High Islands and Atoll Lagoons (French Polynesia) during 5 Years of Exposure. J. Exp. Mar. Biol. Ecol. 2002, 276, 109–127.
- Kiene, W.E.; Hutchings, P.A. Bioerosion Experiments at Lizard Island, Great Barrier Reef. Coral Reefs 1994, 13, 91–98.
- Carreiro-Silva, M.; McClanahan, T.R.; Kiene, W.E. The Role of Inorganic Nutrients and Herbivory in Controlling Microbioerosion of Carbonate Substratum. Coral Reefs 2005, 24, 214–221.
- Carreiro-Silva, M.; McClanahan, T.; Kiene, W. Effects of Inorganic Nutrients and Organic Matter on Microbial Euendolithic Community Composition and Microbioerosion Rates. Mar. Ecol. Prog. Ser. 2009, 392, 1–15.
- Carreiro-Silva, M.; Kiene, W.E.; Golubic, S.; McClanahan, T.R. Phosphorus and Nitrogen Effects on Microbial Euendolithic Communities and Their Bioerosion Rates. Mar. Pollut. Bull. 2012, 64, 602–613.
- Godinot, C.; Tribollet, A.; Grover, R.; Ferrier-Pagès, C. Bioerosion by Euendoliths Decreases in Phosphate-Enriched Skeletons of Living Corals. Biogeosciences Discuss. 2012, 9, 2425–2444.
- Laukner, G. Diseases of Mollusca: Bivalvia. In Diseases of Marine Animals; Kinne, O., Ed.; Biologische Anstalt Helgoland: Hamburg, Germany, 1983; Volume II, pp. 477–961.
- Kaehler, S.; McQuaid, C.D. Lethal and Sub-Lethal Effects of Phototrophic Endoliths Attacking the Shell of the Intertidal Mussel Perna Perna. Mar. Biol. 1999, 135, 497–503.
- Hassenrück, C.; Jantzen, C.; Försterra, G.; Häussermann, V.; Willenz, P. Rates of Apical Septal Extension of Desmophyllum Dianthus: Effect of Association with Endolithic Photo-Autotrophs. Mar. Biol. 2013, 160, 2919–2927.
- Massé, A.; Domart-Coulon, I.; Golubic, S.; Duché, D.; Tribollet, A. Early Skeletal Colonization of the Coral Holobiont by the Microboring Ulvophyceae Ostreobium Sp. Sci. Rep. 2018, 8, 2293.
- Ndhlovu, A.; McQuaid, C.D.; Monaco, C.J. Ectoparasites Reduce Scope for Growth in a Rocky-Shore Mussel (Perna Perna) by Raising Maintenance Costs. Sci. Total Environ. 2021, 753, 142020.
- Curry, G.B. Microborings in Recent Brachiopods and the Functions of Caeca. Lethaia 1983, 16, 119–127.
- Schlichter, D.; Zscharnack, B.; Krisch, H. Transfer of Photoassimilates from Endolithic Algae to Coral Tissue. Naturwissenschaften 1995, 82, 1–564.
- Schlichter, D.; Kampmann, H.; Conrady, S. Trophic Potential and Photoecology of Endolithic Algae Living within Coral Skeletons. Mar. Ecol. 1997, 18, 299–317.
- Marquet, N.; Nicastro, K.R.; Gektidis, M.; McQuaid, C.D.; Pearson, G.A.; Serrão, E.A.; Zardi, G.I. Comparison of Phototrophic Shell-Degrading Endoliths in Invasive and Native Populations of the Intertidal Mussel Mytilus Galloprovincialis. Biol. Invasions 2013, 15, 1253–1272.
- Ndhlovu, A.; McQuaid, C.D.; Nicastro, K.R.; Zardi, G.I. Parasitism by Endolithic Cyanobacteria Reduces Reproductive Output and Attachment Strength of Intertidal Ecosystem Engineers. Mar. Biol. 2022, 169, 37.
- Goldberg, W.M.; Makemson, J.C.; Colley, S.B. Entocladia Endozoica Sp. Nov., a Pathogenic Chlorophyte: Structure, Life History, Physiology, and Effect on Its Coral Host. Biol. Bull. 1984, 166, 368–383.
- Massé, A.; Tribollet, A.; Meziane, T.; Bourguet-Kondracki, M.; Yéprémian, C.; Sève, C.; Thiney, N.; Longeon, A.; Couté, A.; Domart-Coulon, I. Functional Diversity of Microboring Ostreobium Algae Isolated from Corals. Environ. Microbiol. 2020, 22, 4825–4846.
- Nicastro, K.R.; McQuaid, C.D.; Zardi, G.I. Between a Rock and a Hard Place: Combined Effect of Trampling and Phototrophic Shell-Degrading Endoliths in Marine Intertidal Mussels. Mar. Biodivers. 2019, 49, 1581–1586.
- Nolan, C.P. Size, Shape and Shell Morphology in the Antarctic Limpet Nacella Concinna at Signy Island, South Orkney Islands. J. Molluscan Stud. 1991, 57, 225–238.
- Fine, M.; Loya, Y. Endolithic Algae: An Alternative Source of Photoassimilates during Coral Bleaching. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1205–1210.
- Zardi, G.I.; Nicastro, K.R.; McQuaid, C.D.; Ng, T.P.T.; Lathlean, J.; Seuront, L. Enemies with Benefits: Parasitic Endoliths Protect Mussels against Heat Stress. Sci. Rep. 2016, 6, 31413.
- Fine, M.; Steindler, L.; Loya, Y. Endolithic Algae Photoacclimate to Increased Irradiance during Coral Bleaching. Mar. Freshw. Res. 2004, 55, 115.
- Fine, M.; Meroz-Fine, E.; Hoegh-Guldberg, O. Tolerance of Endolithic Algae to Elevated Temperature and Light in the Coral Montipora Monasteriata from the Southern Great Barrier Reef. J. Exp. Biol. 2005, 208, 75–81.
- Reyes-Nivia, C.; Diaz-Pulido, G.; Kline, D.; Guldberg, O.-H.; Dove, S. Ocean Acidification and Warming Scenarios Increase Microbioerosion of Coral Skeletons. Glob. Change Biol. 2013, 19, 1919–1929.
- Risk, M.J.; Sammarco, P.W.; Edinger, E.N. Bioerosion in Acropora across the Continental Shelf of the Great Barrier Reef. Coral Reefs 1995, 14, 79–86.
- Le Campion-Alsumard, T.; Golubic, S.; Priess, K. Fungi in Corals: Symbiosis or Disease? Interaction between Polyps and Fungi Causes Pearl-like Skeleton Biomineralization. Mar. Ecol. Prog. Ser. 1995, 117, 137–147.
- Morse, D.E.; Morse, A.; Duncan, H.; Trench, R.K. Algal Tumors in the Caribbean Octocorallian, Gorgonia Ventalina: II. Biochemical Characterization of the Algae, and First Epidemiological Observations. Bull. Mar. Sci. 1981, 31, 399–409.
- Morse, D.E.; Morse, A.; Duncan, H. Algal Tumors in the Caribeean Sea-Fan, Gorgonia Ventalina. In Proceeding of Third International Coral Reef Symposium; Taylor, D.L., Ed.; Rosenstiel School of Marine and Atmospheric Science: Miami, FL, USA, 1977; Volume 1, pp. 623–629.
- Nicastro, K.R.; Seuront, L.; McQuaid, C.D.; Zardi, G.I. Symbiont-Induced Intraspecific Phenotypic Variation Enhances Plastic Trapping and Ingestion in Biogenic Habitats. Sci. Total Environ. 2022, 826, 153922.
- Fine, M.; Zibrowius, H.; Loya, Y. Oculina Patagonica: A Non-Lessepsian Scleractinian Coral Invading the Mediterranean Sea. Mar. Biol. 2001, 138, 1195–1203.
- Raven, J.A. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide; The Royal Society: London, UK, 2005.
- Andersson, A.J. Coastal Ocean and Carbonate Systems in the High CO2 World of the Anthropocene. Am. J. Sci. 2005, 305, 875–918.
- Schönberg, C.H.L.; Fang, J.K.H.; Carreiro-Silva, M.; Tribollet, A.; Wisshak, M. Bioerosion: The Other Ocean Acidification Problem. ICES J. Mar. Sci. 2017, 74, 895–925.
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J. Living on the Edge of Two Changing Worlds: Forecasting the Responses of Rocky Intertidal Ecosystems to Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404.
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar. Freshw. Res. 1999, 50, 839–866.
- Gattuso, J.-P.; Allemand, D.; Frankignoulle, M. Photosynthesis and Calcification at Cellular, Organismal and Community Levels in Coral Reefs: A Review on Interactions and Control by Carbonate Chemistry. Am. Zool. 1999, 39, 160–183.
- Petes, L.E.; Menge, B.A.; Murphy, G.D. Environmental Stress Decreases Survival, Growth, and Reproduction in New Zealand Mussels. J. Exp. Mar. Biol. Ecol. 2007, 351, 83–91.
- O’Donnell, M.J.; George, M.N.; Carrington, E. Mussel Byssus Attachment Weakened by Ocean Acidification. Nat. Clim. Chang. 2013, 3, 587–590.
- Enochs, I.C.; Manzello, D.P.; Tribollet, A.; Valentino, L.; Kolodziej, G.; Donham, E.M.; Fitchett, M.D.; Carlton, R.; Price, N.N. Elevated Colonization of Microborers at a Volcanically Acidified Coral Reef. PLoS ONE 2016, 11, e0159818.
- Marcelino, V.R.; Morrow, K.M.; Oppen, M.J.H.; Bourne, D.G.; Verbruggen, H. Diversity and Stability of Coral Endolithic Microbial Communities at a Naturally High pCO2 Reef. Mol. Ecol. 2017, 26, 5344–5357.





