Photoautotrophic euendoliths, including cyanobacteria, and red and green microalgae, are part of the endolithic community. The term ‘endolith’ refers to a morphologically and physiologically heterogenous group of microorganisms living within a rock or other stony matter, such as coral skeletons or animal shells, and more specifically, to organisms that actively bore into relatively soluble substrates, such as phosphate and carbonate substrates. Euendoliths are ubiquitous, as they can be found in almost every environment, geographical location, or depth, where the appropriate substratum (e.g., relatively soluble carbonate and phosphate substrates) is available and the requirements for photosynthesis are met. The most diverse and abundant modern euendolithic communities can be found in the marine environment.
Euendoliths, as microorganisms infesting inanimate substrates, were first thought to be ecologically irrelevant. Numerous studies have subsequently shown that euendoliths can colonize living marine calcifying organisms, such as coral skeletons and bivalve shells, causing both sub-lethal and lethal damage. Moreover, under suitable environmental conditions, their presence can have surprising benefits for the host. Thus, infestation by photoautotrophic euendoliths has significant consequences for calcifying organisms that are of particular importance in the case of ecosystems underpinned by calcifying ecosystem engineers.
- bioerosion
- ecosystem engineers
- parasitism
- mutualism
- boring microflora
1. What Are Euendoliths and How Are They Observed?
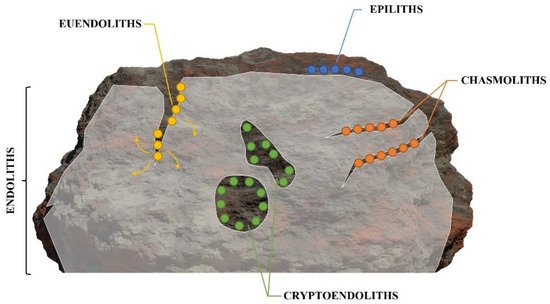
-
Epiliths that live on the surface of the substrate;
-
Chasmoliths (chasm = cleft) that adhere to the surface of fissures and cracks in the substrate;
-
Cryptoendoliths (crypto = hidden) that adhere to the surface of pre-existing cavities within porous rocks, including spaces produced and vacated by euendoliths, with no dissolution action;
-
Euendoliths (eu = true) that actively penetrate carbonate (and phosphate) substrates and reside partially or completely inside cavities of their own making.
- Cultivation on inoculated agar plates
2. Incidence of Photoautotrophic Euendoliths in Marine Ecosystems
Photoautotrophic euendoliths have a cosmopolitan geographical distribution and have been recorded in a variety of habitats, including terrestrial [43][44][9,91], freshwater and volcanic lakes [45][46][11,92], brackish [47][93], and marine environments [48][49][4,94]. Euendoliths are ubiquitous in the marine environment, occurring in enclosed seas, such as the Adriatic Sea [50][16] and the Mediterranean Sea [51][52][95,96], in cold-temperate [53][54][55][97,98,99], tropical waters [56][57][58][59][13,100,101,102], as well as in the Arctic and Antarctic [49][60][94,103]. Present essentially anywhere, there is sufficient light to allow for photosynthesis and a carbonate substrate to bore into; euendolithic communities play an important role in ecological processes in the marine environment [48][61][4,104]. Although they appear to erode virtually all suitable substrates, the distribution of euendoliths and the composition of euendolithic communities are extremely variable and depend on light availability, the nature of the substrate, and a variety of abiotic and biotic environmental factors acting in synergy [22][62][63][19,52,105].2.1. Light Availability
As photosynthetic organisms, light availability is the major determinant of euendolithic activity and distribution and has a strong influence on the composition of euendolithic communities [63][105], reflecting the specific light requirements of different species [64][106]. As boring by euendoliths is an active mechanism, it is often restricted to environmental conditions optimal for growth [65][116]. In most marine habitats, light availability is highly variable and is influenced by the topography of the area, the presence of 3D structures, the nature of the substrate, and water depth. Euendoliths are more abundant, and erosion more severe, in microhabitats with high light availability, such as sun-exposed surfaces in the intertidal, mostly horizontal, and moderately inclined surfaces high on the shore [66][67][68][69][14,107,108,109], or in shallow waters [63][105], compared to microhabitats with low light availability, such as down-facing and shaded substrates [67][70][71][107,110,111]. Reduction of light availability at polluted sites [72][73][112,113] or in habitats at greater depths [58][63][101,105] similarly reduces euendolith abundance. Geographically, photoautotrophic euendoliths are more abundant, and erosion more severe, at lower latitudes than higher latitudes, where unfavorable environmental conditions slow down endolithic infestation in both the intertidal and underwater [49][60][71][74][75][76][77][33,94,103,111,114,115,117]. While the composition of euendolithic communities shifts as light availability decreases with increasing depth, their bathymetric distribution is consistent around the world (see Tables 1 and 2 in [11][42]) [7][62][63][70][71][75][78][79][80][81][82][83][8,19,105,110,111,114,118,119,120,121,122,123]. Euendoliths are ubiquitous in the supratidal, intertidal, and wave spray zones [84][124], where assemblages are dominated by cyanobacteria and chlorophytes, referred to as the CyChlo-association [63][105], in sediments [58][101] as well as mollusk shells and coral skeletons [53][63][66][14,97,105]. In the shallow photic zones, the additional conchocelis stages of rhodophytes can be observed (CyChloRho-association) in the early stages of colonization [63][105]. In the disphotic zone or in shaded microhabitats, where light availability is dramatically reduced, only heterotrophs and low-light specialists amongst the photoautotrophs occur, forming the so-called OstPleHet-association [53][63][85][10,97,105]. These include the cyanobacterium Plectonema terebans Bornet and Flahault ex Gomont (1892) and the chlorophyte Ostreobium quekettii that have been recorded down to about 300 m [86][87][88][125,126,127]. Finally, heterotrophic organisms (i.e., fungi and bacteria) dominate the benthic assemblages of the deep, aphotic zone. At a finer scale, different clades within the same euendolithic species can be distributed along a depth gradient, suggesting different physiological traits [33][63]. Not only does the composition of euendolithic communities change with depth, but also with time, as mature euendolithic communities, even in shallow, clear waters, are dominated by the OstPleHet-association [63][105].2.2. Nature of the Substrate
Photoautotrophic euendoliths colonize a wide range of carbonate substrates, from compact limestone and loose sediments to living calcifying organisms or their fragmented remains [7][8]. Euendoliths have been recorded in the skeletons of corals [56][89][90][13,32,128] and coralline algae [18][50][91][15,16,129], in the shells of mollusks [57][66][92][14,100,130] and brachiopods [93][131], in the tests of foraminifera [84][124], in the calcareous tubes of annelids [55][99] and the plates of barnacles [60][103], and in sclerosponges [94][132]. Colonization by and distribution of euendoliths is intrinsically influenced by the nature and physical properties of the substrate, such as its mineralogy, porosity, translucency, density, or architecture [8][22][58][39,52,101]. While most euendoliths appear to be generalists, substrate preferences are found in some, such as the cyanobacterium Mastigocoleus testarum that bores into calcium carbonate substrates but not into other carbonates [8][65][39,116]. Skeletal remains of calcifying organisms are more susceptible to euendolithic infestation than other carbonate substrates [12][17][58][95][43,50,101,133], with the highest levels of an infestation occurring in the densest and least porous substrates. Within skeletal remains, the high-Mg calcite skeleton of crustose coralline algae is more susceptible to dissolution than the skeletons of massive or branching corals or bivalve shells, which are mostly composed of aragonite [96][97][134,135]. In bivalves, the presence of organic lamellae (i.e., conchiolin) within shells slows down excavation by photoautotrophic euendoliths [57][58][59][62][19,100,101,102] and can only be penetrated by heterotrophic euendoliths fungi [17][57][98][50,100,136]. In live calcifying hosts (e.g., corals, coralline algae, bivalves), a wide range of defenses prevents the colonization of the calcified parts by euendoliths. Coral skeletons are protected by the polyp tissue [56][81][99][13,121,137], while coralline algae have the capacity of sloughing their protective epithelial cells to prevent biofouling [100][138]. Bivalve, brachiopod, and other mollusk shells have a protective layer, the periostracum, which deters fouling organisms [101][102][103][104][105][139,140,141,142,143]. Nonetheless, the incidence of euendolithic infestation in live calcifying organisms is high around the world, independent of the location or the substrate. Nearly all corals around the world have been recorded as infested by euendoliths [56][90][94][13,128,132], while up to 90% of bivalves shells are infested on rocky shores worldwide [52][66][67][68][14,96,107,108]. After the death of the calcifying organisms, colonization becomes more intense, as euendoliths do not have to overcome the active or passive defense mechanisms of the host or adjust their boring performances to carbonate accretion rates of living organisms [18][56][58][99][13,15,101,137]. In newly available dead carbonate substrates, a succession of microborer communities can be observed, boring from the surface down into the substrate [56][106][107][108][13,144,145,146]: (i) pioneer species, such as the large chlorophyte Phaeophila sp. Hauck (1876) and the cyanobacterium Mastigocoleus testarum, settle within the first three months, (ii) these are followed by an intermediate stage, between 3 and 6 months, where the chlorophyte Ostreobium sp. starts to dominate euendolithic communities, and (iii) the final stage, largely dominated by Ostreobium, after more than 6 months of exposure.2.3. Biotic and Abiotic Environmental Factors
In addition to light, biotic, and abiotic environmental factors can act in synergy to influence the composition and density of euendolithic communities, as well as their rates of microbioerosion. In the intertidal, the abrasive effects of sand and other sediments carried by the winds or the waves favor the initial colonization of the substrate by euendoliths, and ultimately increase the severity of infestation in mussels [66][14]. Meanwhile, in shallow waters, nutrient concentration, epilithic cover, and the presence of macroborers and macrograzers interact to shape euendolithic communities [62][109][110][111][19,147,148,149]. This interaction operates through several mechanisms:-
Grazers are attracted to the substrate by the presence of photoautotrophic euendoliths, as these represent a renewable source of food [18][62][113][15,19,151]. The boring activity of euendoliths weakens the superficial layers of the substrate, which can facilitate the settlement of macroborers with their own bioerosive activity, as well as grazing;
-
On the one hand, macrograzers constantly remove the superficial layers of the substrate, thus extending the depth to which the light can penetrate and, therefore, the depth to which the endoliths can bore, increasing microboring rates [112][114][150,152]. Grazing also reduces the settlement and growth of epilithic organisms that compete with euendoliths for space and diminish light availability [115][17]. On the other hand, macroborers excrete different waste products within the infested substrate, such as ammonium, phosphates, or CO2. Such waste products act as fertilizer for euendolithic communities, which increase in abundance, biomass, and productivity in the vicinity of macroborers [111][114][116][149,152,153].
3. Euendolithic Infestation in Marine Bioengineered Ecosystems
While numerous studies have assessed euendolith-induced biodegradation of carbonate skeletal materials, until recently, severe harm to living host organisms was understood to be limited to the erosive activity of invertebrates or fungal borers [124][21]. Due to low light penetration within the substratum, photoautotrophic euendoliths were generally thought to be unable to inflict significant structural damage on live organisms, as they eroded only the uppermost layers of the carbonate substrate [124][21].
Over the last three decades, mounting evidence has shown that the eroding activity of photoautotrophic euendoliths can be the source of severe, often lethal, damage to living calcifying organisms [see Table 1 in Dievart et al. (2022)].
| Responses to Endolithic Infestation | Live Calcifying Hosts | References | |||
|---|---|---|---|---|---|
| Corals | Coralline Algae | Bivalves | Others | ||
| Physiological Parameters | |||||
| Growth | ↓ = | ↓ | ↓ | [105][125][126][127][128][129] | |
| General condition | = | ↓ | [52][67][125][127][130][131][132] | ||
| Reproduction | ↓ | = | [125][128][133][134] | ||
| Attachment strength | ↓ | [67][132][133] | |||
| General survival | ↑ = | ↓ ✞ | ↓ | [66][105][106][125][130][131][133][135][136][137] | |
| Individual survival to heat stress | ↑ (lim) | ↑ (lim) | [68][69][74][76][138][139][140][141] | ||
| Calcified structures | |||||
| Microbioerosion | ↑ | ↑↓ | ↑ | ↑ | [18][56][68][91][105][125][129][142] |
| Thickness | ↑ | ↓ ✞ | ↓ | [54][105][125][126] | |
| Strength | ↓ | ↓ ✞ | ↓ | [67][105][125][129][132][134][136][137][143] | |
| Porosity | ↑ | ↑ | ↑ | [18][56][68][126] | |
| Deformations | ↑ | ↑ ✞ | ↑ | [52][66][126][134][144][145][146] | |
| Maintenance costs | ↑ | ↑ | ↑ | [52][54][105][125][126][128][144] | |
| Mineralogy | ~ | ~ | [52][97] | ||
| Biological interactions | |||||
| Epibionts | ↑ | [132] | |||
| Predators | ↑ | ↑ | [67] | ||
| Grazers | ↑ | ↑ | ↑ | [113][137] | |
| Photoautotrophic euendoliths | ↔ | ↔ | ↔ | [18][68][106][126][130][131] | |
| Bioengineered ecosystems | |||||
| Architectural complexity | ↑↓ | ↓ | [91][97] | ||
| Coastal protection from waves and other stressors | ↓ | ↑↓ | ↓ | ↓ | [67][91][97][134][136][142] |
| Mitigation of environmental stressors for associated species | ↑ | [69][74][76] | |||
| Resistance to anthropogenic stressors | ↓ | [136][147] | |||
Symbols for effects: (=)—no effect; (↑)—positive effect, reinforcement; (↓)—negative effect, reduction; (↓↑)—variable responses depending on the host species and/or environmental conditions; (~)—alteration in the composition of the parameter; (✞)—mortality observed in the host species; (↔)—mutualistic relationship; (lim)—effect observed during unusual harsh environmental conditions (i.e., heatwaves). Please note that some effects presented in this table are based on observations of a single species or different life cycle stages of the host species.
However, the presence of euendoliths has also been observed to have beneficial effects. In corals, photoassimilates are translocated directly from the euendoliths to the host until symbiotic zooxanthellae recolonize the coral tissue [130][131][138][148][23,170,171,210]. However, this mutualistic relationship is limited in the case of rapid heat wave-induced bleaching events, as high light intensities coupled with high temperatures inhibit euendolithic photosynthetic activity [141][178]. In bivalves, photoautotrophic euendoliths indirectly enhance the albedo of the shell, thus reducing the overall body temperature and the mortality rates experienced by infested bivalves [68][76][139][25,108,115]. The beneficial effects of euendoliths can extend to neighboring mussels, further increasing the thermal buffering provided by mussel beds to associated species on rocky shores [74][76][33,115]. In CCA crusts, photoautotrophic euendoliths preferentially remove the highly dissoluble fraction of the carbonate skeleton, thus increasing its resistance to bioerosion, either due to OA or photoautotrophic euendoliths themselves [65][91][96][97][116,129,134,135].
4. Photoautotrophic Euendoliths and Marine Calcifiers in the Anthropocene
Marine calcifiers and their future relationship with photoautotrophic euendoliths will be influenced by global climate change (GCC) [89][149][150][151][32,242,243,244]. For marine calcifiers, rising sea surface temperatures (SST)SST, ocean acidification, and the increase in solar radiation will negatively impact calcification, survival, growth, and reproduction, and diminish their resistance to other environmental stressors, such as pollution [152][153][154][155][156][30,250,251,252,253]. With decreasing calcification and a weakening of existing calcified structures due to passive dissolution in a more acidic ocean, marine calcifying organisms will become more susceptible to bioerosion [151][244]. Euendolithic infestation by photoautotrophs of carbonate substrates, especially those of live calcifying organisms, is expected to increase in prevalence with increased SST, solar radiation, and OA [69][76][91][142][157][158][109,115,129,179,254,255]. As the negative effects of euendolithic infestation on live calcifying organisms are expected to increase in intensity under future oceanic conditions, so might the beneficial effects. Photoautotrophic euendoliths can contribute to host survival under OA and heat waves. Both detrimental and beneficial effects of euendolithic infestation in live calcifying organisms are expected to increase in intensity with the ongoing GCC and OA. Nevertheless, in the long term, euendolithic infestation is detrimental to its calcifying hosts, ultimately leading to their death.
