You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haiming Wei | -- | 2108 | 2022-10-14 11:36:43 | | | |
| 2 | Jessie Wu | + 7 word(s) | 2115 | 2022-10-17 03:33:35 | | | | |
| 3 | Jessie Wu | Meta information modification | 2115 | 2022-10-17 03:36:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, D.; Fu, B.; Wei, H. Advances in Immunotherapy for Hepatitis B. Encyclopedia. Available online: https://encyclopedia.pub/entry/29369 (accessed on 15 December 2025).
Wang D, Fu B, Wei H. Advances in Immunotherapy for Hepatitis B. Encyclopedia. Available at: https://encyclopedia.pub/entry/29369. Accessed December 15, 2025.
Wang, Dongyao, Binqing Fu, Haiming Wei. "Advances in Immunotherapy for Hepatitis B" Encyclopedia, https://encyclopedia.pub/entry/29369 (accessed December 15, 2025).
Wang, D., Fu, B., & Wei, H. (2022, October 14). Advances in Immunotherapy for Hepatitis B. In Encyclopedia. https://encyclopedia.pub/entry/29369
Wang, Dongyao, et al. "Advances in Immunotherapy for Hepatitis B." Encyclopedia. Web. 14 October, 2022.
Copy Citation
Hepatitis B virus (HBV) is a hepatotropic virus with the potential to cause chronic infection, and it is one of the common causes of liver disease worldwide. Chronic HBV infection leads to liver cirrhosis and, ultimately, hepatocellular carcinoma (HCC). Progress in Hepatitis-B-Specific Immunotherapy is discussed.
HBV
NRTI
DNA
1. The Etiology for Hepatitis B
Hepatitis B virus (HBV) DNA is considered to be a major biomarker of viral replication and has been regarded as an important endpoint of clinical trials using nucleoside analog therapy [1][2]. Hepatitis B virions have an envelope containing three viral gene products, including hepatitis B surface antigen (HBsAg) determinant [3][4]. The HBV envelope has been found to enclose an inner nucleocapsid particle, which in most virions, is composed of 120 core protein (i.e., HBcAg) homodimers. Furthermore, it has been shown that the nucleocapsid particles include a copy of a partially double-stranded relaxed circular DNA (rcDNA) genome (Figure 1) [5][6]. Approximately 10% of the nucleocapsid particles are thought to contain double-stranded linear DNA (dslDNA) in place of an rcDNA genome [3][7][8]. After HBV entry, the nucleocapsids (with their rcDNA) are transported into the nucleus, where the host enzymes participate in the repair of the viral genome and its conversion into the cccDNA [9][10][11].
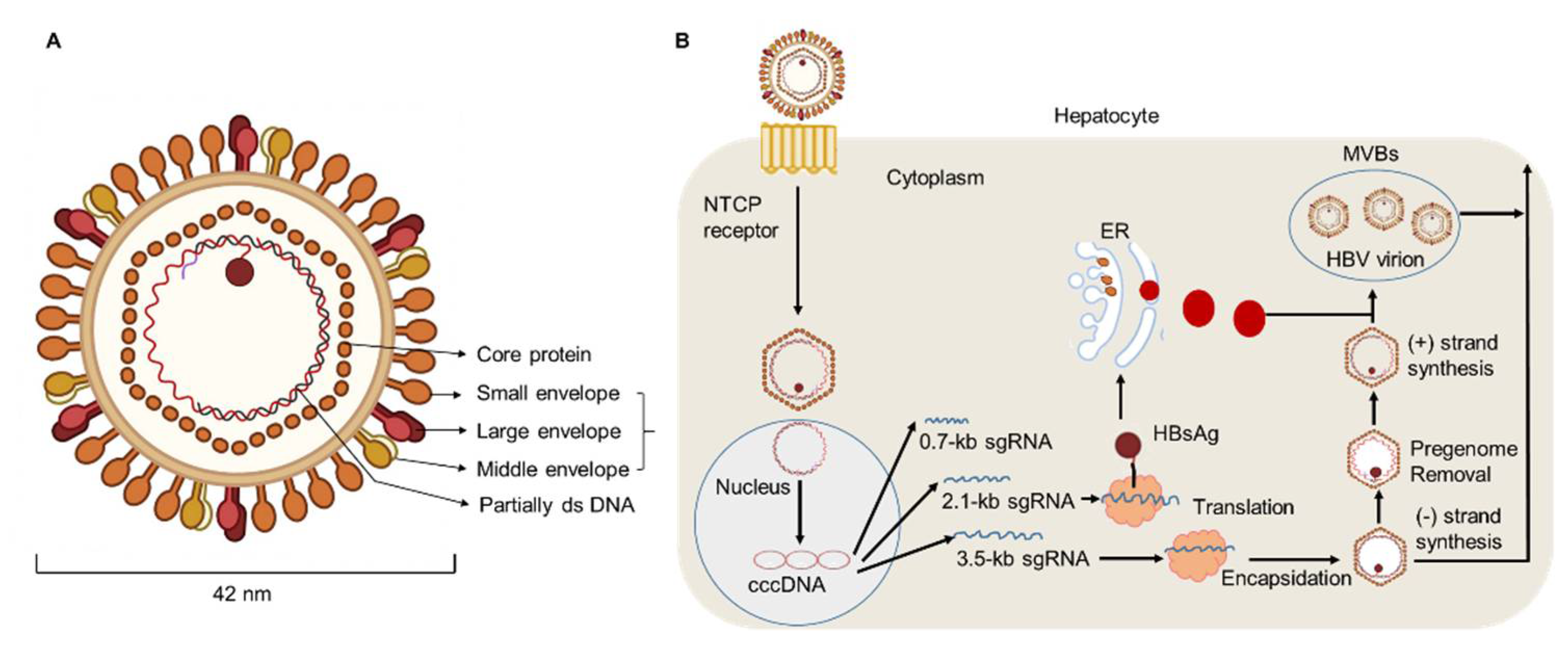
Figure 1. HBV particle and life cycle. (A) Hepatitis B virions are about 42 nm in diameter. The envelope of HBV virion contains three forms of HBsAg: large (L), middle (M), and small (S) envelope proteins. The capsid encapsidates a partially double stranded (ds) DNA. The HBV envelope has an inner nucleocapsid particle that always consists of 120 core protein. (B) Firstly, HBV attaches to the host cell membrane through its envelope proteins and the sodium taurocholate co-transporting polypeptide (NTCP). Next, the viral genome reaches the cytoplasm of hepatocytes and enters the nucleus, where host enzymes will repair the genome into the covalently closed circular DNA (cccDNA). In addition, transcription and nuclear export of mRNA to the hepatocellular cytoplasm for translation are observed. HBsAg are produced via the endoplasmic reticulum (ER)-Golgi complex and then assembled in the cytoplasm, while HBV virions are formed by budding from multivesicular bodies (MVBs). The new virions will exit the host and infect new hepatocytes.
2. Progress in Hepatitis-B-Specific Immunotherapy
Existing treatment regimens have achieved remarkable cure rates for patients infected with the hepatitis C virus (HCV). However, the current regimens for treating HBV remain suboptimal. Current therapeutic approaches include nucleoside analogues (NA) and nucleotide drugs (NUCs), which both efficiently inhibit HBV replication. Lamivudine, the first nucleoside reverse transcriptase inhibitor (NRTI), obtained Food and Drug Administration (FDA) approval in 1998. Since then, other NRTIs such as adefovir and telbivudine have been developed but these are not used as first-line therapies due to drug-associated resistance. Currently, entecavir (ETV), tenofovir alafenamide (TAF), and tenofovir disoproxil fumarate (TDF) are used as the first-line oral drugs against HBV infection [12][13]. These agents can optimally lower HBV DNA levels in the serum of patients and reduce liver failure. However, current antiviral agents have minimal impact directly on cccDNA in primary human hepatocytes [14]. With the persistence of long-lived cccDNA, the potential for relapse of HBV exists, even after the clearance of viremia [15]. In addition, integrated viral DNA may survive immune clearance and the potential for relapse also exists in patients with resolved HBV infection. To date, the study of HBV cccDNA is still hampered by the lack of an appropriate model [16][17]. A deeper understanding of cccDNA might provide new perspectives to find a functional cure. Additionally, the loss of HBeAg or HBsAg with prolonged therapy occurs in very few patients [18][19]. For HBeAg-positive patients, oral antiviral drugs are regarded as the most common treatment strategy because of their effectiveness and ability to provide sustained viral suppression. The decision to treat HBeAg-positive CHB patients with one of the NUCs (such as lamivudine, entecavir, or tenofovir) should be individualized [20][21]. Additionally, the number of HBeAg-negative CHB patients is increasing and these patients have become the majority in terms of the form of chronic HBV, especially in Middle Eastern and north African countries. Indeed, few patients who are HBeAg-negative would achieve the loss of HBsAg, and a large quantity of these patients may experience HBV recurrence after discontinuation of therapy. Therefore, most guidelines suggest lifelong treatment, with the goal of achieving high rates of viral suppression [22]. Marcellin et al. carried out a study of HBeAg-negative patients with TDF treatment for up to 10 years and demonstrated that TDF therapy resulted in persistent maintenance of viral suppression and was well tolerated [23]. Furthermore, through a study from 17 countries, Maria Buti found that more than 90% of patients who were HBeAg-negative and receiving TDF had an HBV DNA of less than 29 IU/mL after treatment for 48 weeks [24].
IFN-α has antiviral properties and can regulate immune function. To date, IFN-α has been regarded as the first-choice therapy for treating CHB [25]. A multi-center study reported that, after 48 weeks of treatment with a combination of PEG-IFN-α plus TDF, a 9.1% HBsAg loss was observed at week 72 post treatment initiation [26][27][28]. In addition, Fu et al. reported that, after PEG-IFN-α-2b treatment, approximately 30% of patients with CHB underwent HBeAg seroconversion by week 72 [29][30]. However, there are some limitations of therapeutic IFN-α administration. For example, IFN-α therapy may have some side effects and contraindications, especially in patients with advanced liver disease [31]. To achieve a functional cure for HBV, a properly orchestrated activation of anti-HBV immunity is required. As patients with CHB have low numbers of HBV-specific CD8+ T cells, which are frequently exhausted, existing immunotherapeutic approaches designed to promote antiviral immunity may not be adequate [32][33][34]. After analyzing the characteristics of innate and adaptive immune response during HBV infection, some promising immuno-dependent therapeutic strategies to achieve a functional cure for CHB were proposed. These included IL-2, checkpoint inhibitors (e.g., anti-PD-1 and anti-CTLA-4), and therapeutic vaccines. Authors demonstrated that non-responder (NR) patients who failed to respond to PEG-IFN-α treatment, benefited from a sequential low dose of IL-2 (1 × 106 IU) therapy, which caused a decrease in the frequency of PD-1+ CD8+ T cells and Tregs [35]. Furthermore, authors found sequential IL-2 therapy significantly restored the frequency of HBV-specific CD8+ T cells and the HBsAg-specific effector function of CD8+ T cells. Importantly, authors found that, in the majority of NR patients, HBeAg levels were markedly decreased after sequential IL-2 therapy [25] (Figure 2).
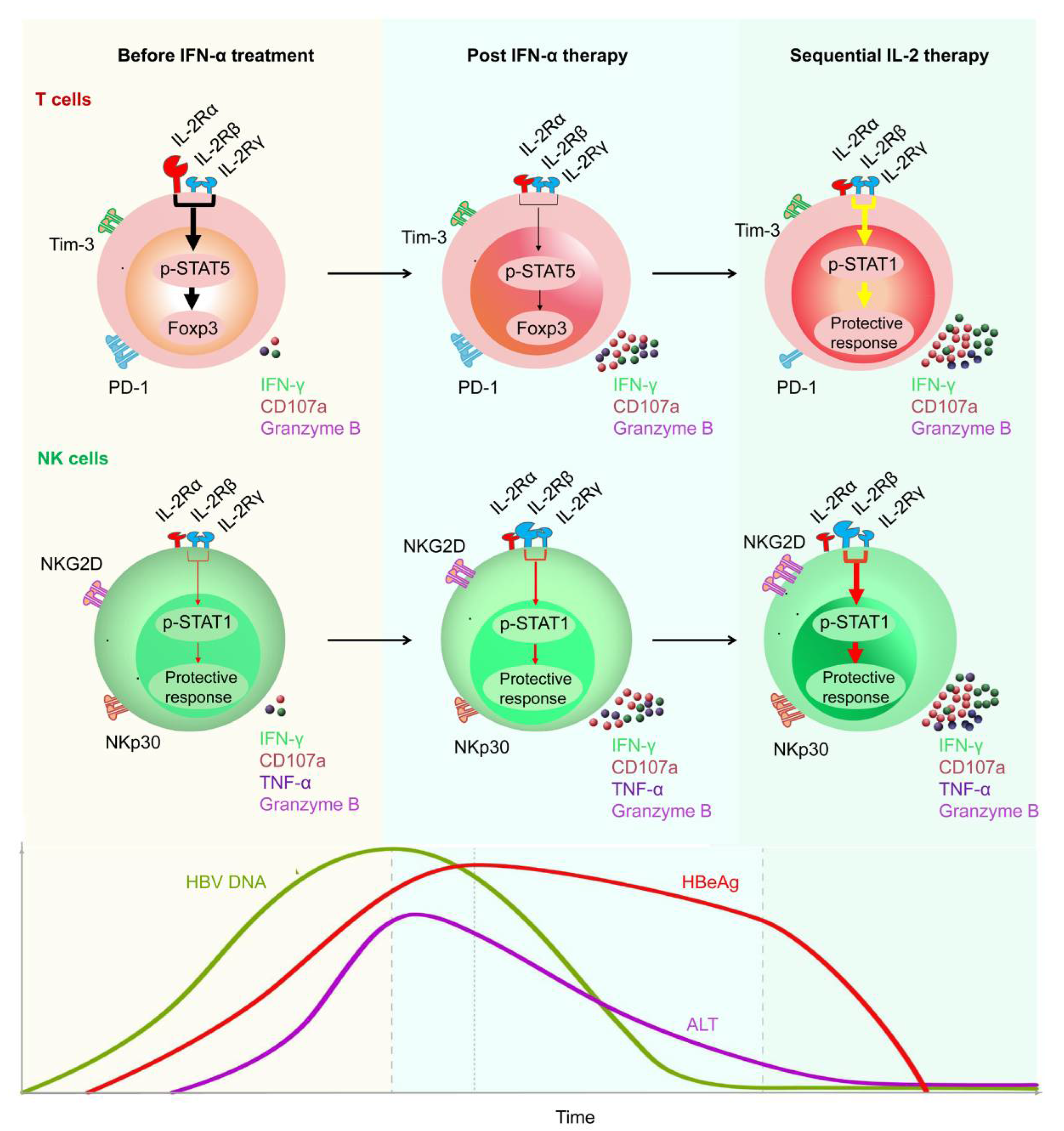
Figure 2. Restoration of HBV-specific CD8+ T cell and NK cell responses by sequential IL-2 treatment in non-responder patients after IFN-α therapy. IL-2Rα was high expressed, and NKp30 was low expressed on T cells and NK cells, respectively, of non-responder (NR) patients, in whom IFN-α therapy had failed. Those NR patients were treated with low-dose IL-2 for 24 weeks. A decrease in IL-2Rα expression on their CD4+ T cells was verified, suggesting that IFN-α therapy may provide a rationale for sequential IL-2 treatment without increasing regulatory T cells (Tregs). In addition, non-responders experienced a decrease in the numbers of PD-1 expression. Furthermore, sequential IL-2 administration restored effective immune function, involving STAT1 activation in both T cells and NK cells. Moreover, IL-2 therapy increased the function of HBV-specific T cells and NK cells, which translated into improved clinical outcomes, including HBeAg seroconversion, among the non-responder CHB patients.
Checkpoint inhibitors and therapeutic vaccination have also been proposed to restore the antiviral immune response of patients with CHB [36]. However, so far, treatments with checkpoint inhibitors have only been applied usefully in some solid malignancies including melanoma and renal cell carcinoma. In vitro studies have shown that anti-PD1/PDL-1 blockade could partially restore the function of exhausted HBV-specific CD8+ T cells [37][38][39]. Gane et al. found that nivolumab (a PD-1 inhibitor) with or without GS-4774 (a therapeutic vaccine) was well-tolerated and would contribute to an HBsAg decrease in virally suppressed HBeAg-negative patients in a phase Ib study [40]. In addition, trials of several vaccine candidates have been carried out in patients with CHB [41][42][43]. Boni et al. reported that the administration of tenofovir plus GS-4774 therapy was well tolerated and could improve HBV-specific T cell responses in CHB patients. In addition, the production of TNF-α, IFN-γ, as well as IL-2, obviously increased. Furthermore, data have suggested that combination treatments including vaccines may be regarded as sequential administration that is able to increase the antiviral immune response in the future [44].
The role of the innate immune system should not be ignored in the process of HBV eradication. However, in the context of HBV infection, the innate immune response is often poorly activated due to immune evasion. RIG-I or Toll-like receptor (TLR) agonists, such as TLR-7 and TLR-8 agonists, have been used to induce the activation of innate immunity. GS-9620, a TLR-7 agonist, has been found to induce the production of IFN-α, especially by pDCs. In addition, the treatment of RO7020531 triggered obvious immune activation in patients with CHB [45][46][47]. Moreover, recently developed TLR-8 agonists may contribute to the activation of PRRs present in the liver, and GS-9688 has been shown to promote the production of IL-12 and IL-18 from monocytes or DCs [48][49][50]. Furthermore, as cytokines such as IL-12 also contribute to NK cell activation, which have been demonstrated to kill both HBV-infected hepatocytes and HBV-specific CD8+ T cells, it is necessary to comprehensively evaluate the function of activated innate immunity in the process of HBV eradication. Furthermore, there are a large quantity of new therapeutic drugs for patients with CHB under investigation. GLS4 is a core protein allosteric modulator. A total of 20 weeks of treatment with GLS4 resulted in reduced DNA levels (1.48–6.09 log decrease) [51]. Several capsid assembly modulators have been under development for CHB therapy. For example, ABI-H0731, was found to cause a significant decrease in HBV DNA levels at 12 weeks, when combined with entecavir [52]. In addition, the administration of RO7049389 not only reduced HBV DNA levels, but also decreased HBsAg, as well as HBeAg levels in the serum [51][53]. Additionally, the effects of siRNAs in clinical trials also appear encouraging. Treatment with JNJ3989 achieved a 1.3–3.8 log decrease in HBsAg levels [51]. Furthermore, several other new therapies that have been investigated have been reported as safe and well tolerated in healthy volunteers, such as GSK3389404 [54] (Table 1).
Table 1. Select new therapeutic strategies for patients with CHB under development.
| Drug Names | Mechanism of Function | Effects | References |
|---|---|---|---|
| GLS4 | Core binding | Data of 20 weeks demonstrated DNA level log decrease of 1.48–5.58 after administration (twice daily, BID) | [51] |
| ABI-H0731 | Core binding | Data showed mean maximum NA level log reduction from baseline were 1.7, 2.1, and 2.8 in the 100, 200, and 300 mg dose group, respectively | [51][52] |
| RO7049389 | Core binding | Median DNA level declines of 2.7 (200 mg, BID) and 3.2 (400 mg, BID) demonstrated at 28 days | [51][55] |
| REP 2165 | HBsAg binding | Obviously higher percentages of CHB patients in REP 2165 group had reduction in HBsAg to below 1 IU/mL and HBsAg seroconversion during the first 24 weeks of TDF and PEG-IFN-α treatment | [51][56][57] |
| TG-1050 | Transgene | HBV specific T cell responses were induced. Data at day 197 showed mean 0.45 log reduction in HBsAg levels | [58] |
| RO7020531 | TLR7 agonist | Safety and tolerability in healthy Chinese donors with a 150 mg q.o.d. | [59] |
| GS-9688 | TLR8 | The antiviral efficacy of 3 mg/kg (weekly) was confirmed in a woodchuck study | [60] |
| JNJ3989 | mRNA degradation | Data showed HBsAg level log reduction of 1.3–3.8 | [61] |
| CRV431 | Blocks NTCP | Data showed a significantly decreased liver HBV DNA levels with the treatment (50 mg/kg/day) for 16 days | [62] |
| GSK3389404 | mRNA degradation | Data showed safe and target engagement, with dose-dependent reductions in HBsAg | [63] |
HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; BID, twice daily; anti-HBs, anti-hepatitis B surface protein; TLR, Toll-like receptor; IU, infectious units; NTCP, sodium–taurocholate cotransporting polypeptide.
Furthermore, immunological approaches against HBV infection, which involve the use of T cells engineered with a classical T cell receptor (TCR) specific for human leukocyte antigens (HLA)-class I restricted HBV epitopes or a chimeric antigen receptor (CAR), have shown some promise [36]. Despite the encouraging preliminary results of such T cell therapies, they are associated with a risk of inducing fatal hepatic inflammation. Thus, the adoptive transfer of engineered T cells and the manufacturing techniques used must be evaluated with more caution. In addition, more robust experimental and clinical trial data are needed. Platelets play important roles in inflammatory and immune-mediated disorders. Aiolfi et al. reported that platelets contributed to the pathogenesis of HBV-related liver disease by their ability to promote the homing of effector CD8+ T cells in the liver, expression of pro-angiogenic mediators (such as VEGF and TGF-β1) and the production of pro-inflammatory cytokines (such as IFN-γ) [64]. Aspirin is a widely used anti-platelet drug. Notably, the suppression of platelet activation using aspirin would significantly reduce the number of HBV-specific CD8+ T cells and the recruitment of inflammatory cells in the liver, which contributes to alleviating liver injury and the likelihood of HCC [64][65]. Therefore, anti-platelet therapy might be another promising approach for the treatment of patients with CHB. Collectively, to achieve the goal of developing a functional cure, more knowledge derived from the accumulation of experiment and clinical trials is needed.
References
- Mommeja-Marin, H.; Mondou, E.; Blum, M.R.; Rousseau, F. Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: Analysis and review of the literature. Hepatology 2003, 37, 1309–1319.
- Crignis, E.D.; Hossain, T.; Romal, S.; Carofiglio, F.; Moulos, P.; Khalid, M.M.; Rao, S.; Bazrafshan, A.; Verstegen, M.M.; Pourfarzad, F.; et al. Application of human liver organoids as a pa-tient-derived primary model for HBV infection and related hepatocellular carcinoma. Elife 2021, 10, e60747.
- Iannacone, M.; Guidotti, L.G. Immunobiology and pathogenesis of hepatitis B virus infection. Nat. Rev. Immunol. 2022, 22, 19–32.
- Guidotti, L.G.; Chisari, F.V. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 2006, 1, 23–61.
- Lucifora, J.; Delphin, M. Current knowledge on Hepatitis Delta Virus replication. Antivir. Res. 2020, 179, 104812.
- Tsukuda, S.; Watashi, K. Hepatitis B virus biology and life cycle. Antivir. Res. 2020, 182, 104925.
- Shih, C.; Yang, C.C.; Choijilsuren, G.; Chang, C.H.; Liou, A.T. Hepatitis B Virus. Trends Microbiol. 2018, 26, 386–387.
- Blondot, M.L.; Bruss, V.; Kann, M. Intracellular transport and egress of hepatitis B virus. J. Hepatol. 2016, 64, S49–S59.
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell Mol. Life Sci. 2019, 76, 1779–1794.
- Wei, L.; Ploss, A. Core components of DNA lagging strand synthesis machinery are essential for hepatitis B virus cccDNA for-mation. Nat. Microbiol. 2020, 5, 715–726.
- Wei, L.; Ploss, A. Mechanism of Hepatitis B Virus cccDNA Formation. Viruses 2021, 13, 1463.
- Nguyen, M.H.; Atsukawa, M.; Ishikawa, T.; Yasuda, S.; Yokohama, K.; Trinh, H.N.; Arai, T.; Fukunishi, S.; Ogawa, E.; Hsu, Y.-C.; et al. Outcomes of Sequential Therapy with Tenofovir Alafenamide After Long-term Entecavir. Am. J. Gastroenterol. 2021, 116, 1264–1273.
- Charlton, M.R.; Alam, A.; Shukla, A.; Dashtseren, B.; Lesmana, C.R.A.; Duger, D.; Payawal, D.A.; Cuong, D.D.; Jangalsaikhan, G.; Cua, I.H.Y.; et al. An expert review on the use of tenofovir ala-fenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020, 55, 811–823.
- Fung, S.; Choi, H.S.J.; Gehring, A.; Janssen, H.L.A. Getting to HBV cure: The promising paths forward. Hepatology 2022, 76, 233–250.
- Shi, Y.; Zheng, M. Hepatitis B virus persistence and reactivation. BMJ 2020, 370, m2200.
- Wang, Y.; Li, Y.; Zai, W.; Hu, K.; Zhu, Y.; Deng, Q.; Wu, M.; Li, Y.; Chen, J.; Yuan, Z. HBV covalently closed circular DNA minichromosomes in distinct epigenetic transcriptional states differ in their vulnerability to damage. Hepatology 2022, 75, 1275–1288.
- Zhang, X.; Lu, W.; Zheng, Y.; Wang, W.; Bai, L.; Chen, L.; Feng, Y.; Zhang, Z.; Yuan, Z. In situ analysis of intrahepatic virological events in chronic hepatitis B virus infection. J. Clin. Investig. 2016, 126, 1079–1092.
- Kuipery, A.; Gehring, A.J.; Isogawa, M. Mechanisms of HBV immune evasion. Antivir. Res. 2020, 179, 104816.
- Zhou, L.; He, R.; Fang, P.; Li, M.; Yu, H.; Wang, Q.; Yu, Y.; Wang, F.; Zhang, Y.; Chen, A.; et al. Hepatitis B virus rigs the cellular metabolome to avoid innate immune recog-nition. Nat. Commun. 2021, 12, 98.
- Leung, N. Treatment of HBeAg-positive chronic hepatitis B with nucleos(t)ide analogues. Liv. Int. 2011, 31, 85–89.
- Zarski, J.P.; Marcellin, P.; Cohard, M.; Lutz, J.M.; Bouche, C.; Rais, A. Comparison of anti-HBe-positive and HBe-antigen-positive chronic hepatitis B in France. French Multicentre Group. J. Hepatol. 1994, 20, 636–640.
- García-López, M.; Lens, S.; Pallett, L.J.; Testoni, B.; Rodríguez-Tajes, S.; Mariño, Z.; Bartres, C.; Gracia-Pras, E.; Leonol, T.; Perpinan, E.; et al. Viral and immune factors associated with suc-cessful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J. Hepatol. 2021, 74, 1064–1074.
- Marcellin, P.; Wong, D.K.; Sievert, W.; Buggisch, P.; Petersen, J.; Flisiak, R.; Manns, M.; Kaita, K.; Krastev, Z.; Lee, S.S.; et al. Ten-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B virus infection. Liv. Int. 2019, 39, 1868–1875.
- Buti, M.; Gane, E.; Seto, W.K.; Chan, H.L.; Chuang, W.L.; Stepanova, T.; Hui, A.-J.; Lim, Y.-S.; Mehta, R.; Janssen, H.L.A.; et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: A randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol. Hepatol. 2016, 1, 196–206.
- Wang, D.; Fu, B.; Shen, X.; Guo, C.; Liu, Y.; Zhang, J.; Sun, J.; Ye, Y.; Li, J.; Tian, Z.; et al. Restoration of HBV-specific CD8 (+) T-cell responses by sequential low-dose IL-2 treatment in non-responder patients after IFN-α therapy. Signal Transduct. Target Ther. 2021, 6, 376.
- Perrillo, R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 2009, 49, S103–S111.
- Dienstag, J.L. Benefits and risks of nucleoside analog therapy for hepatitis B. Hepatology 2009, 49, S112–S121.
- Marcellin, P.; Ahn, S.H.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkashab, M.; Chuang, W.-L.; Lim, S.-G.; Tabak, F.; Mehta, R.; et al. Combination of Tenofovir Disoproxil Fumarate and Pegin-terferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients with Chronic Hepatitis, B. Gastroenterology 2016, 150, 134–144.e110.
- Fu, B.; Wang, D.; Shen, X.; Guo, C.; Liu, Y.; Ye, Y.; Sun, R.; Li, J.; Tian, Z.; Wei, H. Immunomodulation Induced During Interferon-α Therapy Impairs the Anti-HBV Immune Response Through CD24 (+) CD38 (hi) B Cells. Front. Immunol. 2020, 11, 591269.
- Wang, D.; Wang, D.; Huang, M.; Zheng, X.; Shen, Y.; Fu, B.; Zhao, H.; Chen, X.; Peng, P.; Zhu, Q.; et al. Transcriptomic characteristics and impaired immune function of pa-tients who retest positive for SARS-CoV-2 RNA. J. Mol. Cell Biol. 2021, 13, 748–759.
- Vlachogiannakos, J.; Papatheodoridis, G.V. HBeAg-negative chronic hepatitis B: Why do I treat my patients with pegylated inter-feron-alfa? Liv. Int. 2014, 34, 127–132.
- Wu, W.; Shi, Y.; Li, S.; Zhang, Y.; Liu, Y.; Wu, Y.; Chen, Z. Blockade of Tim-3 signaling restores the virus-specific CD8⁺ T-cell response in patients with chronic hepatitis B. Eur. J. Immunol. 2012, 42, 1180–1191.
- Kurktschiev, P.D.; Raziorrouh, B.; Schraut, W.; Backmund, M.; Wächtler, M.; Wendtner, C.M.; Bengsch, B.; Thimme, R.; Denk, G.; Zachoval, R.; et al. Dysfunctional CD8+ T cells in hepa-titis B and C are characterized by a lack of antigen-specific T-bet induction. J. Exp. Med. 2014, 211, 2047–2059.
- Bengsch, B.; Martin, B.; Thimme, R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier pa-tients is linked to T cell differentiation. J. Hepatol. 2014, 61, 1212–1219.
- Wang, F.S.; Fan, J.G.; Zhang, Z.; Gao, B.; Wang, H.Y. The global burden of liver disease: The major impact of China. Hepatology 2014, 60, 2099–2108.
- Bertoletti, A.; Tan, A.T. HBV as a target for CAR or TCR-T cell therapy. Curr. Opin. Immunol. 2020, 66, 35–41.
- Schurich, A.; Khanna, P.; Lopes, A.R.; Han, K.J.; Peppa, D.; Micco, L.; Nebbia, G.; Kennedy, P.T.F.; Geretti, A.-M.; Dusheiko, G.; et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011, 53, 1494–1503.
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 4215–4225.
- Fisicaro, P.; Valdatta, C.; Massari, M.; Loggi, E.; Biasini, E.; Sacchelli, L.; Cavallo, M.C.; Silini, E.M.; Andreone, P.; Missale, G.; et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010, 138, 682–693, 693.e1–4.
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907.
- Mancini-Bourgine, M.; Fontaine, H.; Scott-Algara, D.; Pol, S.; Bréchot, C.; Michel, M.-L. Induction or expansion of T-cell responses by a hepatitis B DNA vaccine administered to chronic HBV carriers. Hepatology 2004, 40, 874–882.
- Michel, M.L.; Pol, S.; Brechot, C.; Tiollais, P. Immunotherapy of chronic hepatitis B by anti HBV vaccine: From present to future. Vaccine 2001, 19, 2395–2399.
- Vandepapelière, P.; Lau, G.K.; Leroux-Roels, G.; Horsmans, Y.; Gane, E.; Tawandee, T.; Merican, M.I.B.; Win, K.M.; Trepo, C.; Cooksley, G.; et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: A randomized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007, 25, 8585–8597.
- Boni, C.; Janssen, H.L.A.; Rossi, M.; Yoon, S.K.; Vecchi, A.; Barili, V.; Yoshida, E.M.; Trinh, H.; Rodell, T.C.; Laccabue, D.; et al. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients with Chronic Hepatitis. Gastroenterology 2019, 157, 227–241.e227.
- Niu, C.; Li, L.; Daffis, S.; Lucifora, J.; Bonnin, M.; Maadadi, S.; Salas, E.; Chu, R.; Ramos, H.; Livingston, C.M.; et al. Toll-like receptor 7 agonist GS-9620 induces prolonged inhibition of HBV via a type I interferon-dependent mechanism. J. Hepatol. 2018, 68, 922–931.
- Lanford, R.E.; Guerra, B.; Chavez, D.; Giavedoni, L.; Hodara, V.L.; Brasky, K.M.; Fosdick, A.; Frey, C.R.; Zheng, J.; Wolfgang, G.; et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology 2013, 144, 1508–1517, 1517.e1–10.
- Block, T.M.; Chang, K.M.; Guo, J.T. Prospects for the Global Elimination of Hepatitis, B. Annu. Rev. Virol. 2021, 8, 437–458.
- Jo, J.; Tan, A.T.; Ussher, J.E.; Sandalova, E.; Tang, X.Z.; Tan-Garcia, A.; To, N.; Hong, M.; Chia, A.; Gill, U.S.; et al. Toll-like receptor 8 agonist and bacteria trigger potent activa-tion of innate immune cells in human liver. PLoS Pathog. 2014, 10, e1004210.
- Xun, Z.; Yao, X.; Zhu, C.; Ye, Y.; Wu, S.; Chen, T.; Zeng, Y.; Lin, C.; Yang, B.; Ou, Q.; et al. Proteomic characterization of the natural history of chronic HBV infection re-vealed by tandem mass tag-based quantitative proteomics approach. Mater. Today Bio. 2022, 15, 100302.
- Perrillo, R.; Lin, H.S.; Schwarz, K.B.; Rosenthal, P.; Lisker-Melman, M.; Chung, R.T.; Prokunina-Olsson, L.; Coherty, G.; Feld, J.; Hepatitis B Research Network (HBRN). Changes in serum hepatitis B surface and e antigen, interferon-inducible protein 10, and aminotransferase levels during combination therapy of immune-tolerant chronic hepatitis B. Hepatology 2022, 76, 775–787.
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844.
- Yuen, M.F.; Agarwal, K.; Gane, E.J.; Schwabe, C.; Ahn, S.H.; Kim, D.J.; Lim, Y.-S.; Cheng, W.; Sievert, W.; Visvanathan, K.; et al. Safety, pharmacokinetics, and antiviral effects of ABI-H0731, a hepatitis B virus core inhibitor: A randomised, placebo-controlled phase 1 trial. Lancet Gastroenterol. Hepatol. 2020, 5, 152–166.
- Lahlali, T.; Berke, J.M.; Vergauwen, K.; Foca, A.; Vandyck, K.; Pauwels, F.; Zoulim, F.; Durantel, D. Novel Potent Capsid Assembly Modulators Regulate Multiple Steps of the Hepatitis B Virus Life Cycle. Antimicrob. Agents Chemother. 2018, 62, e00835-18.
- Lee, H.W.; Lee, J.S.; Ahn, S.H. Hepatitis B Virus Cure: Targets and Future Therapies. Int. J. Mol. Sci. 2020, 22, 213.
- Yuen, M.F.; Zhou, X.; Gane, E.; Schwabe, C.; Tanwandee, T.; Feng, S.; Jin, Y.; Triyani, M.; Lemenuel-Diot, A.; Cosson, V.; et al. Safety, pharmacokinetics, and antiviral activity of RO7049389, a core protein allosteric modulator, in patients with chronic hepatitis B virus infection: A multicentre, random-ised, placebo-controlled, phase 1 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 723–732.
- Bazinet, M.; Pântea, V.; Placinta, G.; Moscalu, I.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Larovoi, L.; Smesnoi, V.; Musteata, T.; et al. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients with Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology 2020, 158, 2180–2194.
- Vaillant, A. HBsAg, Subviral Particles, and Their Clearance in Establishing a Functional Cure of Chronic Hepatitis B Virus Infec-tion. ACS Infect. Dis. 2021, 7, 1351–1368.
- Zoulim, F.; Fournier, C.; Habersetzer, F.; Sprinzl, M.; Pol, S.; Coffin, C.S.; Leroy, V.; MA, M.; Wedemeyer, H.; Lohse, A.W.; et al. Safety and immunogenicity of the therapeutic vaccine TG1050 in chronic hepatitis B patients: A phase 1b placebo-controlled trial. Hum. Vaccin. Immunother. 2020, 16, 388–399.
- Luk, A.; Jiang, Q.; Glavini, K.; Triyatni, M.; Zhao, N.; Racek, T.; Zhu, Y.; Grippo, J. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin. Transl. Sci. 2020, 13, 985–993.
- Daffis, S.; Balsitis, S.; Chamberlain, J.; Zheng, J.; Santos, R.; Rowe, W.; Ramakrishnan, D.; Pattabiraman, D.; Spurlock, S.; Chu, R.; et al. Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis, B. Hepatology 2021, 73, 53–67.
- Pan, J.L.; Xu, X.Y. Research progress of siRNA in reducing serum HBsAg levels in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 2020, 28, 179–182.
- Gallay, P.; Ure, D.; Bobardt, M.; Chatterji, U.; Ou, J.; Trepanier, D.; Foster, R. The cyclophilin inhibitor CRV431 inhibits liver HBV DNA and HBsAg in transgenic mice. PLoS ONE 2019, 14, e0217433.
- Yuen, M.F.; Heo, J.; Kumada, H.; Suzuki, F.; Suzuki, Y.; Xie, Q.; Jia, J.; Karino, Y.; Hou, J.; Chayama, K.; et al. Phase IIa, randomised, double-blind study of GSK3389404 in pa-tients with chronic hepatitis B on stable nucleos(t)ide therapy. J. Hepatol. 2022, 77, 967–977.
- Aiolfi, R.; Sitia, G. Chronic hepatitis B: Role of anti-platelet therapy in inflammation control. Cell Mol. Immunol. 2015, 12, 264–268.
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; Chisari, F.V.; Ruggeri, Z.M.; Guidotti, L.G. Platelets mediate cytotoxic T lympho-cyte-induced liver damage. Nat. Med. 2005, 11, 1167–1169.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
861
Revisions:
3 times
(View History)
Update Date:
17 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




