Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Hepatitis B virus (HBV) is a hepatotropic virus with the potential to cause chronic infection, and it is one of the common causes of liver disease worldwide. Chronic HBV infection leads to liver cirrhosis and, ultimately, hepatocellular carcinoma (HCC). Progress in Hepatitis-B-Specific Immunotherapy is discussed.
- HBV
- NRTI
- DNA
1. The Etiology for Hepatitis B
HBV DNA is considered to be a major biomarker of viral replication and has been regarded as an important endpoint of clinical trials using nucleoside analog therapy [28,29]. Hepatitis B virions have an envelope containing three viral gene products, including HBsAg determinant [2,30]. The HBV envelope has been found to enclose an inner nucleocapsid particle, which in most virions, is composed of 120 core protein (i.e., HBcAg) homodimers. Furthermore, it has been shown that the nucleocapsid particles include a copy of a partially double-stranded relaxed circular DNA (rcDNA) genome (Figure 1) [31,32]. Approximately 10% of the nucleocapsid particles are thought to contain double-stranded linear DNA (dslDNA) in place of an rcDNA genome [2,33,34]. After HBV entry, the nucleocapsids (with their rcDNA) are transported into the nucleus, where the host enzymes participate in the repair of the viral genome and its conversion into the cccDNA [35,36,37].
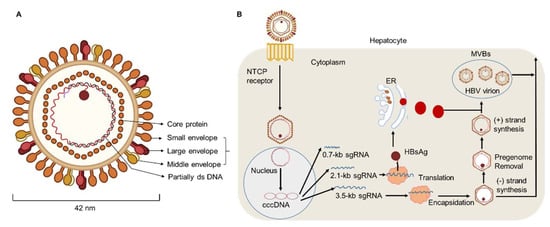
Figure 1. HBV particle and life cycle. (A) Hepatitis B virions are about 42 nm in diameter. The envelope of HBV virion contains three forms of HBsAg: large (L), middle (M), and small (S) envelope proteins. The capsid encapsidates a partially double stranded (ds) DNA. The HBV envelope has an inner nucleocapsid particle that always consists of 120 core protein. (B) Firstly, HBV attaches to the host cell membrane through its envelope proteins and the sodium taurocholate co-transporting polypeptide (NTCP). Next, the viral genome reaches the cytoplasm of hepatocytes and enters the nucleus, where host enzymes will repair the genome into the covalently closed circular DNA (cccDNA). In addition, transcription and nuclear export of mRNA to the hepatocellular cytoplasm for translation are observed. HBsAg are produced via the endoplasmic reticulum (ER)-Golgi complex and then assembled in the cytoplasm, while HBV virions are formed by budding from multivesicular bodies (MVBs). The new virions will exit the host and infect new hepatocytes.
2. Progress in Hepatitis-B-Specific Immunotherapy
Existing treatment regimens have achieved remarkable cure rates for patients infected with the hepatitis C virus (HCV). However, the current regimens for treating HBV remain suboptimal. Current therapeutic approaches include nucleoside analogues (NA) and nucleotide drugs (NUCs), which both efficiently inhibit HBV replication. Lamivudine, the first nucleoside reverse transcriptase inhibitor (NRTI), obtained Food and Drug Administration (FDA) approval in 1998. Since then, other NRTIs such as adefovir and telbivudine have been developed but these are not used as first-line therapies due to drug-associated resistance. Currently, entecavir (ETV), tenofovir alafenamide (TAF), and tenofovir disoproxil fumarate (TDF) are used as the first-line oral drugs against HBV infection [89,90]. These agents can optimally lower HBV DNA levels in the serum of patients and reduce liver failure. However, current antiviral agents have minimal impact directly on cccDNA in primary human hepatocytes [80]. With the persistence of long-lived cccDNA, the potential for relapse of HBV exists, even after the clearance of viremia [91]. In addition, integrated viral DNA may survive immune clearance and the potential for relapse also exists in patients with resolved HBV infection. To date, the study of HBV cccDNA is still hampered by the lack of an appropriate model [92,93]. A deeper understanding of cccDNA might provide new perspectives to find a functional cure. Additionally, the loss of HBeAg or HBsAg with prolonged therapy occurs in very few patients [17,27]. For HBeAg-positive patients, oral antiviral drugs are regarded as the most common treatment strategy because of their effectiveness and ability to provide sustained viral suppression. The decision to treat HBeAg-positive CHB patients with one of the NUCs (such as lamivudine, entecavir, or tenofovir) should be individualized [94,95]. Additionally, the number of HBeAg-negative CHB patients is increasing and these patients have become the majority in terms of the form of chronic HBV, especially in Middle Eastern and north African countries. Indeed, few patients who are HBeAg-negative would achieve the loss of HBsAg, and a large quantity of these patients may experience HBV recurrence after discontinuation of therapy. Therefore, most guidelines suggest lifelong treatment, with the goal of achieving high rates of viral suppression [96]. Marcellin et al. carried out a study of HBeAg-negative patients with TDF treatment for up to 10 years and demonstrated that TDF therapy resulted in persistent maintenance of viral suppression and was well tolerated [97]. Furthermore, through a study from 17 countries, Maria Buti found that more than 90% of patients who were HBeAg-negative and receiving TDF had an HBV DNA of less than 29 IU/mL after treatment for 48 weeks [98].
IFN-α has antiviral properties and can regulate immune function. To date, IFN-α has been regarded as the first-choice therapy for treating CHB [10]. A multi-center study reported that, after 48 weeks of treatment with a combination of PEG-IFN-α plus TDF, a 9.1% HBsAg loss was observed at week 72 post treatment initiation [99,100,101]. In addition, Fu et al. reported that, after PEG-IFN-α-2b treatment, approximately 30% of patients with CHB underwent HBeAg seroconversion by week 72 [53,54]. However, there are some limitations of therapeutic IFN-α administration. For example, IFN-α therapy may have some side effects and contraindications, especially in patients with advanced liver disease [102]. To achieve a functional cure for HBV, a properly orchestrated activation of anti-HBV immunity is required. As patients with CHB have low numbers of HBV-specific CD8+ T cells, which are frequently exhausted, existing immunotherapeutic approaches designed to promote antiviral immunity may not be adequate [103,104,105]. After analyzing the characteristics of innate and adaptive immune response during HBV infection, some promising immuno-dependent therapeutic strategies to achieve a functional cure for CHB were proposed. These included IL-2, checkpoint inhibitors (e.g., anti-PD-1 and anti-CTLA-4), and therapeutic vaccines. We demonstrated that non-responder (NR) patients who failed to respond to PEG-IFN-α treatment, benefited from a sequential low dose of IL-2 (1 × 106 IU) therapy, which caused a decrease in the frequency of PD-1+ CD8+ T cells and Tregs [9]. Furthermore, we found sequential IL-2 therapy significantly restored the frequency of HBV-specific CD8+ T cells and the HBsAg-specific effector function of CD8+ T cells. Importantly, we found that, in the majority of NR patients, HBeAg levels were markedly decreased after sequential IL-2 therapy [10] (Figure 2).
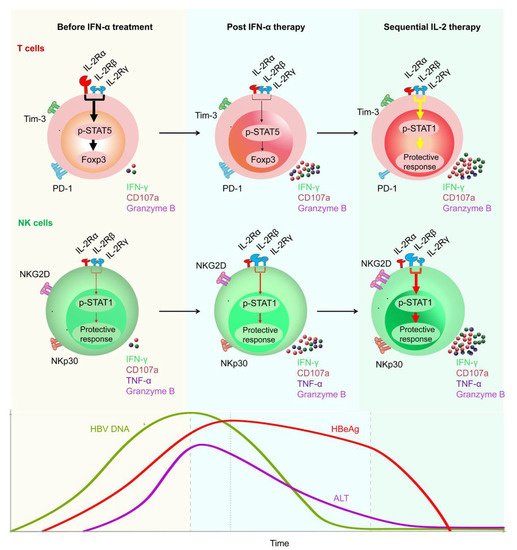
Figure 2. Restoration of HBV-specific CD8+ T cell and NK cell responses by sequential IL-2 treatment in non-responder patients after IFN-α therapy. IL-2Rα was high expressed, and NKp30 was low expressed on T cells and NK cells, respectively, of non-responder (NR) patients, in whom IFN-α therapy had failed. Those NR patients were treated with low-dose IL-2 for 24 weeks. A decrease in IL-2Rα expression on their CD4+ T cells was verified, suggesting that IFN-α therapy may provide a rationale for sequential IL-2 treatment without increasing regulatory T cells (Tregs). In addition, non-responders experienced a decrease in the numbers of PD-1 expression. Furthermore, sequential IL-2 administration restored effective immune function, involving STAT1 activation in both T cells and NK cells. Moreover, IL-2 therapy increased the function of HBV-specific T cells and NK cells, which translated into improved clinical outcomes, including HBeAg seroconversion, among the non-responder CHB patients.
Checkpoint inhibitors and therapeutic vaccination have also been proposed to restore the antiviral immune response of patients with CHB [106]. However, so far, treatments with checkpoint inhibitors have only been applied usefully in some solid malignancies including melanoma and renal cell carcinoma. In vitro studies have shown that anti-PD1/PDL-1 blockade could partially restore the function of exhausted HBV-specific CD8+ T cells [62,107,108]. Gane et al. found that nivolumab (a PD-1 inhibitor) with or without GS-4774 (a therapeutic vaccine) was well-tolerated and would contribute to an HBsAg decrease in virally suppressed HBeAg-negative patients in a phase Ib study [109]. In addition, trials of several vaccine candidates have been carried out in patients with CHB [18,110,111]. Boni et al. reported that the administration of tenofovir plus GS-4774 therapy was well tolerated and could improve HBV-specific T cell responses in CHB patients. In addition, the production of TNF-α, IFN-γ, as well as IL-2, obviously increased. Furthermore, data have suggested that combination treatments including vaccines may be regarded as sequential administration that is able to increase the antiviral immune response in the future [112].
The role of the innate immune system should not be ignored in the process of HBV eradication. However, in the context of HBV infection, the innate immune response is often poorly activated due to immune evasion. RIG-I or Toll-like receptor (TLR) agonists, such as TLR-7 and TLR-8 agonists, have been used to induce the activation of innate immunity. GS-9620, a TLR-7 agonist, has been found to induce the production of IFN-α, especially by pDCs. In addition, the treatment of RO7020531 triggered obvious immune activation in patients with CHB [113,114,115]. Moreover, recently developed TLR-8 agonists may contribute to the activation of PRRs present in the liver, and GS-9688 has been shown to promote the production of IL-12 and IL-18 from monocytes or DCs [116,117,118]. Furthermore, as cytokines such as IL-12 also contribute to NK cell activation, which have been demonstrated to kill both HBV-infected hepatocytes and HBV-specific CD8+ T cells, it is necessary to comprehensively evaluate the function of activated innate immunity in the process of HBV eradication. Furthermore, there are a large quantity of new therapeutic drugs for patients with CHB under investigation. GLS4 is a core protein allosteric modulator. A total of 20 weeks of treatment with GLS4 resulted in reduced DNA levels (1.48–6.09 log decrease) [20]. Several capsid assembly modulators have been under development for CHB therapy. For example, ABI-H0731, was found to cause a significant decrease in HBV DNA levels at 12 weeks, when combined with entecavir [119]. In addition, the administration of RO7049389 not only reduced HBV DNA levels, but also decreased HBsAg, as well as HBeAg levels in the serum [20,120]. Additionally, the effects of siRNAs in clinical trials also appear encouraging. Treatment with JNJ3989 achieved a 1.3–3.8 log decrease in HBsAg levels [20]. Furthermore, several other new therapies that have been investigated have been reported as safe and well tolerated in healthy volunteers, such as GSK3389404 [19] (Table 1).
Table 1. Select new therapeutic strategies for patients with CHB under development.
| Drug Names | Mechanism of Function | Effects | References |
|---|---|---|---|
| GLS4 | Core binding | Data of 20 weeks demonstrated DNA level log decrease of 1.48–5.58 after administration (twice daily, BID) | [20] |
| ABI-H0731 | Core binding | Data showed mean maximum NA level log reduction from baseline were 1.7, 2.1, and 2.8 in the 100, 200, and 300 mg dose group, respectively | [20,119] |
| RO7049389 | Core binding | Median DNA level declines of 2.7 (200 mg, BID) and 3.2 (400 mg, BID) demonstrated at 28 days | [20,121] |
| REP 2165 | HBsAg binding | Obviously higher percentages of CHB patients in REP 2165 group had reduction in HBsAg to below 1 IU/mL and HBsAg seroconversion during the first 24 weeks of TDF and PEG-IFN-α treatment | [20,122,123] |
| TG-1050 | Transgene | HBV specific T cell responses were induced. Data at day 197 showed mean 0.45 log reduction in HBsAg levels | [124] |
| RO7020531 | TLR7 agonist | Safety and tolerability in healthy Chinese donors with a 150 mg q.o.d. | [125] |
| GS-9688 | TLR8 | The antiviral efficacy of 3 mg/kg (weekly) was confirmed in a woodchuck study | [126] |
| JNJ3989 | mRNA degradation | Data showed HBsAg level log reduction of 1.3–3.8 | [127] |
| CRV431 | Blocks NTCP | Data showed a significantly decreased liver HBV DNA levels with the treatment (50 mg/kg/day) for 16 days | [128] |
| GSK3389404 | mRNA degradation | Data showed safe and target engagement, with dose-dependent reductions in HBsAg | [129] |
HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; BID, twice daily; anti-HBs, anti-hepatitis B surface protein; TLR, Toll-like receptor; IU, infectious units; NTCP, sodium–taurocholate cotransporting polypeptide.
Furthermore, immunological approaches against HBV infection, which involve the use of T cells engineered with a classical T cell receptor (TCR) specific for human leukocyte antigens (HLA)-class I restricted HBV epitopes or a chimeric antigen receptor (CAR), have shown some promise [106]. Despite the encouraging preliminary results of such T cell therapies, they are associated with a risk of inducing fatal hepatic inflammation. Thus, the adoptive transfer of engineered T cells and the manufacturing techniques used must be evaluated with more caution. In addition, more robust experimental and clinical trial data are needed. Platelets play important roles in inflammatory and immune-mediated disorders. Aiolfi et al. reported that platelets contributed to the pathogenesis of HBV-related liver disease by their ability to promote the homing of effector CD8+ T cells in the liver, expression of pro-angiogenic mediators (such as VEGF and TGF-β1) and the production of pro-inflammatory cytokines (such as IFN-γ) [130]. Aspirin is a widely used anti-platelet drug. Notably, the suppression of platelet activation using aspirin would significantly reduce the number of HBV-specific CD8+ T cells and the recruitment of inflammatory cells in the liver, which contributes to alleviating liver injury and the likelihood of HCC [130,131]. Therefore, anti-platelet therapy might be another promising approach for the treatment of patients with CHB. Collectively, to achieve the goal of developing a functional cure, more knowledge derived from the accumulation of experiment and clinical trials is needed.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11101116
This entry is offline, you can click here to edit this entry!
