
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yun-Yang Chao | -- | 1763 | 2022-10-11 12:54:52 | | | |
| 2 | Beatrix Zheng | + 39 word(s) | 1802 | 2022-10-12 08:35:54 | | | | |
| 3 | Beatrix Zheng | + 3 word(s) | 1805 | 2022-10-14 06:15:34 | | | | |
| 4 | Beatrix Zheng | Meta information modification | 1805 | 2022-10-14 06:18:40 | | |
Video Upload Options
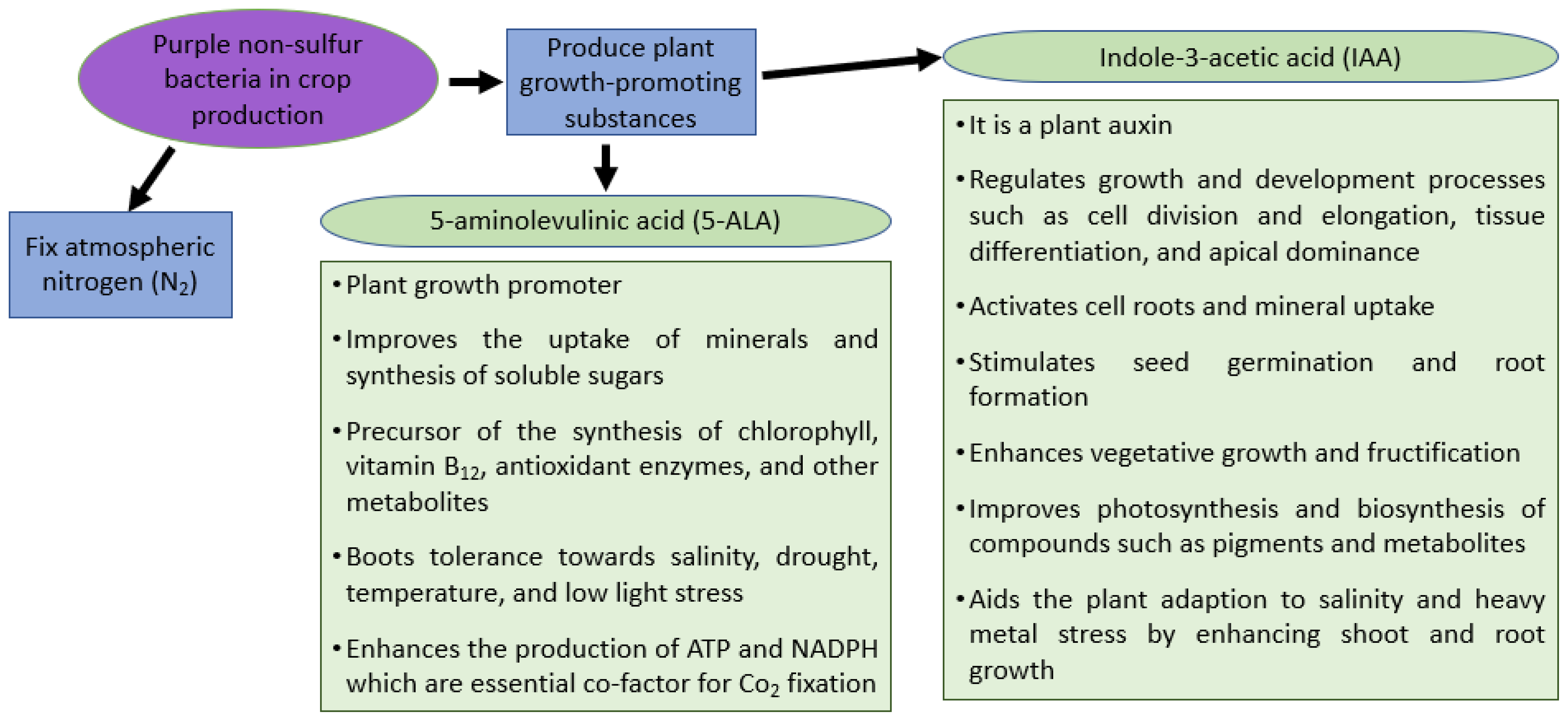
Photosynthetic bacteria (PSB) are procaryotes capable of carrying out photosynthesis by converting light energy into chemical energy. These photosynthetic bacteria can either grow in the presence or absence of oxygen (aerobic and anaerobic conditions) and can either use organic or inorganic substances as an electron donor to fix the atmospheric nitrogen (N2) and carbon dioxide (CO2). The purple non-sulfur bacteria belong to the anoxygenic group of PSB, and their major groups include Rhodopseudomonas spp. and Rhodobacter spp. They are naturally present in wastewater ponds, lagoons, lakes, sediments, wetland ecosystems, moist soils, hypersaline systems, and marine ecosystems. They possess versatile metabolic pathways and, therefore, are widely used in the livestock and fisheries industries , in bioremedial methods for heavy metals and sewage, and in biofuel production (electricity or photohydrogen). Studies have also shown that purple non-sulfur bacteria (PNSB) help boost soil fertility when applied directly to the soil, whereas PNSB applied to plants help improve crop growth and yield.
1. Plant Growth Promotion and Yield Improvement in Rice by PNSB
2. PNSB Helps Alleviate Plant Stress in Rice
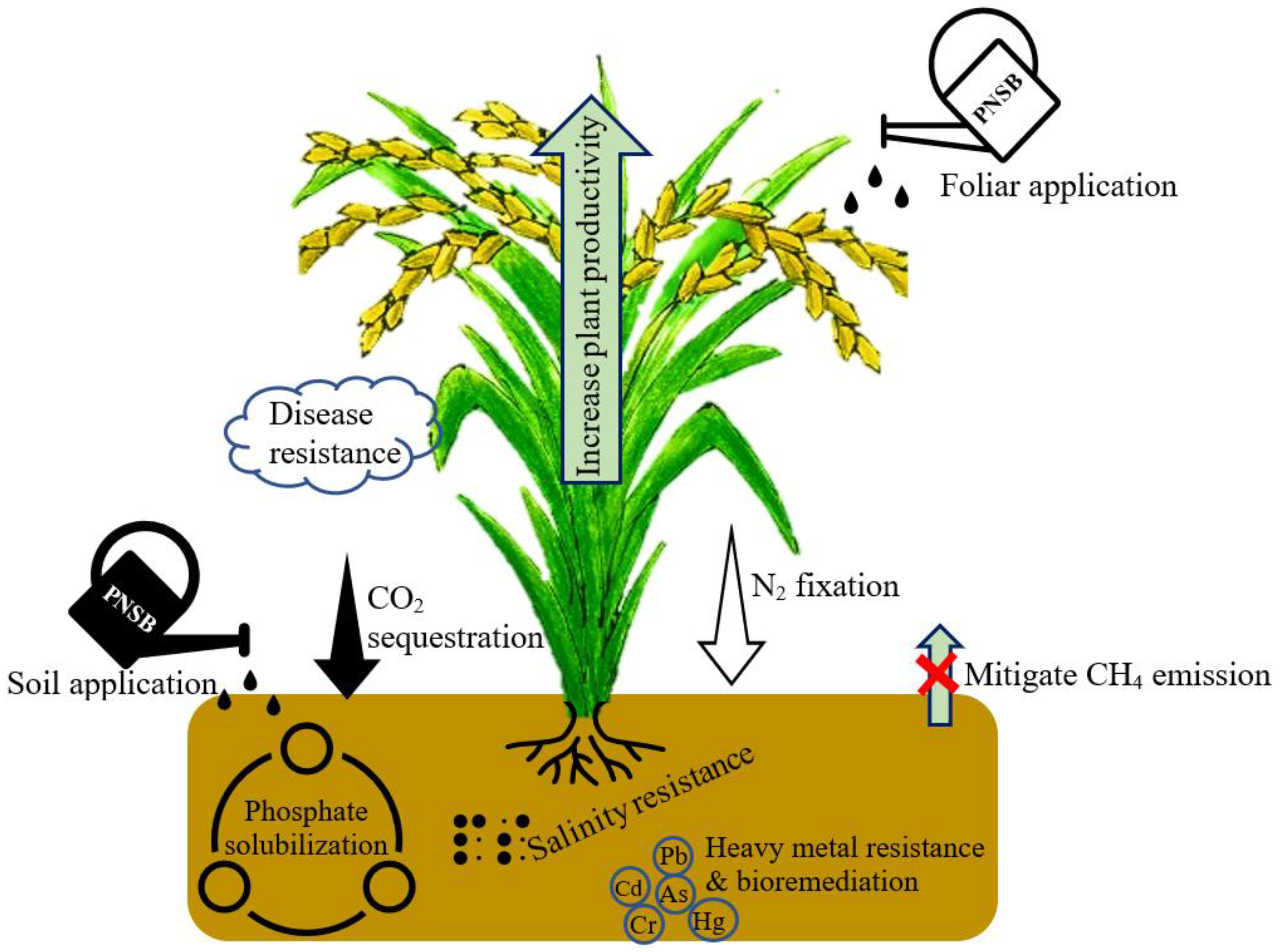
3. PNSB Inoculation Reduces CH4 Emissions from Rice Fields
References
- Sakarika, M.; Spanoghe, J.; Sui, Y.; Wambacq, E.; Grunert, O.; Haesaert, G.; Spiller, M.; Vlaeminck, S.E. Purple Non-Sulphur Bacteria and Plant Production: Benefits for Fertilization, Stress Resistance and the Environment. Microb. Biotechnol. 2020, 13, 1336–1365.
- Sakpirom, J.; Kantachote, D.; Nunkaew, T.; Khan, E. Characterizations of Purple Non-Sulfur Bacteria Isolated from Paddy Fields, and Identification of Strains with Potential for Plant Growth-Promotion, Greenhouse Gas Mitigation and Heavy Metal Bioremediation. Res. Microbiol. 2017, 168, 266–275.
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial Producers of Plant Growth Stimulators and Their Practical Use: A Review. Appl. Biochem. Microbiol. 2006, 42, 117–126.
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176.
- Kazan, K. Auxin and the Integration of Environmental Signals into Plant Root Development. Ann. Bot. 2013, 112, 1655–1665.
- Bending, G.D.; Rodríguez-Cruz, M.S.; Lincoln, S.D. Fungicide Impacts on Microbial Communities in Soils with Contrasting Management Histories. Chemosphere 2007, 69, 82–88.
- Kaymak, H.C. Potential of PGPR in Agricultural Innovations. In Plant Growth and Health Promoting Bacteria; Maheshwari, D.K., Ed.; Microbiology Monographs; Springer: Berlin/Heidelberg, Germany, 2011; pp. 45–79. ISBN 978-3-642-13612-2.
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586.
- Kobayashi, M.; Haque, M.Z. Contribution to Nitrogen Fixation and Soil Fertility by Photosynthetic Bacteria. Plant Soil 1971, 35, 443–456.
- Maudinas, B.; Chemardin, M.; Yovanovitch, E.; Gadal, P. Gnotobiotic Cultures of Rice Plants up to Ear Stage in the Absence of Combined Nitrogen Source but in the Presence of Free Living Nitrogen Fixing Bacteria Azotobacter Vinelandii and Rhodopseudomonas Capsulata. Plant Soil 1981, 60, 85–97.
- Yoshida, T.; Tabata, T.; Saraswati, R.; Kobayashi, M. Study on Resourceful Disposal of Organic Waste and High-Yielding Culture of Rice Plant. J. Environ. Conserv. Eng. 1991, 20, 607–610.
- Elbadry, M.; Gamal-Eldin, H.; Elbanna, K. Effects of Rhodobacter Capsulatus Inoculation in Combination with Graded Levels of Nitrogen Fertilizer on Growth and Yield of Rice in Pots and Lysimeter Experiments. World J. Microbiol. Biotechnol. 1999, 15, 393–395.
- Elbadry, M.; Elbanna, K. Response of Four Rice Varieties to Rhodobacter Capsulatus at Seedling Stage. World J. Microbiol. Biotechnol. 1999, 15, 363–367.
- Harada, N.; Nishiyama, M.; Otsuka, S.; Matsumoto, S. Effects of Inoculation of Phototrophic Purple Bacteria on Grain Yield of Rice and Nitrogenase Activity of Paddy Soil in a Pot Experiment. Soil Sci. Plant Nutr. 2005, 51, 361–367.
- Gamal-Eldin, H.; Elbanna, K. Field Evidence for the Potential of Rhodobacter Capsulatus as Biofertilizer for Flooded Rice. Curr. Microbiol. 2011, 62, 391–395.
- Yen, K.S.; Sundar, L.S.; Chao, Y.-Y. Foliar Application of Rhodopseudomonas Palustris Enhances the Rice Crop Growth and Yield under Field Conditions. Plants 2022, 11, 2452.
- Zhao, Y.; Sun, Y.; Pei, M.; Fu, J.; Ji, H.; Zhao, L.; Xiao, X. Enhanced Rice Yields Are Related to Pronounced Shifts in Soil Resident Bacterial Community Structures in Response to Rhodopseudomonas Palustris and Bacillus Subtilis Inoculation. J. Soils Sediments 2021, 21, 2369–2380.
- Arashida, H.; Kugenuma, T.; Watanabe, M.; Maeda, I. Nitrogen Fixation in Rhodopseudomonas Palustris Co-Cultured with Bacillus Subtilis in the Presence of Air. J. Biosci. Bioeng. 2019, 127, 589–593.
- Khan, M.R.; Khan, N.; Khan, S.M. Evaluation of Agricultural Materials as Substrate for Mass Culture of Fungal Biocontrol Agents of Fusarial Wilt and Root-Knot Nematode Diseases. Tests Agrochem. Cultiv. 2001, 22, 50–51.
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic Role of Microbial Plant Biostimulants in Abiotic Stress Relief in Crop Plants. J. Plant Interact. 2022, 17, 705–718.
- Lee, S.-K.; Lur, H.-S.; Liu, C.-T. From Lab to Farm: Elucidating the Beneficial Roles of Photosynthetic Bacteria in Sustainable Agriculture. Microorganisms 2021, 9, 2453.
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of Salt-Induced Photosynthesis and Growth Inhibition by Salicylic Acid Involves Glycinebetaine and Ethylene in Mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74.
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and Sulfur Influence Ethylene Formation and Alleviate Cadmium-Induced Oxidative Stress by Improving Proline and Glutathione Production in Wheat. J. Plant Physiol. 2015, 173, 9–18.
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Mobin, M.; Anjum, N.A.; Khan, N.A. Modulation and Significance of Nitrogen and Sulfur Metabolism in Cadmium Challenged Plants. Plant Growth Regul. 2016, 78, 1–11.
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen Peroxide Alleviates Nickel-Inhibited Photosynthetic Responses through Increase in Use-Efficiency of Nitrogen and Sulfur, and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 44.
- Khan, M.I.R.; Khan, N.A. Ethylene Reverses Photosynthetic Inhibition by Nickel and Zinc in Mustard through Changes in PS II Activity, Photosynthetic Nitrogen Use Efficiency, and Antioxidant Metabolism. Protoplasma 2014, 251, 1007–1019.
- Farnese, F.S.; Menezes-Silva, P.E.; Gusman, G.S.; Oliveira, J.A. When Bad Guys Become Good Ones: The Key Role of Reactive Oxygen Species and Nitric Oxide in the Plant Responses to Abiotic Stress. Front. Plant Science 2016, 7, 471.
- Feigl, G.; Lehotai, N.; Molnár, A.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc Induces Distinct Changes in the Metabolism of Reactive Oxygen and Nitrogen Species (ROS and RNS) in the Roots of Two Brassica Species with Different Sensitivity to Zinc Stress. Ann. Bot. 2015, 116, 613–625.
- Silveira, N.M.; de Oliveira, J.A.; Ribeiro, C.; Canatto, R.A.; Siman, L.; Cambraia, J.; Farnese, F. Nitric Oxide Attenuates Oxidative Stress Induced by Arsenic in Lettuce (Lactuca sativa) Leaves. Water Air Soil Pollut. 2015, 226, 379.
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.-S.P. Role of Ethylene and Its Cross Talk with Other Signaling Molecules in Plant Responses to Heavy Metal Stress. Plant Physiol. 2015, 169, 73–84.
- Wang, L.; Su, H.; Han, L.; Wang, C.; Sun, Y.; Liu, F. Differential Expression Profiles of Poplar MAP Kinase Kinases in Response to Abiotic Stresses and Plant Hormones, and Overexpression of PtMKK4 Improves the Drought Tolerance of Poplar. Gene 2014, 545, 141–148.
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037.
- Dar, M.I.; Naikoo, M.I.; Khan, F.A.; Rehman, F.; Green, I.D.; Naushin, F.; Ansari, A.A. An Introduction to Reactive Oxygen Species Metabolism Under Changing Climate in Plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 25–52. ISBN 978-981-10-5254-5.
- Ashraf, M.; Harris, P.J.C. Potential Biochemical Indicators of Salinity Tolerance in Plants. Plant Sci. 2004, 166, 3–16.
- Chen, J.; Wang, Y.; Guo, X.; Rao, D.; Zhou, W.; Zheng, P.; Sun, J.; Ma, Y. Efficient Bioproduction of 5-Aminolevulinic Acid, a Promising Biostimulant and Nutrient, from Renewable Bioresources by Engineered Corynebacterium Glutamicum. Biotechnol. Biofuels 2020, 13, 41.
- Zhen, A.; Bie, Z.L.; Huang, Y.; Liu, Z.X.; Fan, M.L. Effects of 5-Aminolevulinic Acid on the H2O2-Content and Antioxidative Enzyme Gene Expression in NaCl-Treated Cucumber Seedlings. Biol. Plant. 2012, 56, 566–570.
- Sun, Y.-P.; Zhang, Z.-P.; Wang, L.-J. Promotion of 5-Aminolevulinic Acid Treatment on Leaf Photosynthesis Is Related with Increase of Antioxidant Enzyme Activity in Watermelon Seedlings Grown under Shade Condition. Photosynthetica 2009, 47, 347–354.
- Naeem, M.S.; Rasheed, M.; Liu, D.; Jin, Z.L.; Ming, D.F.; Yoneyama, K.; Takeuchi, Y.; Zhou, W.J. 5-Aminolevulinic Acid Ameliorates Salinity-Induced Metabolic, Water-Related and Biochemical Changes in Brassica napus L. Acta Physiol. Plant. 2011, 33, 517–528.
- Nunkaew, T.; Kantachote, D.; Kanzaki, H.; Nitoda, T.; Ritchie, R.J. Effects of 5-Aminolevulinic Acid (ALA)-Containing Supernatants from Selected Rhodopseudomonas Palustris Strains on Rice Growth under NaCl Stress, with Mediating Effects on Chlorophyll, Photosynthetic Electron Transport and Antioxidative Enzymes. Electron. J. Biotechnol. 2014, 17, 4.
- Wongkantrakorn, N.; Sunohara, Y.; Matsumoto, H. Mechanism of Growth Amelioration of NaCl-Stressed Rice (Oryza sativa L.) by δ-Aminolevulinic Acid. J. Pestic. Sci. 2009, 34, 89–95.
- Akram, N.A.; Ashraf, M. Regulation in Plant Stress Tolerance by a Potential Plant Growth Regulator, 5-Aminolevulinic Acid. J. Plant Growth Regul. 2013, 32, 663–679.
- Sasaki, K.; Watanabe, M.; Tanaka, T. Biosynthesis, Biotechnological Production and Applications of 5-Aminolevulinic Acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29.
- Sasikala, C.H.; Ramana, C.V.; Rao, P.R. 5-Aminolevulinic Acid: A Potential Herbicide/Insecticide from Microorganisms. Biotechnol. Prog. 1994, 10, 451–459.
- Tan, S.; Cao, J.; Xia, X.; Li, Z. Advances in 5-Aminolevulinic Acid Priming to Enhance Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2022, 23, 702.
- Bindu, R.C.; Vivekanandan, M. Hormonal Activities of 5-Aminolevulinic Acid in Callus Induction and Micropropagation. Plant Growth Regul. 1998, 26, 15–18.
- Saikeur, A.; Choorit, W.; Prasertsan, P.; Kantachote, D.; Sasaki, K. Influence of Precursors and Inhibitor on the Production of Extracellular 5-Aminolevulinic Acid and Biomass by Rhodopseudomonas Palustris KG31. Biosci. Biotechnol. Biochem. 2009, 73, 987–992.
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) Alleviated Salinity Stress in Cucumber Seedlings by Enhancing Chlorophyll Synthesis Pathway. Front. Plant Sci. 2018, 9, 635.
- Kantha, T.; Kantachote, D.; Klongdee, N. Potential of Biofertilizers from Selected Rhodopseudomonas Palustris Strains to Assist Rice (Oryza sativa L. Subsp. Indica) Growth under Salt Stress and to Reduce Greenhouse Gas Emissions. Ann. Microbiol. 2015, 65, 2109–2118.
- Kantachote, D.; Nunkaew, T.; Kantha, T.; Chaiprapat, S. Biofertilizers from Rhodopseudomonas Palustris Strains to Enhance Rice Yields and Reduce Methane Emissions. Appl. Soil Ecol. 2016, 100, 154–161.
- Batool, K.; Rehman, Y. Arsenic-Redox Transformation and Plant Growth Promotion by Purple Nonsulfur Bacteria Rhodopseudomonas Palustris CS2 and Rhodopseudomonas Faecalis SS5. BioMed Res. Int. 2017, 2017, 625327.
- Nookongbut, P.; Kantachote, D.; Megharaj, M.; Naidu, R. Reduction in Arsenic Toxicity and Uptake in Rice (Oryza sativa L.) by As-Resistant Purple Nonsulfur Bacteria. Environ. Sci. Pollut. Res. 2018, 25, 36530–36544.
- Panwichian, S.; Kantachote, D.; Wittayaweerasak, B.; Mallavarapu, M. Removal of Heavy Metals by Exopolymeric Substances Produced by Resistant Purple Nonsulfur Bacteria Isolated from Contaminated Shrimp Ponds. Electron. J. Biotechnol. 2011, 14, 2.
- Xiao, X.; Zhu, Y.; Gao, Y.; Fu, J.; Zhao, Y.; Zhao, L. Inoculation of Paddy Soils with Rhodopseudomonas Palustris Enhanced Heavy Metal Immobilisation. Plant Soil Environ. 2021, 67, 55–60.
- Zeng, J.; Li, X.; Wang, X.; Zhang, K.; Wang, Y.; Kang, H.; Chen, G.; Lan, T.; Zhang, Z.; Yuan, S. Cadmium and Lead Mixtures Are Less Toxic to the Chinese Medicinal Plant Ligusticum Chuanxiong Hort. than Either Metal Alone. Ecotoxicol. Environ. Safety 2020, 193, 110342.
- Cheng, W.; Sakai, H.; Hartley, A.; Yagi, K.; Hasegawa, T. Increased Night Temperature Reduces the Stimulatory Effect of Elevated Carbon Dioxide Concentration on Methane Emission from Rice Paddy Soil. Glob. Change Biol. 2008, 14, 644–656.





