Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junya Tanaka | -- | 2977 | 2022-10-11 05:45:57 | | | |
| 2 | Catherine Yang | -1 word(s) | 2976 | 2022-10-11 06:13:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ozaki, S.; Mikami, K.; Kunieda, T.; Tanaka, J. Chloride Intracellular Channel Proteins and Malignant Tumor Progression. Encyclopedia. Available online: https://encyclopedia.pub/entry/28803 (accessed on 14 January 2026).
Ozaki S, Mikami K, Kunieda T, Tanaka J. Chloride Intracellular Channel Proteins and Malignant Tumor Progression. Encyclopedia. Available at: https://encyclopedia.pub/entry/28803. Accessed January 14, 2026.
Ozaki, Saya, Kanta Mikami, Takeharu Kunieda, Junya Tanaka. "Chloride Intracellular Channel Proteins and Malignant Tumor Progression" Encyclopedia, https://encyclopedia.pub/entry/28803 (accessed January 14, 2026).
Ozaki, S., Mikami, K., Kunieda, T., & Tanaka, J. (2022, October 11). Chloride Intracellular Channel Proteins and Malignant Tumor Progression. In Encyclopedia. https://encyclopedia.pub/entry/28803
Ozaki, Saya, et al. "Chloride Intracellular Channel Proteins and Malignant Tumor Progression." Encyclopedia. Web. 11 October, 2022.
Copy Citation
Chloride intracellular channel proteins (CLICs are the dimorphic protein present in both soluble and membrane fractions. As an integral membrane protein, CLICs potentially possess ion channel activity. In vertebrates, CLICs are classified into six classes: CLIC1, 2, 3, 4, 5, and 6. CLIC2 is expressed at higher levels in benign tumors than in malignant ones, most likely preventing tumor cell invasion into surrounding tissues. CLIC2 is also expressed in the vascular endothelial cells of normal tissues and maintains their intercellular adhesive junctions, presumably suppressing the hematogenous metastasis of malignant tumor cells.
metastasis
invasion
MMP
MT1-MMP
CLIC4
glioma
1. Introduction
1.1. CLIC Family
Although chloride intracellular channel proteins (CLICs) have been identified as chloride ion channels, it remains unclear whether they indeed function as ion channels [1][2][3]. In vertebrates, there are six CLIC family members (CLIC 1–6) with well-conserved molecular structures (Table 1). Among the six CLICs, CLIC1 and CLIC4 are the most widely studied with respect to their localization, functions, and expression [1][3]. CLICs are localized in soluble fractions in the cytosol and nuclei rather than in membrane fractions [3][4][5]. CLICs are widely expressed in metazoans and have well-conserved molecular structures. Therefore, CLICs are assumed to play an important role in physiological processes. Many roles of CLICs other than acting as ion channels have been proposed [6][7][8][9], although a recent study reported that CLICs activate NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome probably by increasing the efflux of chloride ions [10].
Table 1. CLICs: their distribution, ion channel activities, and biological functions.
| CLICs | Distribution | Ion Channel | Biological Function | References |
|---|---|---|---|---|
| CLIC1 | Various organs | Poorly selective anion channels | Participates in inflammatory processes Activation of MYC signaling Enzymatic activity Redox regulation |
[11][12][13][14][15][16][17] |
| CLIC2 | Blood vessels. heart, liver | Anion channels | Modulation of ryanodine receptor Inhibition of MMP14 activity |
[4][5][13][18][19] |
| CLIC3 | Muscles, heart, lung, kidney | Component of anion channel, regulator of channel | Endosomal trafficking Promote invasive behavior |
[20][21][22][23] |
| CLIC4 | Various organs | Poorly selective ion channels | Enhance tumor invasiveness Enhance TGF-β signaling Induce apoptosis Involved in angiogenesis Stimulation of MMP14 activity |
[24][25][26][27][28][29] |
| CLIC5 | Kidney, heart, lung, colon | Poorly selective ion channels | Actin cytoskeleton-dependent membrane remodeling | [9][25][30] |
| CLIC6 | Soluble and membrane fractions | Unknown | Interact with dopamine receptors | [31][32][33] |
CLICs are relatively small globular proteins with a molecular mass of approximately 30 kDa. Because CLICs possess a molecular structure resembling glutathione-S-transferase (GST), they have been investigated for enzymatic activity, although most researchers do not support this view [3][34]. In addition to the formation of chloride ion channels [10], CLICs are thought to play various roles, including ryanodine receptor (RyR) modulation [18][35][36][37], plasma membrane remodeling [38], intracellular trafficking [29][39], intracellular tubule formation [7], actin cytoskeleton reorganization [9], inflammasome activation [10], and TGFβ-mediated signal modification [40][41]. CLICs have been found in various intracellular locations, including the cytoplasm, mitochondria, endosome, nuclei, endoplasmic reticulum, and secretory granules [3][4][5][42][43]. CLICs are mainly found in soluble fractions, but they also occur in membrane fractions [1][3][34]. It is necessary to elucidate how these two localizations are regulated [44]. Recombinant CLICs are well known for their high solubility in aqueous buffers [1]. CLIC2 is secreted extracellularly in significant amounts, and its role in the extracellular fluid has been postulated, as described below; CLIC4 is also secreted in small amounts [4]. Regarding the distribution, functions, and structures of CLICs expressed in vertebrates, see Table 1 and well summarized reviews [3][42][45][46][47].
1.2. Known Structures of CLIC2
CLICs exist as both soluble globular proteins and integral membrane proteins that potentially possess ion channel activity. Because of the presence of reactive cystein residues, pH and redox conditions in most instances affect the transition between these two states [1]. Highly soluble recombinant CLIC proteins possess a putative transmembrane domain. When recombinant CLIC proteins are added to artificial synthetic lipid bilayers, they are integrated into the bilayer and reproducible ion channel activity is detected by electrophysiological measurements. CLIC2 is also demonstrated to form ion channels in lipid bilayers in a pH-dependent manner; a marked ion channel activity of CLIC2 was observed at pH 5.0 over the pH range of 5.0–9.0 [19]. However, the selectivity for anions of the channels is very poor and the electrophysiological characterization is not sufficient to call chloride ion channels. Therefore, the question is still being raised as to whether CLICs, including CLIC2, can function as an ion channel. CLICs have homology with GST omega protein and may be involved in regulating ion channels rather than forming ion channels themselves [1][3][36]. The well-known regulatory function of CLIC2 on RyRs support the notion that CLICs modulates channel activities [18]. The three-dimensional structure of human CLIC2 in its water-soluble form has been determined by X-ray crystallography, and two crystal forms have been reported [19]. CLIC2 has an intramolecular disulfide bridge remaining monomeric, whereas an intramolecular disulfide of CLIC1 forms a dimer state. CLIC2 has a highly charged region called foot loop on the C-terminal side. A possibility has been indicated that CLIC2 may interact with other molecules through the highly charged C-terminal region [19]. Although the biological significance of such characteristic molecular structures is still to be elucidated, it may be suggestive of the unique functions of CLIC2, such as the regulation of RyR or binding to MMP14 [4].
1.3. Known Functions of CLIC2
CLIC1 and CLIC4 can be inserted into artificial phospholipid membranes to form ion channels with low selectivity under non-physiological acidic conditions [1][46][48]. However, it remains unclear whether they can form ion channels under physiological conditions. Similarly, CLIC2 forms ion channels in artificial membranes with ion conductance similar to that of CLIC4 [46]. However, CLIC2 is scarcely localized in membranous structures including plasma and organellar membranes [4][5], suggesting that the majority of CLIC2 does not form ion channels. Tang et al. [10] have reported that CLICs 1, 4, and 5 can activate NLRP3 inflammasome by their actions as chloride ion channels or as modulators for the ion channels. Since they used murine macrophages, it is not clear whether CLIC2 acts in a similar way as an ion channel.
In humans, CLIC2 is located at the telomeric region of Xq28, the end of the X chromosome. Human cases with deletions or mutations of this gene, leading to intellectual disabilities predominantly in men, have been reported [48][49]. CLIC2 can bind directly to RyRs while inhibiting its Ca2+ channel functions [18][50][51]. One of the mutations of CLIC2 (c.303C>G, p.H101Q) causes the activation rather than inhibition of RyRs, accelerating intracellular Ca2+-induced Ca2+ release and leading to the abnormal activation of neurons and cardiomyocytes. Cases with this mutation show symptoms of intellectual disability as well as cardiomegaly [48][49]. CLIC2′s only known action at the molecular level is the inhibition of RyRs by directly interacting with them. However, because CLIC2 is widely expressed in various organs and cells (https://www.proteomicsdb.org/proteomicsdb/#human/proteinDetails/O15247/expression), it may have functions other than inhibiting RyRs.
1.4. CLIC2 and Malignancy
The findings of a previous report about the relationships between the survival periods of patients with various cancers and expression levels of six CLICs, based on in silico analyses, are summarized in Table 2 [42]. The effects of high or low expression levels of CLICs on the survival rate varies significantly depending on the type of cancer, suggesting that each type of cancer uses different mechanisms for their progression, metastasis, or invasion. In general, the high expression of CLICs 2, 5, and 6 tends to improve the prognosis of cancer, while CLICs 3 and 4 may be detrimental and CLIC 1 appears to have both positive and negative effects. However, only few molecular biological studies [4][5][10][52] have attempted to elucidate how CLICs are involved in cancer progression, making it difficult to clarify the relationship between cancer prognosis and CLICs at the molecular and cellular levels.
Table 2. Correlation between CLIC expression levels and cancer mortality.
| Cancer | Breast | Ovarian | Lung | Gastric | Liver | Pancreatic |
|---|---|---|---|---|---|---|
| CLIC1 | detrimental | detrimental | n.s. | ameliorative | detrimental | detrimental |
| CLIC2 | ameliorative | detrimental | ameliorative | ameliorative | ameliorative | n.s. |
| CLIC3 | detrimental | detrimental | n.s. | detrimental | n.s. | detrimental |
| CLIC4 | n.s. | detrimental | detrimental | detrimental | n.s. | detrimental |
| CLIC5 | ameliorative | detrimental | ameliorative | ameliorative | n.s. | n.s. |
| CLIC6 | ameliorative | ameliorative | ameliorative | ameliorative | n.s. | n.s. |
As shown in Table 1, CLIC4 is a probable detrimental factor for cancer prognosis, and this may be attributable to its stimulating effect on MMP activities, opposite to CLIC2 [29]. Different mechanisms may also underlie the enhancing effects of CLIC4 on malignant tumor growth [52]. Comparing the actions of CLIC2 and CLIC4 may be of help to understand the novel roles of CLICs.
1.5. Why CLIC2?
The transplantation of C6 cells in the back of Wistar rat neonates results in their death beginning from four weeks after the transplantation due to the hematogenous metastasis of C6 cells from the back tumors to the lungs [53]. Enhanced green fluorescent protein (EGFP)-expressing C6 cells were prepared and transplanted into the back subcutaneous tissue of Wistar rat neonates. Four weeks later, metastatic tumor masses in the lungs were dissected, and the EGFP-expressing C6 cells were isolated by cell sorting (Figure 1). The isolated cells were expanded in vitro before they were re-transplanted into the back of Wistar rat neonates. The gene expression of EGFP-expressing C6 cells from the metastatic lung tumors and primary back tumors was thoroughly examined using RNA-Seq [4]. The gene expression of C6 cells in the metastatic tumors differed markedly from that of C6 cells in the primary tumors, despite the fact that both cells were obtained from the same culture plates. Differentially expressed genes (DEGs) in the metastatic tumors were associated with the cell adhesion, including periostin (Postn), cadherin 15 (Cdh15), and dermatopontin (Dpt), as well as angiogenesis-related signaling and regulation of biological quality as the nodes were notably larger in the network. DEGs in the primary tumors were involved in pathways associated with extracellular matrix regulators, such as chondroitin sulfate N-acetylgalactosaminyltransferase 1 (Csgalnact1), and inflammation-related genes such as oncostatin M receptor (Osmr). (Figure 1). The increase in the expression of inflammation-related genes in the primary tumors may be in accordance with a report demonstrating that CLIC2 improves the prognosis of breast cancer by increasing the number of infiltrated lymphocytes [54]. Therefore, there may be a positive relationship between CLIC2 and anticancer immunity. CLICs 1, 4, and 5 have been linked to NLRP3 inflammasome activation in mouse macrophages, and CLIC2 might exhibit similar proinflammatory activation [10].
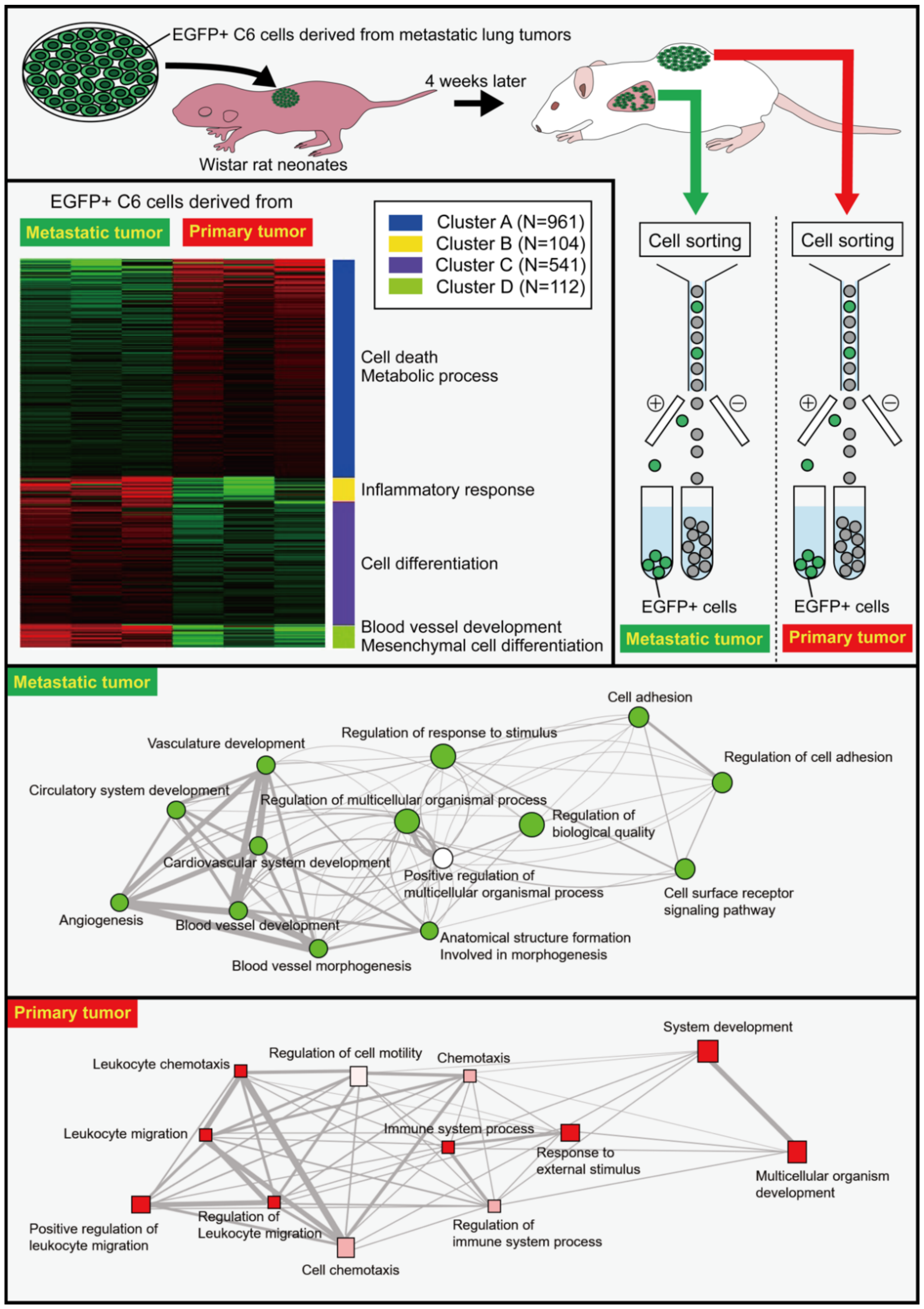
Figure 1. Differential gene expression in cells derived from metastatic and primary tumors as revealed by RNA-Seq. EGFP-expressing C6 glioma cells were transplanted in the back of Wistar rat neonates. Four weeks later, the metastatic tumor masses were dissected before the isolation of EGFP+ C6 cells from the tumor masses using a fluorescence-activating cell sorter (FACS). The isolated cells were cultured to expand the cell number, and the expanded cells were re-transplanted into the back of Wistar rat neonates. EGFP+ cells were isolated from lung metastatic tumors and back primary tumors four weeks later. Isolated cells were subjected to RNA-Seq analysis that revealed marked differences between the cells from the metastatic and the primary tumors. Gene ontology analyses showed the differential pathways of genes between metastatic and primary tumors in biological processes. For more detailed information, see the literature by Ozaki et al. [4].
2. Roles of Secreted CLIC2; Relationship with MMPs
CLIC2 was not detectable in the culture supernatant of parent C6 cells, but MMP2 was abundant. CLIC2 was found in high concentrations in the culture supernatant of CC cells, whereas MMP2 was found in low concentrations. MMP14, which activates MMP2 by partially degrading proMMP2, was not expressed differentially in C6 and CC cells. Active MMP14 is a plasma membrane-associated protein, but in CC cells, MMP14 localization to the plasma membrane was reduced, while secretion to the extracellular space was significantly increased, implying that CLIC2 may inhibit MMP14 plasma membrane localization. It was found that CLIC2 binds to MMP14 by immunoprecipitation, albeit weakly. Recombinant CLIC2 protein prepared by a cell-free wheat germ protein synthesis system inhibited MMP14 activity in a concentration-dependent manner, and the inhibitory effects were comparable to those of the same concentration of N-Isobutyl-N-[4-methoxyphenylsulfonyl] glycyl hydroxamic acid, a broad-spectrum and water-soluble MMP inhibitor. The inhibitory effect of CLIC2 on MMP14 activity was stronger than that of the tissue inhibitor of metalloproteinase 2 (TIMP2), an endogenous inhibitory protein for MMPs. The mechanism of MMP14 inhibition by CLIC2 and TIMP2 may be similar because no synergistic effect was observed when TIMP2 and CLIC2 were mixed and added to the MMP14 activity assay system.
MMP14 is responsible for malignant cell invasion and metastasis by activating MMP activities. Then, can the CLIC2 protein prevent invasion and metastasis by inhibiting MMP14 activities? CLIC2 protein was added to the culture medium during an invasion assay with U251 human glioblastoma cell line using the Boyden chamber with Matrigel-coated cell culture inserts. CLIC2 significantly reduced the invasion of U251 cells, despite the cells demonstrating strong invasive activity by degrading the Matrigel in the absence of CLIC2. Similar preventive effects on invasion were observed when parent C6 cells or another human glioblastoma cell line SFC-2 was used. The recombinant CLIC4 protein did not show any suppressive effects on the invasion of parent C6 cells. CLIC4 is highly expressed by many tumor cells, but it did not show inhibitory effects on MMP14.
Primary cultured human meningioma cells, which originally expressed CLIC2 abundantly, showed little invasive activity, but the knockdown of CLIC2 resulted in marked invasion in the Boyden chamber assay. This knockdown experiment suggests that the suppressive effects of CLIC2 on MMP activities are exerted both intracellularly and extracellularly, but it is unclear which one plays a more central role. This issue can be resolved through experiments such as suppressing the action of CLIC2 outside the cell by adding neutralizing antibodies.
However, the observation that recombinant CLIC2 can inhibit malignant cell invasion by suppressing MMP activity helps to explain why normal tissues and benign tumors do not undergo metastatic invasion. CLIC2 or related endogenous mechanisms may be used to create new therapeutic strategies against the invasion and metastasis of malignant tumors.
3. Intercellular Adhesive Structures and CLIC2
There are three possible mechanisms by which CLIC2 suppresses distant metastasis of malignant cells. The first possibility is that CLIC2 causes the stabilization of intercellular adhesion between normal blood vessel endothelial cells, thereby preventing the invasion of malignant cells into circulation. In cancer tissues, scarce CLIC2 expression may lead to unstable intercellular adhesion, enabling the hematogenous spread of malignant cells. The second possibility is that tumor cells express CLIC2 that binds to MMP14 intracellularly while inhibiting the enzyme activity. The third possibility is that tumor cells secrete CLIC2 that inhibits MMP activities in the extracellular milieu, resulting in the maintenance of intercellular adhesion and extracellular matrix leading to the prevention of invasion and metastasis of tumor cells.
MMPs are responsible for the destruction of blood vessel tight junctions, which leads to the disruption of the blood–brain barrier (BBB) [55]. For example, MMP activities are elevated in ischemic brain lesions, and an MMP9 inhibitor reduces stroke volume [56], leading to the prevention of BBB disruption [57]. Normal blood vessels in the brain express CLIC2, which may suppress MMP activities in homeostatic conditions, leading to the maintenance of tight junctions in BBB. Various claudins, on the other hand, activate MMP2 through MMP14 [58]. This finding suggests that tight junctions activate MMPs, which cause tight junction disruption, implying that MMPs must be homeostatically suppressed by some mechanism. CLIC2 is a likely candidate for suppressing MMP activities to maintain barrier functions of normal blood vessels. Rat models of stroke or traumatic brain injury accompanied by increased vascular permeability and elevated MMP activities may be required to investigate changes in CLIC2 expression levels. It is worthwhile to investigate whether the administration of CLIC2 into circulation can prevent BBB breakdown in rodent ischemic or traumatic injury models.
CLICs 1 and 4 also stimulate barrier functions of endothelial cells through the activation of small guanosine triphosphatase (GTPase) Rac1 or RhoA in response to sphingosine 1-phosphate [59]. Although this is a distinct mechanism from the CLIC2-mediated mechanism through the inhibition of MMPs, the resultant enhanced barrier function of blood vessels leads to the suppressed invasion and metastasis of malignant cells. However, CLICs 1 and 4 are also involved in angiogenesis [28][60] and the enhancement of sprouting of blood vessel endothelial cells [28][61]. Therefore, CLICs 1 and 4 may be involved in both the suppression and progression of malignancy regarding blood vessels.
4. Modulation of MMP Activities by CLIC4
As described above, CLIC2 inhibits tumor cell invasion and metastasis by suppressing MMP14 activity. On the other hand, CLIC4 may stimulate the progression of malignant tumors [42][52]. CLIC4, a more widely expressed CLIC than CLIC2, has an opposite effect on MMP14 [29]. CLIC4 is reportedly colocalized with MMP14 in late endosomes of a human normal retinal pigment epithelial-derived cell line, ARPE19 cells. CLIC4 may play a role in the maintenance of MMP14 activity. MMP14 is localized in lipid rafts, which are detergent-resistant cholesterol-rich membrane microdomains on plasma membrane. However, when CLIC4 is knocked down in ARPE19 cells, MMP14 localization in lipid rafts is prevented. An immunoprecipitation assay revealed that CLIC4 can bind to MMP14. Furthermore, CLIC4 stimulates the extracellular secretion of MMP2. When ARPE19 cells are cultured on gelatin, the cells show gelatinolytic activities, which are abolished if CLIC4 is knocked down. Thus, CLIC4 may be necessary for the activities of MMPs. The report by Hsu et al. [29] is the first one to show the capability of CLIC to modulate MMP activities. CLIC2 binds to MMP14 and regulates MMP2 activity, indicating the similarity between CLIC2 and CLIC4 in terms of the modulation of MMP activities. It is necessary to further investigate whether CLICs are generally responsible for the regulation of MMP activities. The observation that CLIC2 inhibits MMPs, which is the opposite of what CLIC4 does, is particularly intriguing. As shown in Table 1, generally, CLIC4 exerts detrimental effects on cancer progression, while CLIC2 shows ameliorative effects. The distinct effects of CLICs on cancer progression may be associated with their different effects on MMPs. Although mice lack CLIC2, CLIC5 and CLIC6 may be the CLIC genes that can substitute CLIC2 in mice. It is necessary to investigate the effect of CLIC5 and CLIC6 on MMP activities in the future to reveal whether CLIC5 or CLIC6 can substitute the suppressive CLIC2 functions on MMPs. C6 glioma cells strongly express CLIC4, and it will also be necessary to examine whether the forced expression of CLIC2 and the knockdown of CLIC4 have a synergistic suppressive effect on invasion and metastatic potential.
References
- Littler, D.R.; Harrop, S.J.; Goodchild, S.C.; Phang, J.M.; Mynott, A.V.; Jiang, L.; Valenzuela, S.M.; Mazzanti, M.; Brown, L.J.; Breit, S.N.; et al. The enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins? FEBS Lett. 2010, 584, 2093–2101.
- Zeng, J.; Li, Z.; Lui, E.Y.; Lam, S.H.; Swaminathan, K. Tilapia and human CLIC2 structures are highly conserved. Biochem. Biophys. Res. Commun. 2018, 495, 1752–1757.
- Argenzio, E.; Moolenaar, W.H. Emerging biological roles of Cl- intracellular channel proteins. J. Cell Sci. 2016, 129, 4165–4174.
- Ozaki, S.; Umakoshi, A.; Yano, H.; Ohsumi, S.; Sumida, Y.; Hayase, E.; Usa, E.; Islam, A.; Choudhury, M.E.; Nishi, Y.; et al. Chloride intracellular channel protein 2 is secreted and inhibits MMP14 activity, while preventing tumor cell invasion and metastasis. Neoplasia 2021, 23, 754–765.
- Ueno, Y.; Ozaki, S.; Umakoshi, A.; Yano, H.; Choudhury, M.E.; Abe, N.; Sumida, Y.; Kuwabara, J.; Uchida, R.; Islam, A.; et al. Chloride intracellular channel protein 2 in cancer and non-cancer human tissues: Relationship with tight junctions. Tissue Barriers 2019, 7.
- Ulmasov, B.; Bruno, J.; Gordon, N.; Hartnett, M.E.; Edwards, J.C. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am. J. Pathol. 2009, 174, 1084–1096.
- Berry, K.L.; Bulow, H.E.; Hall, D.H.; Hobert, O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science 2003, 302, 2134–2137.
- Fernandez-Salas, E.; Sagar, M.; Cheng, C.; Yuspa, S.H.; Weinberg, W.C. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J. Biol. Chem. 1999, 274, 36488–36497.
- Berryman, M.; Bretscher, A. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 2000, 11, 1509–1521.
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat. Commun. 2017, 8, 202.
- Singh, H.; Ashley, R.H. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys. J. 2006, 90, 1628–1638.
- Tulk, B.M.; Kapadia, S.; Edwards, J.C. CLIC1 inserts from the aqueous phase into phospholipid membranes, where it functions as an anion channel. Am. J. Physiol. Cell Physiol. 2022, 282, C1103–C1112.
- Littler, D.R.; Harrop, S.J.; Fairlie, W.D.; Brown, L.J.; Pankhurst, G.J.; Pankhurst, S.; DeMaere, M.Z.; Campbell, T.J.; Bauskin, A.R.; Tonini, R.; et al. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 2004, 279, 9298–9305.
- Peter, B.; Fanucchi, S.; Dirr, H.W. A conserved cationic motif enhances membrane binding and insertion of the chloride intracellular channel protein 1 transmembrane domain. Eur. Biophys. J. 2014, 43, 405–414.
- Jiang, L.; Salao, K.; Li, H.; Rybicka, J.M.; Yates, R.M.; Luo, X.W.; Shi, X.X.; Kuffner, T.; Tsai, V.W.; Husaini, Y.; et al. Intracellular chloride channel protein CLIC1 regulates macrophage function through modulation of phagosomal acidification. J. Cell Sci. 2012, 125 Pt 22, 5479–5488.
- Salao, K.; Jiang, L.; Li, H.; Tsai, V.W.; Husaini, Y.; Curmi, P.M.; Brown, L.J.; Brown, D.A.; Breit, S.N. CLIC1 regulates dendritic cell antigen processing and presentation by modulating phagosome acidification and proteolysis. Biol. Open 2016, 5, 620–630.
- Al Khamici, H.; Brown, L.J.; Hossain, K.R.; Hudson, A.L.; Sinclair-Burton, A.A.; Ng, J.P.; Daniel, E.L.; Hare, J.E.; Cornell, B.A.; Curmi, P.M.; et al. Members of the chloride intracellular ion channel protein family demonstrate glutaredoxin-like enzymatic activity. PLoS ONE 2015, 10, e115699.
- Board, P.G.; Coggan, M.; Watson, S.; Gage, P.W.; Dulhunty, A.F. CLIC-2 modulates cardiac ryanodine receptor Ca2+ release channels. Int. J. Biochem. Cell Biol. 2004, 36 Pt 18, 1599–1612.
- Cromer, B.A.; Gorman, M.A.; Hansen, G.; Adams, J.J.; Coggan, M.; Littler, D.R.; Brown, L.J.; Mazzanti, M.; Breit, S.N.; Curmi, P.M.; et al. Structure of the Janus protein human CLIC2. J. Mol. Biol. 2007, 374, 719–731.
- Qian, Z.; Okuhara, D.; Abe, M.K.; Rosner, M.R. Molecular cloning and characterization of a mitogen-activated protein kinase-associated intracellular chloride channel. J. Biol. Chem. 1999, 274, 1621–1627.
- Dozynkiewicz, M.A.; Jamieson, N.B.; Macpherson, I.; Grindlay, J.; van den Berghe, P.V.; von Thun, A.; Morton, J.P.; Gourley, C.; Timpson, P.; Nixon, C.; et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell 2012, 22, 131–145.
- Macpherson, I.R.; Rainero, E.; Mitchell, L.E.; van den Berghe, P.V.; Speirs, C.; Dozynkiewicz, M.A.; Chaudhary, S.; Kalna, G.; Edwards, J.; Timpson, P.; et al. CLIC3 controls recycling of late endosomal MT1-MMP and dictates invasion and metastasis in breast cancer. J. Cell Sci. 2014, 127, 3893–3901.
- Hernandez-Fernaud, J.R.; Ruengeler, E.; Casazza, A.; Neilson, L.J.; Pulleine, E.; Santi, A.; Ismail, S.; Lilla, S.; Dhayade, S.; MacPherson, I.R.; et al. Secreted CLIC3 drives cancer progression through its glutathione-dependent oxidoreductase activity. Nat. Commun. 2017, 8, 14206.
- Duncan, R.R.; Westwood, P.K.; Boyd, A.; Ashley, R.H. Rat brain p64H1, expression of a new member of the p64 chloride channel protein family in endoplasmic reticulum. J. Biol. Chem. 1997, 272, 23880–23886.
- Singh, H.; Ashley, R.H. CLIC4 (p64H1) and its putative transmembrane domain form poorly selective, redox-regulated ion channels. Mol. Membr. Biol. 2007, 24, 41–52.
- Shukla, A.; Edwards, R.; Yang, Y.; Hahn, A.; Folkers, K.; Ding, J.; Padmakumar, V.C.; Cataisson, C.; Suh, K.S.; Yuspa, S.H. CLIC4 regulates TGF-beta-dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 2014, 33, 842–850.
- Fernandez-Salas, E.; Suh, K.S.; Speransky, V.V.; Bowers, W.L.; Levy, J.M.; Adams, T.; Pathak, K.R.; Edwards, L.E.; Hayes, D.D.; Cheng, C.; et al. mtCLIC/CLIC4, an organellular chloride channel protein, is increased by DNA damage and participates in the apoptotic response to p53. Mol. Cell. Biol. 2002, 22, 3610–3620.
- Tung, J.J.; Hobert, O.; Berryman, M.; Kitajewski, J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis 2009, 12, 209–220.
- Hsu, K.S.; Otsu, W.; Li, Y.; Wang, H.C.; Chen, S.; Tsang, S.H.; Chuang, J.Z.; Sung, C.H. CLIC4 regulates late endosomal trafficking and matrix degradation activity of MMP14 at focal adhesions in RPE cells. Sci Rep. 2019, 9, 12247.
- Salles, F.T.; Andrade, L.R.; Tanda, S.; Grati, M.; Plona, K.L.; Gagnon, L.H.; Johnson, K.R.; Kachar, B.; Berryman, M.A. CLIC5 stabilizes membrane-actin filament linkages at the base of hair cell stereocilia in a molecular complex with radixin, taperin, and myosin VI. Cytoskeleton 2014, 71, 61–78.
- Ferofontov, A.; Strulovich, R.; Marom, M.; Giladi, M.; Haitin, Y. Inherent flexibility of CLIC6 revealed by crystallographic and solution studies. Sci. Rep. 2018, 8, 6882.
- Griffon, N.; Jeanneteau, F.; Prieur, F.; Diaz, J.; Sokoloff, P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Mol. Brain Res. 2003, 117, 47–57.
- Friedli, M.; Guipponi, M.; Bertrand, S.; Bertrand, D.; Neerman-Arbez, M.; Scott, H.S.; Antonarakis, S.E.; Reymond, A. Identification of a novel member of the CLIC family, CLIC6, mapping to 21q22.12. Gene 2003, 320, 31–40.
- Littler, D.R.; Assaad, N.N.; Harrop, S.J.; Brown, L.J.; Pankhurst, G.J.; Luciani, P.; Aguilar, M.I.; Mazzanti, M.; Berryman, M.A.; Breit, S.N.; et al. Crystal structure of the soluble form of the redox-regulated chloride ion channel protein CLIC4. FEBS J. 2005, 272, 4996–5007.
- Jalilian, C.; Gallant, E.M.; Board, P.G.; Dulhunty, A.F. Redox potential and the response of cardiac ryanodine receptors to CLIC-2, a member of the glutathione S-transferase structural family. Antioxid. Redox. Signal. 2008, 10, 1675–1686.
- Dulhunty, A.; Gage, P.; Curtis, S.; Chelvanayagam, G.; Board, P. The glutathione transferase structural family includes a nuclear chloride channel and a ryanodine receptor calcium release channel modulator. J. Biol. Chem. 2001, 276, 3319–3323.
- Molina-Navarro, M.M.; Rosello-Lleti, E.; Ortega, A.; Tarazon, E.; Otero, M.; Martinez-Dolz, L.; Lago, F.; Gonzalez-Juanatey, J.R.; Espana, F.; Garcia-Pavia, P.; et al. Differential gene expression of cardiac ion channels in human dilated cardiomyopathy. PLoS ONE 2013, 8, e79792.
- Pierchala, B.A.; Munoz, M.R.; Tsui, C.C. Proteomic analysis of the slit diaphragm complex: CLIC5 is a protein critical for podocyte morphology and function. Kidney Int. 2010, 78, 868–882.
- Rickhag, M.; Wieloch, T.; Gido, G.; Elmer, E.; Krogh, M.; Murray, J.; Lohr, S.; Bitter, H.; Chin, D.J.; von Schack, D.; et al. Comprehensive regional and temporal gene expression profiling of the rat brain during the first 24 h after experimental stroke identifies dynamic ischemia-induced gene expression patterns, and reveals a biphasic activation of genes in surviving tissue. J. Neurochem. 2006, 96, 14–29.
- Suh, K.S.; Mutoh, M.; Mutoh, T.; Li, L.; Ryscavage, A.; Crutchley, J.M.; Dumont, R.A.; Cheng, C.; Yuspa, S.H. CLIC4 mediates and is required for Ca2+-induced keratinocyte differentiation. J. Cell Sci. 2007, 120 Pt 15, 2631–2640.
- Shukla, A.; Malik, M.; Cataisson, C.; Ho, Y.; Friesen, T.; Suh, K.S.; Yuspa, S.H. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat. Cell Biol. 2009, 11, 777–784.
- Gururaja Rao, S.; Patel, N.J.; Singh, H. Intracellular Chloride Channels: Novel Biomarkers in Diseases. Front. Physiol. 2020, 11, 96.
- Ponnalagu, D.; Rao, S.G.; Farber, J.; Xin, W.; Hussain, A.T.; Shah, K.; Tanda, S.; Berryman, M.A.; Edwards, J.C.; Singh, H. Data supporting characterization of CLIC1, CLIC4, CLIC5 and DmCLIC antibodies and localization of CLICs in endoplasmic reticulum of cardiomyocytes. Data Brief. 2016, 7, 1038–1044.
- Edwards, J.C.; Kahl, C.R. Chloride channels of intracellular membranes. FEBS Lett. 2010, 584, 2102–2111.
- Gururaja Rao, S.; Ponnalagu, D.; Patel, N.J.; Singh, H. Three Decades of Chloride Intracellular Channel Proteins: From Organelle to Organ Physiology. Curr. Protoc. Pharmacol. 2018, 80, 11–21.
- Singh, H. Two decades with dimorphic Chloride Intracellular Channels (CLICs). FEBS Lett. 2010, 584, 2112–2121.
- Kim, H.J.; Lee, P.C.; Hong, J.H. Chloride Channels and Transporters: Roles beyond Classical Cellular Homeostatic pH or Ion Balance in Cancers. Cancers 2022, 14, 856.
- Takano, K.; Liu, D.; Tarpey, P.; Gallant, E.; Lam, A.; Witham, S.; Alexov, E.; Chaubey, A.; Stevenson, R.E.; Schwartz, C.E.; et al. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum. Mol. Genet. 2012, 21, 4497–4507.
- Witham, S.; Takano, K.; Schwartz, C.; Alexov, E. A missense mutation in CLIC2 associated with intellectual disability is predicted by in silico modeling to affect protein stability and dynamics. Proteins 2011, 79, 2444–2454.
- Richardson, S.J.; Steele, G.A.; Gallant, E.M.; Lam, A.; Schwartz, C.E.; Board, P.G.; Casarotto, M.G.; Beard, N.A.; Dulhunty, A.F. Association of FK506 binding proteins with RyR channels-effect of CLIC2 binding on sub-conductance opening and FKBP binding. J. Cell Sci. 2017, 130, 3588–3600.
- Meng, X.; Wang, G.; Viero, C.; Wang, Q.; Mi, W.; Su, X.D.; Wagenknecht, T.; Williams, A.J.; Liu, Z.; Yin, C.C. CLIC2-RyR1 interaction and structural characterization by cryo-electron microscopy. J. Mol. Biol. 2009, 387, 320–334.
- Suh, K.S.; Mutoh, M.; Gerdes, M.; Crutchley, J.M.; Mutoh, T.; Edwards, L.E.; Dumont, R.A.; Sodha, P.; Cheng, C.; Glick, A.; et al. Antisense suppression of the chloride intracellular channel family induces apoptosis, enhances tumor necrosis factor α-induced apoptosis, and inhibits tumor growth. Cancer Res. 2005, 65, 562–571.
- Kuwabara, J.; Umakoshi, A.; Abe, N.; Sumida, Y.; Ohsumi, S.; Usa, E.; Taguchi, K.; Choudhury, M.E.; Yano, H.; Matsumoto, S.; et al. Truncated CD200 stimulates tumor immunity leading to fewer lung metastases in a novel Wistar rat metastasis model. Biochem. Biophys. Res. Commun. 2018, 496, 542–548.
- Xu, T.; Wang, Z.; Dong, M.; Wu, D.; Liao, S.; Li, X. Chloride intracellular channel protein 2: Prognostic marker and correlation with PD-1/PD-L1 in breast cancer. Aging 2020, 12, 17305–17327.
- Liu, J.; Jin, X.; Liu, K.J.; Liu, W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J. Neurosci. 2012, 32, 3044–3057.
- Romanic, A.M.; White, R.F.; Arleth, A.J.; Ohlstein, E.H.; Barone, F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998, 29, 1020–1030.
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709.
- Miyamori, H.; Takino, T.; Kobayashi, Y.; Tokai, H.; Itoh, Y.; Seiki, M.; Sato, H. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J. Biol. Chem. 2001, 276, 28204–28211.
- Mao, Y.; Kleinjan, M.L.; Jilishitz, I.; Swaminathan, B.; Obinata, H.; Komarova, Y.A.; Bayless, K.J.; Hla, T.; Kitajewski, J.K. CLIC1 and CLIC4 mediate endothelial S1P receptor signaling to facilitate Rac1 and RhoA activity and function. Sci. Signal. 2021, 14, eabc0425.
- Tung, J.J.; Kitajewski, J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J. Angiogenes Res. 2010, 2, 23.
- Lucitti, J.L.; Tarte, N.J.; Faber, J.E. Chloride intracellular channel 4 is required for maturation of the cerebral collateral circulation. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1141–H1150.
More
Information
Subjects:
Neurosciences; Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
11 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




