
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hristo Popov | -- | 1419 | 2022-10-10 03:08:48 | | | |
| 2 | Amina Yu | Meta information modification | 1419 | 2022-10-10 03:18:22 | | |
Video Upload Options
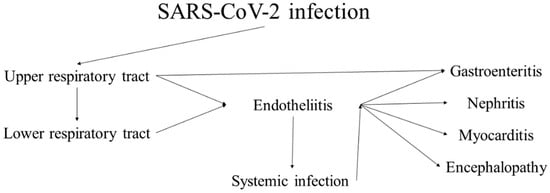
Coronaviruses are a large group of RNA viruses, the most notable representatives of which are SARS-CoV, MERS-CoV and SARS-CoV-2. Human coronavirus infections were first documented in the 1960s, when members causing seasonal common colds were successfully replicated in human embryonal trachea and kidney cell cultures and classified based on electron microscopy. The history of coronaviruses stretched far back to that point, however, with some representatives causing disease in animals identified several decades prior and evolutionary data pointing towards the origin of this viral group more than 55 million years ago. In the short time period of research since they were discovered, coronaviruses have shown significant diversity, genetic peculiarities and varying tropism, resulting in the three identified causative agents of severe disease in humans—SARS, MERS and the most recent one, COVID-19, which has surpassed the previous two due to causing a pandemic resulting in significant healthcare, social and political consequences. Coronaviruses are likely to have caused pandemics long before, such as the so-called Asian or Russian influenza. Despite being epitheliotropic viruses and predominantly affecting the respiratory system, these entities affect multiple systems and organs, including the kidneys. In the kidneys, they actively replicate in glomerular podocytes and epithelial cells of the tubules, resulting in acute kidney injury, seen in a significant percentage of severe and fatal cases. Furthermore, the endothelial affinity of the viruses, resulting in endotheliitis, increases the likelihood of thrombotic microangiopathy, damaging the kidneys in a two-hit mechanism. As such, recently, COVAN has been a suggested nomenclature change indicating renal involvement in coronavirus infections and its long-lasting consequences.
References
- Goldsmith, C.S.; Tatti, K.M.; Ksiazek, T.G.; Rollin, P.E.; Comer, J.A.; Lee, W.W.; Rota, P.A.; Bankamp, B.; Bellini, W.J.; Zaki, S.R. Ultrastructural characterization of SARS coronavirus. Emerg. Infect. Dis. 2004, 10, 320–326.
- Masters, P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006, 66, 193–292.
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22.
- Lalchhandama, K. The chronicles of coronaviruses: The electron microscope, the doughnut, and the spike. Sci. Vis. 2020, 20, 78–92.
- Godet, M.; L’Haridon, R.; Vautherot, J.F.; Laude, H. TGEV corona virus ORF4 encodes a membrane protein that is incorporated into virions. Virology 1992, 188, 666.
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23.
- Simmons, G.; Zmora, P.; Gierer, S.; Heurich, A.; Pöhlmann, S. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. Antivir. Res. 2013, 100, 605–614.
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502.
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 1–8.
- Family—Coronaviridae. Virus Taxon. 2012, 806–828.
- Decaro, N. Alphacoronavirus. Springer Index Viruses 2011, 371–383.
- Decaro, N. Betacoronavirus. Springer Index Viruses 2011, 385–401.
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868.
- King, A. An uncommon cold. New Sci. 2020, 246, 32.
- Estola, T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970, 14, 330–336.
- Liu, P.; Shi, L.; Zhang, W.; He, J.; Liu, C.; Zhao, C.; Kong, S.K.; Loo, J.F.C.; Gu, D.; Hu, L. Prevalence and genetic diversity analysis of human coronaviruses among cross-border children. Virol. J. 2017, 14, 1–8.
- Fabricant, J. The early history of infectious bronchitis. Avian Dis. 1998, 42, 648–650.
- Bushnell, L.D.; Brandly, C.A. Laryngotracheitis in Chicks. Poult Sci. 1933, 12, 55–60.
- Decaro, N. Gammacoronavirus‡: Coronaviridae. Springer Index Viruses 2011, 403.
- McIntosh, K. Coronaviruses: A Comparative Review. Curr. Top. Microbiol. Immunol./Ergeb. Mikrobiol. Immun. 1974, 85–129.
- Kahn, J.S.; McIntosh, K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005, 24, S223–S227.
- Mahase, E. Covid-19: First Coronavirus was described in The BMJ in 1965. BMJ 2020, 369, m1547.
- Tyrrell, D.A.J.; Bynoe, M.L. Cultivation of a Novel Type of Common-Cold Virus in Organ Cultures. Br. Med. J. 1965, 1, 1467–1470.
- Hamre, D.; Procknow, J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193.
- Almeida, J.D.; Tyrrell, D.A. The morphology of three previously uncharacterized human respiratory viruses that grow in organ culture. J. Gen. Virol. 1967, 1, 175–178.
- McIntosh, K.; Becker, W.B.; Chanock, R.M. Growth in suckling-mouse brain of ‘IBV-like’ viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. USA 1967, 58, 2268–2273.
- Wertheim, J.O.; Chu, D.K.W.; Peiris, J.S.M.; Kosakovsky Pond, S.L.; Poon, L.L.M. A case for the ancient origin of coronaviruses. J. Virol. 2013, 87, 7039–7045.
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008.
- Fan, Y.; Zhao, K.; Shi, Z.L.; Zhou, P. Bat Coronaviruses in China. Viruses 2019, 11, 210.
- Alfarouk, K.O.; AlHoufie, S.T.S.; Ahmed, S.B.M.; Shabana, M.; Ahmed, A.; Alqahtani, S.S.; Alqahtani, A.S.; Alqahtani, A.M.; Ramadan, A.M.; Ahmed, M.E. Pathogenesis and Management of COVID-19. J. Xenobiot. 2021, 11, 6.
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544.
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science 2020, 368, 1012–1015.
- Guarner, J. Three Emerging Coronaviruses in Two DecadesThe Story of SARS, MERS, and Now COVID-19. Am. J. Clin. Pathol. 2020, 153, 420–421.
- Forgie, S.; Marrie, T.J. Healthcare-associated atypical pneumonia. Semin. Respir. Crit. Care Med. 2009, 30, 67–85.
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163.
- Brüssow, H.; Brüssow, L. Clinical evidence that the pandemic from 1889 to 1891 commonly called the Russian flu might have been an earlier coronavirus pandemic. Microb. Biotechnol. 2021, 14, 1860.
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.M.; Van Ranst, M. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005, 79, 1595.
- Phillips, N. The coronavirus is here to stay - here’s what that means. Nature 2021, 590, 382–384.
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452.
- Dzieciatkowski, T.; Szarpak, L.; Filipiak, K.J.; Jaguszewski, M.; Ladny, J.R.; Smereka, J. COVID-19 challenge for modern medicine. Cardiol. J. 2020, 27, 175–183.
- Lai, J.W.; Cheong, K.H. Superposition of COVID-19 waves, anticipating a sustained wave, and lessons for the future. BioEssays 2020, 42, 2000178.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- Popov, G.T.; Baymakova, M.; Vaseva, V.; Kundurzhiev, T.; Mutafchiyski, V. Clinical Characteristics of Hospitalized Patients with COVID-19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis. 2020, 20, 910–915.
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558.
- Khanna, R.C.; Cicinelli, M.V.; Gilbert, S.S.; Honavar, S.G.; Murthy, G.V.S. COVID-19 pandemic: Lessons learned and future directions. Indian J. Ophthalmol. 2020, 68, 703.
- Buja, L.M.; Wolf, D.; Zhao, B.; Akkanti, B.; McDonald, M.; Lelenwa, L.; Reilly, N.; Ottaviani, G.; Elghetany, M.T.; Trujillo, D.O.; et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020, 48, 107233.
- Siripanthong, B.; Nazarian, S.; Muser, D.; Deo, R.; Santangeli, P.; Khanji, M.Y.; Cooper, L.T., Jr.; Chahal, C.A.A. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm 2020, 17, 1463–1471.
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764.
- Abdel-Mannan, O.; Eyre, M.; Löbel, U.; Bamford, A.; Eltze, C.; Hameed, B.; Hemingway, C.; Hacohen, Y. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020, 77, 1440–1445.





