Coronaviruses are a large group of RNA viruses, the most notable representatives of which are SARS-CoV, MERS-CoV and SARS-CoV-2. Human coronavirus infections were first documented in the 1960s, when members causing seasonal common colds were successfully replicated in human embryonal trachea and kidney cell cultures and classified based on electron microscopy. The history of coronaviruses stretched far back to that point, however, with some representatives causing disease in animals identified several decades prior and evolutionary data pointing towards the origin of this viral group more than 55 million years ago. In the short time period of research since they were discovered, coronaviruses have shown significant diversity, genetic peculiarities and varying tropism, resulting in the three identified causative agents of severe disease in humans—SARS, MERS and the most recent one, COVID-19, which has surpassed the previous two due to causing a pandemic resulting in significant healthcare, social and political consequences. Coronaviruses are likely to have caused pandemics long before, such as the so-called Asian or Russian influenza. Despite being epitheliotropic viruses and predominantly affecting the respiratory system, these entities affect multiple systems and organs, including the kidneys. In the kidneys, they actively replicate in glomerular podocytes and epithelial cells of the tubules, resulting in acute kidney injury, seen in a significant percentage of severe and fatal cases. Furthermore, the endothelial affinity of the viruses, resulting in endotheliitis, increases the likelihood of thrombotic microangiopathy, damaging the kidneys in a two-hit mechanism. As such, recently, COVAN has been a suggested nomenclature change indicating renal involvement in coronavirus infections and its long-lasting consequences.
Coronaviruses are representatives of a large group of RNA viruses [
1]. According to their structure, these viruses have relatively large sizes, varying between 80 and 120 μm, but some members are characterized by smaller and significantly larger sizes—from 50 to 400 μm, with a molecular mass of about 40,000 kilodaltons [
1,
2,
3,
4]. The viral single-stranded RNA is enveloped by a double lipid membrane in which transmembrane and structural proteins are integrated, including the so-called spike proteins [
5]. Formed in this way, the virion has a characteristic rayed surface, visible in electron microscopy and computer-modeled reconstructions [
1]. From this characteristic surface structure of the virions, they derive their name—
corona viruses [
1,
6]. It is through these membrane-associated proteins that the virions bind to cell receptors, during which the viral endocytosis in the host cell takes place. Immediately after virion endocytosis, viral “undressing” occurs in the host cell’s cytoplasm and viral RNA is released [
7]. The structure of this RNA, with a 5′ methylated cap and a 3′ polyadenylated tail, allows it to be recognized by the granulated endoplasmic reticulum as an mRNA and thus initiate direct replication of new structural proteins for the assembly of new virions, by synthesizing a replication–transcription complex [
6].
The replication–transcription complex allows the viral RNA to be replicated in multiple steps, the viral structures to be transcribed and, in the presence of at least two viral RNAs, even from another virus family, in the host cell, to recombine the sources [
6]. Errors in the first two described stages and the third stage of the process give the characteristics of high mutagenicity and emergence of new virus variants; a critical clarification is that such mutant and/or recombined forms are not always vital [
8]. Furthermore, the hijacking of the host cell not only disrupts its metabolism and homeostasis, leading to induced apoptosis or necrosis, but can also lead to structural, functional, or mutation alterations occurring in it [
9].
After the synthesis of the necessary genetic and structural material, the process of virion assembly begins, taking place predominantly in the Golgi complex, after which new virions are released from the cell by mediated exocytosis and/or cell destruction (necrosis) and can directly infect other cells [
6].
Coronaviruses exhibit epithelial tropism, with individual members having specific tissue, organ and even species tropism, as well as different modes of transmission [
10]. The most common infection transmission mechanism is airborne, while there are also data on fecal–oral transmission (alimentary)—coronavirus gastroenteritis in pigs [
6,
10,
11,
12]. Given the diversity of structural proteins, the cellular receptors used for endocytosis vary between different entities, with the most commonly used ones being the ACE-2 and alanine aminopeptidase receptors [
6,
13].
As a group of viruses, they cause diseases in a large proportion of mammals, including humans, as well as in birds, i.e., are a typical example of anthropozoonosis [
6,
14,
15]. Characteristic of the course of these infections is the involvement of the respiratory system, with the majority of representatives involving the upper respiratory tract and causing seasonal common colds with acute, predominantly serous rhinosinusitis, conjunctivitis, otitis, pharyngitis and laryngitis [
14,
16]. Classically, these infections are not severe; they are transient, no specific treatment is available, and if necessary, the treatment is symptomatic—nasal decongestants, antipyretics and vitamins [
16].
The earliest documented data on coronavirus infections date back to the 1920s, when they were described as causing severe bronchitis in newly hatched chicks, with extremely high mortality ranging from 40% to 90% [
15,
17]. Newly hatched chicks had severe respiratory symptoms, and the isolated virus was named infectious bronchitis virus in 1933 and cultivated in 1937 [
18,
19]. In the 1940s, viral infections in mice causing encephalitis and hepatitis were also described, and at a later stage, it was understood that the three viruses described so far belong to the same group [
4,
20]. Human coronaviruses were described in the 1960s as the causes of common seasonal colds, in which it was impossible to cultivate the causative agent using conventional methods used for adenoviruses and rhinoviruses [
21,
22]. Cultivation was achieved only a few years later using tissue cultures from human embryonal trachea [
23]. Descriptions of similar viruses followed in the same decade, some of which were replicated successfully in human embryonal kidney cell cultures [
23,
24,
25]. Utilizing electron microscopy, it was established that all the viral pathogens described so far have a characteristic shape and membrane protrusions, which is why they were united in a joint group called coronaviruses, which later included many more representatives, based on genetic analysis, with varying organ tropism and severity of symptoms [
25,
26].
Evolutionarily, the earliest established ancestor of modern coronaviruses is thought to have existed about 10,000 years ago, with some evidence suggesting the presence of similar viruses about 55 million years ago in bats and birds [
27]. This evolutionary theory of the origin of modern coronaviruses is well supported by the fact that the natural reservoir and source of new variants are most often bats and birds [
28].
The modern classification of viruses defines these viruses in the family
coronaviridae, with two subfamilies,
letovirinae and
orthocoronavirinae, of which the characteristics and entities described so far are representatives [
10,
29]. The
orthocoronavirinae subfamily, in turn, consists of four
genera—alpha, beta, gamma and delta coronavirus [
27]. Alpha- and betacoronaviruses have the greatest infectious affinity for the human population, while gamma and delta are primarily zoonotic infections [
10,
29,
30,
31]. Betacoronaviruses are of fundamental importance to the human population, whose representations include the three most severe infections with such viruses—SARS, MERS and COVID-19 [
12,
32,
33]. It is important to note that while the betacoronavirus family is a close relative, SARS-CoV and SARS-CoV-2 are members of the
subgenus sarbecovirus, while MERS is a more distant relative—a member of the
subgenus of
merbecovirus.
Although the clinical picture in humans is relatively mild, except for some virus types, coronaviruses are thought to have caused epidemic outbreaks of severe disease long before they were identified, most often interpreted as influenza infections [
6,
14,
16,
34,
35]. For example, the last major pandemic of the 19th century—the so-called Asian or Russian influenza, which caused a pandemic outbreak in 1889–1890, long considered to be influenza type 1—had similar symptoms to COVID-19, including neurological symptoms—loss of taste and smell, clouding of consciousness and encephalitis-like symptoms, all rarely seen in influenza [
14,
36,
37]. Of course, serological data from the period do not exist, and proving a phylogenetic relationship is practically impossible.
Since emerging in late 2019, the most recent representative of severe coronaviruses, SARS-CoV-2 has led to multiple consequences, from medical and social to political and philosophical [
38,
39,
40,
41]. The clinical disease the virus causes, coronavirus disease, identified in 2019 (COVID-19), while initially regarded as a respiratory infection, has shown its multisystem nature [
42,
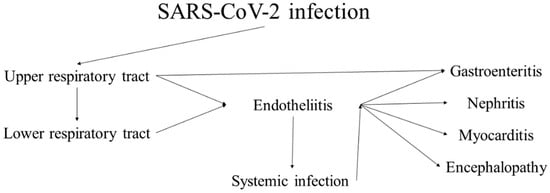
43]. While most cases present with and have the most severe clinical symptoms from the respiratory system, case reports, small cohort studies and systemic research have genuinely shown the systemic nature of the virus due to its epitheliotropism and rapid dissemination (
Figure 1) [
40,
44,
45,
46,
47,
48,
49]. The characteristics of the virus and the disease it causes are broadly representative of coronaviruses, and its pathological effects are highly similar to those of the previous two significant outbreaks—SARS and MERS.
Figure 1. Progression and sequelae of SARS-CoV-2 infection (COVID-19).
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia2040117