Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shiqin Zhao | -- | 3284 | 2022-10-07 03:30:01 | | | |
| 2 | Jason Zhu | -15 word(s) | 3269 | 2022-10-08 06:53:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xiao, S.; Chen, N.; Chai, Z.; Zhou, M.; Xiao, C.; Zhao, S.; Yang, X. Structural Diversity of Secondary Metabolites from Marine-Derived Bacillus. Encyclopedia. Available online: https://encyclopedia.pub/entry/28339 (accessed on 07 February 2026).
Xiao S, Chen N, Chai Z, Zhou M, Xiao C, Zhao S, et al. Structural Diversity of Secondary Metabolites from Marine-Derived Bacillus. Encyclopedia. Available at: https://encyclopedia.pub/entry/28339. Accessed February 07, 2026.
Xiao, Shaoyujia, Nan Chen, Zixue Chai, Mengdie Zhou, Chenghaotian Xiao, Shiqin Zhao, Xiliang Yang. "Structural Diversity of Secondary Metabolites from Marine-Derived Bacillus" Encyclopedia, https://encyclopedia.pub/entry/28339 (accessed February 07, 2026).
Xiao, S., Chen, N., Chai, Z., Zhou, M., Xiao, C., Zhao, S., & Yang, X. (2022, October 07). Structural Diversity of Secondary Metabolites from Marine-Derived Bacillus. In Encyclopedia. https://encyclopedia.pub/entry/28339
Xiao, Shaoyujia, et al. "Structural Diversity of Secondary Metabolites from Marine-Derived Bacillus." Encyclopedia. Web. 07 October, 2022.
Copy Citation
The marine is a highly complex ecosystem including various microorganisms. Bacillus species is a predominant microbialflora widely distributed in marine ecosystems. Bacillus species can grow rapidly and tolerate extremely adverse environmental conditions such as extreme ambient temperature, salinity and pH, high pressure and nutrient deficiency. B. subtilis can adopt several responses when faced with the depletion of essential nutrients, including motility, secretion of extracellular enzymes, genetic transformation, antibiotic production, and finally sporulation. The genus Bacillus is a prolific producer of bioactive metabolites, including more than 350 kinds of rod-shaped and Gram-positive bacteria.
marine microorganism
Bacillus
structural diversity
1. Introduction
The ocean is a highly complex ecosystem, a rich and underdeveloped treasure house containing a wide variety of biological resources including aquatic species and various microorganisms [1][2][3]. Natural products, especially small molecules isolated from biological sources, have long been regarded for their huge potential in human medicine and are still gaining traction [4]. Marine microorganisms produce many undiscovered molecules with unprecedented structures and pharmacological activities in an extreme living environment [2]. Therefore, it is commonly recognized that marine microbes constitute a promising source of novel metabolites with considerable therapeutically potential for new drug screening and development [3][5][6][7][8].
Bacillus species is a predominant microbialflora widely distributed in marine ecosystems [9][10]. Bacillus species can grow rapidly and tolerate extremely adverse environmental conditions such as extreme ambient temperature, salinity and pH, high pressure and nutrient deficiency [11]. B. subtilis can adopt several responses when faced with the depletion of essential nutrients, including motility, secretion of extracellular enzymes, genetic transformation, antibiotic production, and finally sporulation [12]. The genus Bacillus is a prolific producer of bioactive metabolites, including more than 350 kinds of rod-shaped and Gram-positive bacteria [13]. Thereinto, B. subtilis, B. licheniformis and B. amyloliquefaciens possess potential value as therapeutic agent candidates on account of their ability to produce bioactive secondary metabolites [14][15][16][17].
In recent years, a variety of secondary metabolites of marine Bacillus species have been studied, including lipopeptides [18], polyketides [19], non-ribosomal peptides [20], macrolides [16][21], and glycopeptides [9]. Several natural products were isolated from the marine organisms for drug development by traditional means of bioactivity-guided methods and chemical structure elucidation. More precise analytical methods, such as LC-MS and NMR spectroscopy guided metabolic profiling and dereplication of a crude extract, also promoted the emergence of new secondary metabolites [22][23][24]. Some novel technologies, such as improved genome mining methods, have propelled natural products in the field of drug discovery [25]. The marine hosts a huge variety of organisms adapted to the specific environment, which should result in the production of a wide range of unique biomolecules [7]. The production of secondary metabolites is normally associated with the bacterium’s response to a growth-limiting environment; hence, exploring the high diversity of marine environments may uncover multiple compounds with unique structures and biological activities [26]. For example, most of this industrial Bacillus pumilus group (Bp group) have been isolated from terrestrial ecosystems at present. By contrast, members of the Bp group are ubiquitous and diverse in marine environments, but less explored [27].
According to studies, these compounds have a wide range of biological activities, viz., antimicrobial [28][29], anticancer [30][31][32], antivirus [33], antifungal, promotion of plant growth [34], immunosuppressive, antituberculosis, antimycoplasmic and exceptional surfactant [35], indicating their promising medicinal, agricultural and industrial potential. Over the past decades, Firmicutes phylum were found to be the marine-derived bacteria producers of the most antimicrobial activity, Bacillus strains specifically [26]. Sequel of genomic analyses demonstrated the prospect of marine Bacillus species producing wide-ranging polyketide classes of antibiotic agents, such as macrocyclic lactones, bacillaene, macrolactins, and difficidins [36][37]. Polyketide, as conspicuous bactericidal agents in the human health area, was reported to produce by multifunctional microbial polyketide synthase (pks) complex [38]. Difficidins are a polyketide class of polyenes, which were reported to biosynthesize by type I pks encrypted in the dif operon and were recognized to hinder bacterial pathogens [35]. Moreover, polyketides are the most structurally diverse and pharmacologically relevant natural products with low toxicity and high efficacy, many of which exhibit cytotoxic effects on cancer cells.
2. Cyclic Lipopeptides
Cyclic lipopeptides (CLPs) are common secondary metabolites isolated from marine-derived Bacillus. The CLPs are a class of metabolites with structural diversity produced by multifarious bacterial genera [39]. There are three families of CLPs being of particular importance, namely surfactins, iturins and plipastatins, all consisting of a short cyclic oligopeptide linked to the tail of a fatty acid [14]. Surfactin sequences comprise of seven amino acids and a β-hydroxy fatty acid chain containing 12–16 carbons [40]. The iturin family sequences are composed of heptapeptides and a β-amino fatty acid chain of 14–17 carbon atoms, which consists of bacillomycin D, F, L, Lc, iturin A, AL, C and mycosubtilin (Figure 1) [41]. The plipastatin family comprise of ten amino acids and a β-hydroxy fatty acid containing 14–18 carbon atoms [42]. Figure 2 lists the structures of cyclic lipopeptides produced by marine-derived Bacillus species.
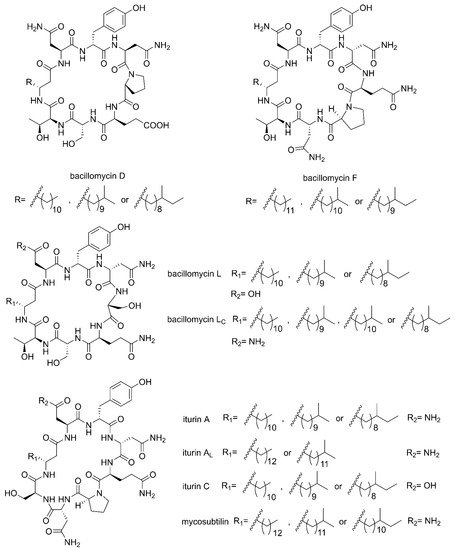
Figure 1. The structures of iturin family members (bacillomycin D, F, L, Lc, iturin A, AL, C and mycosubtilin).
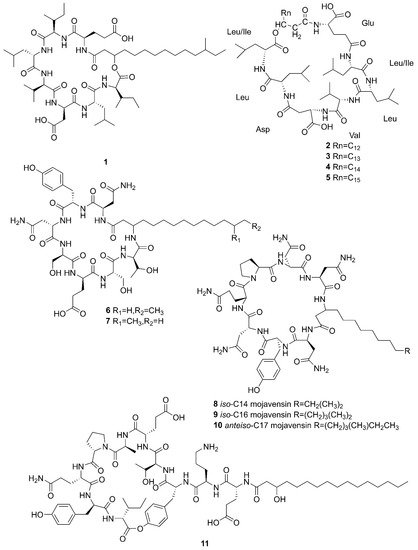
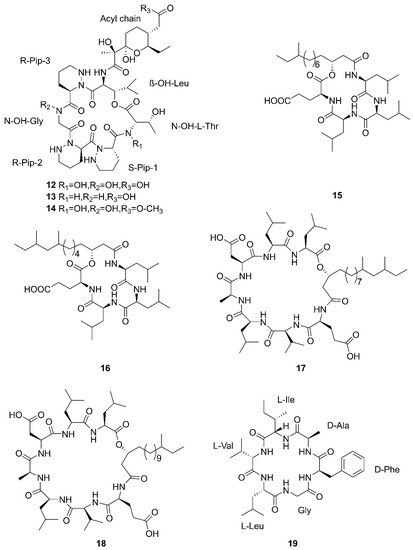
Figure 2. Cyclic lipopeptides produced by marine-derived Bacillus species.
Compounds 1–5 belong to surfactin family. A new CLP surfactin named anteiso-C15 Ile2,7 surfactin (1) was isolated from B. velezensis SH-B74 in the China Center for Type Culture Collection (CCTCC), which collected from the marine sediments, comprising of an anteiso-C15 type saturated fatty acid chain, and a peptidic backbone of L-Glu1, L-Ile2, D-Leu3, L-Val4, L-Asp5, D-Leu6, L-Ile7 [43]. Rn-Glu1-Leu/Ile2-Leu3-Val4-Asp5-Leu6-Leu/Ile7 (2–5) belonging to surfactin homolog were isolated from B. licheniformis MB01 collected from sediments in the Bohai Sea, China [44]. Compounds 6–10 belong to the iturin family. A novel lipopeptide antibiotic bacillopeptin named bacillopeptin B1 (6) and a known compound, bacillopeptin B (7) were detected in the fermentation broth of a marine sediment-derived B. amyloliquefaciens SH-B74 collected from sediments in the South China Sea. More precisely, compound 6 as a member of bacillopeptin family has the same amino-acid sequence and the same molecular weight as compound 7, but has a different fatty-acid residue [22]. Compounds 8–10 were characterized as cyclic lipopeptides with saturated β-amino fatty acid chain residues, iso-C14 mojavensin, iso-C16 mojavensin, and anteiso-C17 mojavensin, all of which were produced by a marine-derived B. mojavensis B0621A obtained from the mantle of a pearl oyster Pinctada martensii in the South China Sea [45]. In addition, plipastatin A1 (11), belonging to the plipastatin family, was obtained by solidphase extraction and reversed-phase high-performance liquid chromatograph (RP-HPLC) from the fermentation broth of a marine sediment-derived B. amyloliquefaciens SH-B74 in the CCTCC [46]. A new cyclic hexapeptide with three piperazic acids (N-OH-Thr, N-OH-Gly, β-OH-Leu) named dentigerumycin E (12) and two reported derivatives, 2-N, 16-N-deoxydenteigerumycin E (13) and dentigerumycin E methyl ester (14), were isolated from coculture of marine Streptomyces and Bacillus strains collected together from the intertidal mudflat in Wando, Republic of Korea. It is worth mentioning that only compound 12 showed antiproliferative and antimetastatic activities against human cancer cells, suggesting that 2-N-OH, 16-N-OH, and 37-OH (carboxylic acid) are essential for the activities [25]. Two novel cyclic lipopeptides, bacilotetrin A (15) and bacilotetrin B (16), possessing three leucines and one glutamic residue cyclized with a lipophilic 3-hydroxyl fatty acid, were isolated from B. subtilis 109GGC020 in the sediments from the Gageocho of southern reef, Republic of Korea [12]. Additionally, gageopeptins A (17) and B (18), two novel cyclic lipopeptides, were isolated from the same strain in the same sediments as above [47]. A new cyclic hexapeptide named bacicyclin (19) was purified from Bacillus sp. BC028 associated with the blue mussel Mytilus edulis collected from the western shore of the Baltic Sea in Germany [48].
3. Diketopiperazines
Cyclicpeptide diketopiperazines consist of residues of two amino acids and mevalonic acid [49]. Figure 3 lists the structures of diketopiperazines that were produced by marine-derived Bacillus species.
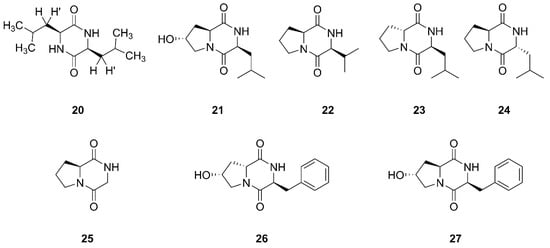
Figure 3. Diketopiperazines produced by marine-derived Bacillus species.
Compound 20 was established as a diketopiperazine (3S, 6S)-3,6-diisobutylpiperazine-2,5-dione, which was isolated from the ethyl acetate extract of the culture broth of Bacillus sp. SPB7. This strain SPB7 was obtained from marine sponge Spongia officinalis collected from the Palk Bay of Bengal, India. In particular, this is the first time that compound 20 had been isolated from a sponge-associated microbe [50]. Finally, seven cyclic dipeptides compounds 21–27 were identified and characterized as cyclo (L-leu-trans-8-hydroxy-L-pro), cyclo (L-val-L-pro), cyclo (D-pro-L-leu), cyclo (L-pro-D-leu), cyclo (gly-L-pro), cyclo (L-phe-cis-8-hydroxy-D-pro), and cyclo (L-phe-trans-8-hydroxy-L-pro), respectively, which were isolated from Bacillus sp. UST050418-715 collected from sponge in the sea near St. Juan Island, Washington, USA [51]. Compound 25 was also found from sponge-endosymbiotic Bacillus species collected from Agatti island located in the Arabian Sea, in the Laksha Archipelago of India [52].
4. Linear Lipopeptides
Linear lipopeptide is a kind of lipopeptide, in which amino acids are connected in turn into linear, unconnected head and tail and no cyclic structure. Fatty acids are connected to α-amino groups or other hydroxyl groups at the N-terminal of the peptide chain [53]. Figure 4 lists the structures of linear lipopeptides that were produced by marine-derived Bacillus species.
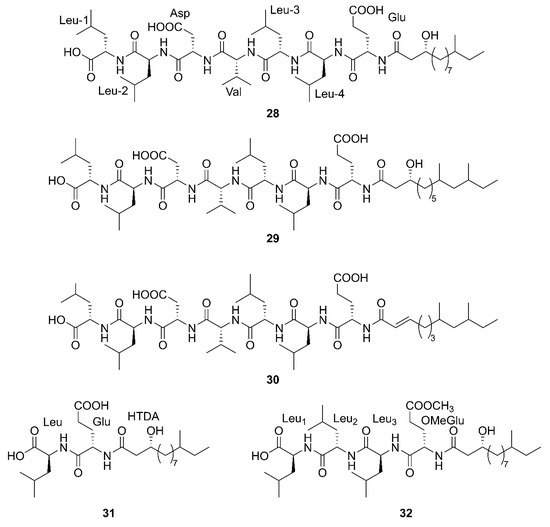
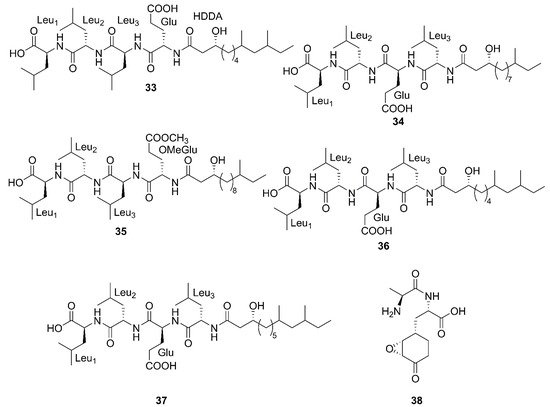
Figure 4. Linear lipopeptides produced by marine-derived Bacillus species.
Three newfound linear lipopeptides named gageostatins A (28), B (29) and C (30), comprising of hepta-peptides and new 3-β-hydroxy fatty acids yielded by a marine-derived bacterium B. subtilis from the culture broth [35]. Furthermore, three novel linear lipopeptides possessing di- and tetrapeptides and a new fatty acid, gageotetrins A (31), B (32) and C (33), were isolated from a marine B. subtilis [54]. Four unreported lipopeptides, gageopeptides A (34), B (35), C (36), and D (37) were isolated and identified from a marine-derived bacterium B. subtilis, which consisted of tetrapeptides and 3-β-hydroxy fatty acids [55]. The fatty acid of 28, 31, 32 and 34 was identical and determined as a 3-β-hydroxy-11-methyltridecanoic acid. Likewise, compounds 29 and 37 both possessed the same fatty acid, 3-β-hydroxy-9,11-dimethyltridecanoic acid. Moreover, the fatty acid unit of 33 and 36 was 3-β-hydroxy-8,10-dimethyldodecanoic acid. In particular, the absolute stereochemistry at C-3 of the fatty acids of linear lipopeptides 28–37 is R configuration except 30. Additionally, the configuration of the amino acid residues in 28–37 was found to be L-form, while Val in 28–30 was D-form. Besides, bacilysin (38), another identified dipeptide, was isolated from seaweed-associated B. amyloliquefaciens MTCC 10456 in Microbial Type Culture Collection and Gene Bank (MTCC) of Chandigarh in India. Notably, this is the first report on the co-production and isolation of anti- Malassezia spp. chemicals from marine Bacillus species [41]. In conclusion, all linear Lipopeptide mentioned above were obtained from the Gageocho in the southern reef (Republic of Korea) except 38.
5. Nonribosomal Peptides
Nonribosomal peptides (NRPs) are large enzyme complexes with a modular structure responsible for binding a particular amino acid. NRPSs of Bacillus are synthesized by large multimodular nonribosomal peptide-synthetase (NRPS) through prolonging the active monomers of amino acid building blocks [56]. Figure 5 lists the structures of nonribosomal peptides produced by marine-derived Bacillus species.
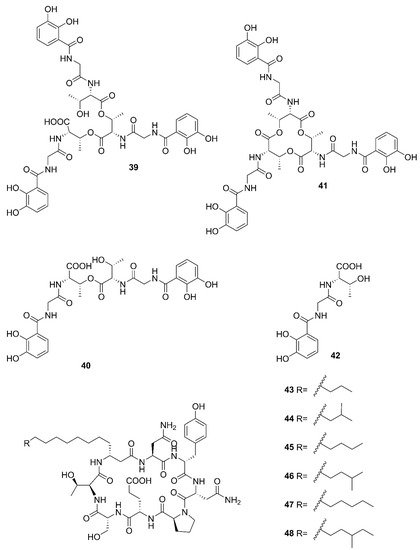
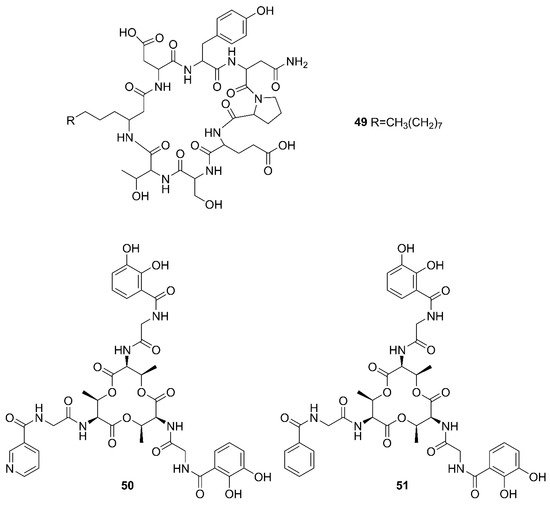
Figure 5. Nonribosomal peptides produced by marine-derived Bacillus species.
Two unreported compounds, bacillibactin B (39) and bacillibactin C (40), along with the known compounds Bacillibactin (41) and SVK21 (42), were discovered from Bacillus sp. named PKU-MA00093 from sponges, corals and sediments in the South China Sea. Additionally, compounds 43–48 were characterized as bacillomycin D, iso-C15 bacillomycin D, C15 bacillomycin D, iso-C16 bacillomycin D, C16 bacillomycin D, and anteiso-C17 bacillomycin D, respectively. They were isolated from Bacillus sp. PKU-MA00092 collected from sponges, corals and sediments in the South China Sea. Notably, this was the first time to report the structures of 45 and 47 with fully specified 1H NMR and 13C NMR data; their structures are highly similar except for the fatty acid moieties [57]. Compounds 45 and 47 in company with C14 bacillomycin D (49), were obtained from seaweed-associated B. amyloliquefaciens MTCC 10456 collected from seaweed in the MTCC, of Chandigarh, India [41]. Moreover, compounds 45 and 49 were also isolated from the methanol extract harvested from marine-derived B. megaterium CGMCC7086 obtained from the intestines of marine fish in the Yellow Sea of East China by two-step ultrafiltration and liquid chromatography-electronic spray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Using a highly-efficient separation technique and identification method, more than 40 lipopeptides variants were identified from a Bacillus strain [23]. Besides, compound 47 was isolated from B. subtilis B38 strain [58]. Two unique bacillibactins, bacillibactins E (50) and F (51) were the first bacterial siderophores containing nicotinic and benzoic acid moieties isolated from a marine sponge Cinachyrella apion associated Bacillus sp. WMMC1349 collected from the west shore of Ramrod Key in Florida [24].
6. Polyketides
Polyketides are a class of extremely large secondary metabolites assembled from simple acyl-coA compounds [59]. Bacillus species of marine origin was a potential source of bioactive compounds of polyketides and bacteriocins with significant antimicrobial activity against human pathogens [60]. Figure 6 lists the structures of polyketides that were produced by marine-derived Bacillus species.
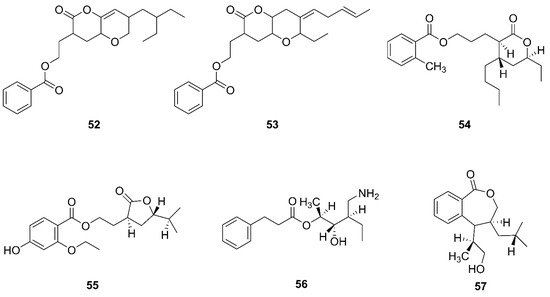
Figure 6. Polyketides produced by marine-derived Bacillus species.
Two novel compounds, O-heterocycle pyrans, 2-(7-(2-Ethylbutyl)-2,3,4,4a,6,7-hexahydro-2-oxopyrano-[3,2b]-pyran-3-yl)-ethyl benzoate (52) and 2-((4Z)-2-ethyl-octahydro-6-oxo-3-((E)-pent-3-enylidene)-pyrano-[3,2b]-pyran-7-yl)-ethyl benzoate (53) were obtained by repeated chromatography from the heterotrophic bacterium B. subtilis MTCC 10407 associated with brown seaweed Sargassum myriocystum on the southeast coast of India [61]. Additionally, 11-(15-butyl-13-ethyl-tetrahydro-12-oxo-2H-pyran-13-yl) propyl-2-methylbenzoate (54), 9-(tetrahydro-2-isopropyl-11-oxofuran-10-yl)-ethyl-4-ethoxy-2-hydroxybenzoate (55), 12-(aminomethyl)-11-hydroxyhexanyl-10-phenylpropanoate (56), and 7-(14-hydroxypropan-13-yl)-8-isobutyl-7, and 8 dihydrobenzo[c]oxepin-1(3H)-one (57) were isolated from a heterotrophic marine bacterium B. amyloliquefaciens. This strain was isolated from the brown seaweed Padina gymnospora collected from the intertidal zone of the Mannar Gulf in Peninsular India [60].
7. Macrolactins
Marine Bacillus species produce abundant polyketide classes of antibiotic agents, such as macrolactins, difficidins, and bacillaenes [13][36][62]. Diffcidin is a highly unsaturated macrocyclic polyene with a 22-membered carbon skeleton and a phosphate moiety, which is rarely found in secondary metabolites of Bacillus species. Bacillaene is a linear structure consisting of a conjugated hexaene. Carbon skeleton of most macrolactins contains three diene groups attached to the carbon backbone of a 24-membered lactone ring [36]. Figure 7 lists the structures of macrolactins produced by marine-derived Bacillus species.
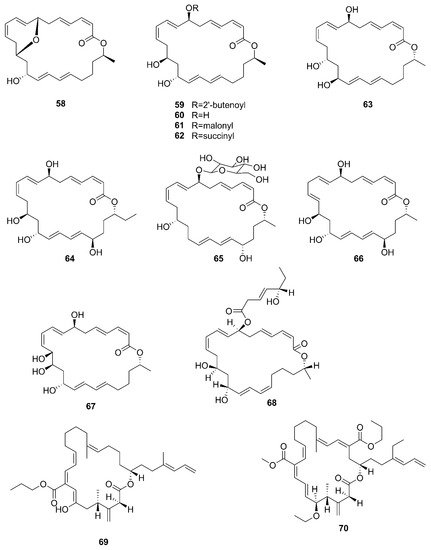
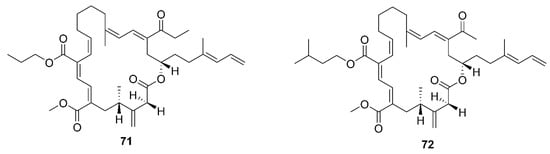
Figure 7. Macrolactins produced by marine-derived Bacillus species.
A new macrolactin derivative, 7,13-epoxyl-macrolactin A (58), along with four known macrolactins, 7-O-2′E-butenoyl macrolactin A (59), Macrolactin A (60), 7-O-malonyl macrolactin A (61), and 7-O-succinyl macrolactin A (62) were isolated from bacteria B. subtilis B5. It is worth emphasizing that this strain was extracted from deep-sea sediments at depths of 3000 m in the Pacific Ocean [63]. Compounds 60 and 62 were also isolated from seaweed-associated B. amyloliquefaciens MTCC 10456 in the MTCC of Chandigarh, India; this is the first report on the co-production and isolation of anti-Malassezia spp. compounds from marine Bacillus species [41]. In particular, the major difference between compounds 58 and 59–62 is in the epoxy ring. Compound 58 displayed a potent inhibitory effect on the expression of interleukin-1β (IL-1β), interleukin-6 (IL-6) and inducible nitric oxide synthase (iNOS), due to the existence of the epoxy ring [63]. Five novel 24-membered macrolactins named bamemacrolactins A (63), B (64), C (65), D (66) and E (67) were produced by B. siamensis, which was isolated from the gorgonian coral Anthogorgia caerulea gathered from Beihai city (Guangxi, China) [64]. The 7-O-methyl-5′-hydroxy-3′-heptenoate−macrolactin (68), a new macrolactin compound, was obtained from B. subtilis MTCC10403 associated with seaweed Anthophycus longifolius collected from the Gulf of Mannar of Peninsular India [65]. Compounds 69–72 were characterized as four homologous difficidin-type 21-membered macrocyclic lactone, isolated from a heterotrophoic B. amyloliquefaciens MTCC12713 associated with an intertidal macroalga Kappaphycus alverezii collected from the Gulf of Mannar in Peninsular India. In addition, they were established as 18,19-dihydro-6-hydroxy-8-propyl carboxylate difficidin, 5-ethoxy-28-methyl-(9-methyl-19propyl dicarboxylate) difficidin, (6-methyl-9-propyl dicarboxylate)-19-propanone difficidin, and 20-acetyl-(6-methyl-9-isopentyl dicarboxylate) difficidin, respectively [13].
8. Other Compounds
Figure 8 lists the other compounds produced by marine-derived Bacillus species. A novel thiopeptide named micrococcin P3 (73) and a known compound named micrococcin P1 (74) were isolated from the fermentation broth of B. stratosphericus [66]. Five new bacillamidins A (75), B (76), C (77), D (78) and E (79), along with two known synthetic analogs, bacillamidins F (80) and G (81), were isolated from the marine-derived B. pumilus strain RJA1515. This strain was extracted from deep-sea sediments at depths of 84 m collected in Bamfield in British Columbia [67]. Ieodoglucomide C (82) and ieodoglycolipid (83), two new glycolipids, were produced by the marine-derived B. licheniformis 09IDYM23 which was isolated from sediments at a depth 20 m collected at Ieodoin the southern reef of the Republic of Korea, both of which were obtained from the fermentation of this strain [68]. According to bioactivity-guided strategy, (-)-sattabacin (84) and (-)-4-hydroxysattabacin (85) were firstly discovered from Bacillus sp. (SCO-147) collected from marine sediments in Suncheon Bay of Korea [69]. Marine-derived B. subtilis AD35, gathered from marine water and sediment at the Alexandria sea shore in Egypt, could yield a previously reported but firstly isolated compound, Di-(2-ethylhexyl) phthalate (DEHP) (86) [70]. Additionally, compound 86 and dibutyl phthalate (DBP) (87) were isolated from the extract broth of marine-derived B. polymyxa L1-9, which was collected in a mud sample from the intertidal mudflat in the Lianyungang Port of China [71].
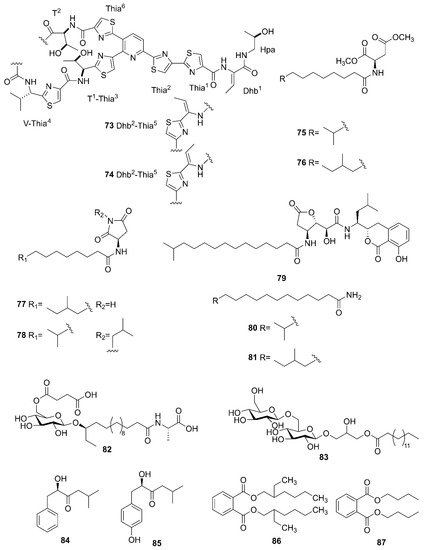
Figure 8. Other compounds produced by marine-derived Bacillus species.
A total of 87 secondary metabolites were reported from marine-derived Bacillus species from January 2014 to December 2021. Their chemical structures were classified into cyclic lipopeptides (1–19, among them, 1–2 surfactins, 3–10 belong to iturins, 11 belongs to plipastatin), diketopiperazines (20–27), linear lipopeptides (28–38), nonribosomal peptides (39–51), polyketides (52–57), macrolactins (58–72, among them, 69–72 belong to difficidins), and other compounds (73–87) according to their putative biogenetic sources. As shown in Figure 9A, 21.84% of the compounds reported were CLPs, and these compounds account for an overwhelming majority of all 87 metabolites, followed by macrolactins with 17.24%. Therefore, CLPs are a class of secondary metabolites with structural diversity and pharmacological perspective.
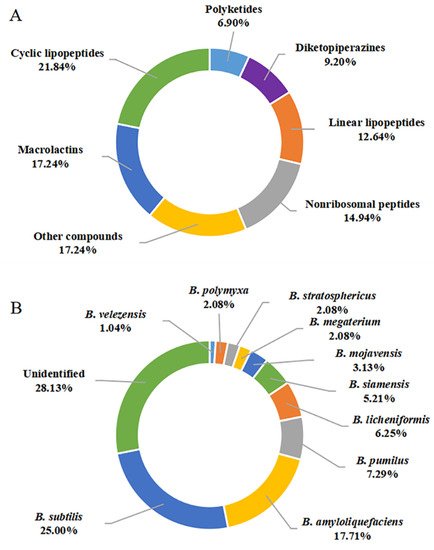
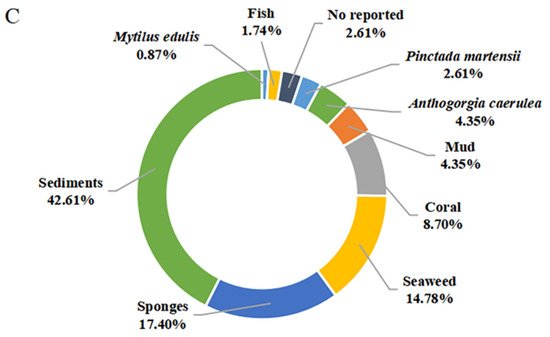
Figure 9. Quantification of studies on secondary metabolites from marine-derived Bacillus: (A) Chemical structures categories; (B) Producing strains; (C) Environment sources.
The genus Bacillus comprises more than 350 species, some of which are used as antifungal agents, while others are promising producers of green pesticide [14]. As discussed above, a total of 10 identified species, including B. subtilis, B. amyloliquefaciens, B. megaterium CGMCC7086, B. mojavensis B0621A, B. licheniformis, B. siamensis, B. stratosphericus, B. pumilus RJA1515, B. polymyxa L1-9, and B. velezensis SH-B74 were reported as the producing strains of these described secondary metabolites. Among them, B. subtilis and B. amyloliquefaciens were the most prolific strains, with 24 (20.00%) and 17 (17.71%) metabolites identified, respectively (Figure 10B). CLPs are ubiquitous in several Bacillus strains. However, linear lipopeptides are found predominantly in B. subtilis species, while the macrolactins are more preponderant in B. amyloliquefaciens species, suggesting the species-specific metabolites.
The secondary metabolites of marine-derived Bacillus species could be isolated from marine sediments, marine invertebrates (sponges, molluscs, and corals), and vertebrates (mainly fishes), as well as marine plants (mainly seaweed). Currently, there are 9 reported sources of Bacillus secondary metabolites. As shown in Figure 9C, a total of 49 compounds {1–7,11,15–18,28–37,39–48,58–62,75–85,86 (B. subtilis AD35)} originated from marine sediments, accounting for 42.61% of the sources of Bacillus species. In particular, the producing strains of 58–62 originated from deep-sea sediment at a depth of 3000 m, while the other strains of 75–83 originated from deep-sea sediment at depths less than 100 m. Moreover, the producing strains of 12–14, 86 (B. polymyxa L1-9), and 87 originated from mud. More precisely, the producing strains of 15–16 and 28–37 originated from the Republic of Korea’s southern reef. Twenty compounds (20–27,39–48,50,51), identified from marine sponges, accounted for 17.40% of the reported environmental sources of Bacillus secondary metabolites. It is worth noting that 20 belonging to diketopiperazine were isolated unprecedentedly from a sponge-associated microbe. Moreover, 45–49, 52–56 and 63–67 were also derived from coral, wherein 63–67, whose producing strains were obtained from the gorgonian coral A. caerulea, accounted for 4.35% of the reported environmental sources of Bacillus secondary metabolites. The 8–10 and 19 that originated from pearl oyster P. martensii and M. edulis, respectively, accounted for 3.48% of the reported ones. A total of 17 secondary metabolites {38,45,47,49,52–57,60,62 (B. amyloliquefaciens MTCC 10456) and 68–72} were identified from Bacillus residing in marine plants, accounted for 14.78% of the reported total amount. Thereinto, the producing strains of 52–57 originated from brown seaweed, while the producing strains of 45,47,49,60,62 (B. amyloliquefaciens MTCC 10456), 38,52–57 and 68–72 were collected from seaweed. In addition to these producing strains, only one Bacillus strain (B. megaterium CGMCC7086), which produced 45 and 49, was obtained from the intestines of marine fish and accounted for 1.74% of the reported total amount. Unfortunately, the environmental sources of the producing strain of 47 (B. subtilis B38), 73 and 74 (B. stratosphericus) were not described. From the above analysis, it can be concluded that marine sediments and sponges are more abundant sources of productive strains of marine-derived Bacillus, and which deserved much more attention in subsequent chemical studies.
References
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69.
- Barzkar, N. Marine Microbial Alkaline Protease: An Efficient and Essential Tool for Various Industrial Applications. Int. J. Biol. Macromol. 2020, 161, 1216–1229.
- Zheng, L.H.; Zhu, X.J.; Yang, K.L.; Zhu, M.H.; Farooqi, A.A.; Kang, D.L.; Sun, M.; Xu, Y.X.; Lin, X.K.; Feng, Y.G.; et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers 2018, 10, 1043.
- De Rop, A.S.; Rombaut, J.; Willems, T.; De Graeve, M.; Vanhaecke, L.; Hulpiau, P.; De Maeseneire, S.L.; De Mol, M.L.; Soetaert, W.K. Novel Alkaloids from Marine Actinobacteria: Discovery and Characterization. Mar. Drugs 2021, 20, 6.
- El-Sersy, N.A.; Abdelwahab, A.E.; Abouelkhiir, S.S.; Abou-Zeid, D.M.; Sabry, S.A. Antibacterial and Anticancer Activity of ε-Poly-L-Lysine (Ε-Pl) Produced by a Marine Bacillus subtilis sp. J. Basic Microbiol. 2012, 52, 513–522.
- Habbu, P.; Warad, V.; Shastri, R.; Madagundi, S.; Kulkarni, V.H. Antimicrobial Metabolites from Marine Microorganisms. Chin. J. Nat. Med. 2016, 14, 101–116.
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231.
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal Potential of Marine Natural Products. Eur. J. Med. Chem. 2017, 126, 631–651.
- Sharma, D.; Singh, S.S.; Baindara, P.; Sharma, S.; Khatri, N.; Grover, V.; Patil, P.B.; Korpole, S. Surfactin Like Broad Spectrum Antimicrobial Lipopeptide Co-Produced with Sublancin from Bacillus Subtilis Strain A52: Dual Reservoir of Bioactives. Front. Microbiol. 2020, 11, 1167.
- Mawlankar, R.; Thorat, M.N.; Krishnamurthi, S.; Dastager, S.G. Bacillus cellulasensis sp. nov., Isolated from Marine Sediment. Arch. Microbiol. 2016, 198, 83–89.
- Liu, R.; Huang, Z.B.; Dong, C.M.; Shao, Z.Z. Lottiidibacillus patelloidae Gen. Nov., Sp. Nov., Isolated from the Intestinal Tract of a Marine Limpet and Reclassification of Bacillus taeanensis as Maribacillus taeanensis Gen. Nov., Comb. Nov. Antonie Leeuwenhoek 2019, 112, 797–807.
- Tareq, F.S.; Shin, H.J. Bacilotetrins A and B, Anti-Staphylococcal Cyclic-Lipotetrapeptides from a Marine-Derived Bacillus subtilis. J. Nat. Prod. 2017, 80, 2889–2892.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Dhara, S. Difficidin Class of Polyketide Antibiotics from Marine Macroalga-Associated Bacillus as Promising Antibacterial Agents. Appl. Microbiol. Biotechnol. 2021, 105, 6395–6408.
- Harwood, C.R.; Mouillon, J.M.; Pohl, S.; Arnau, J. Secondary Metabolite Production and the Safety of Industrially Important members of the Bacillus subtilis group. FEMS. Microbiol. Rev. 2018, 42, 721–738.
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green Chem. Lett. Rev. 2021, 14, 245–270.
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A Valuable Member of Bioactive Molecules Within Plant Microbiomes. Molecules 2019, 24, 1046.
- Bibi, F.; Naseer, M.I.; Azhar, E.I. Assessing the Diversity of Bacterial Communities from Marine Sponges and Their Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 2747–2754.
- Zhang, L.L.; Sun, C.M. Fengycins, Cyclic Lipopeptides from Marine Bacillus subtilis strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing Reactive Oxygen Species Production and Chromatin Condensation. Appl. Environ. Microbiol. 2018, 84, e00418–e00445.
- Kizhakkekalam, V.K.; Chakraborty, K.; Joy, M. Oxygenated Elansolid Type of Polyketide Spanned Macrolides from a Marine Heterotrophic Bacillus as Prospective Antimicrobial Agents Against Multidrug Resistant Pathogens. Int. J. Antimicrob. Agents 2020, 55, 105892.
- Karthik, L.; Sun, W.; Wang, Y.K.; Mulati, N.; Gong, S.Q.; Zhang, F.L.; Li, Z.Y.; Li, Y.X. Biosynthesis In Vitro of Bacillamide Intermediate-Heterocyclic Alacysthiazole by Heterologous Expression of Nonribosomal Peptide Synthetase (NRPS). J. Biotechnol. 2019, 292, 5–11.
- Yi, X.X.; Gan, Y.M.; Jiang, L.; Yu, L.; Liu, Y.H.; Gao, C.H. Rapid Improvement in the Macrolactins Production of Bacillus Sp. Combining Atmospheric Room Temperature Plasma with the Specific Growth Rate Index. J. Biosci. Bioeng. 2020, 130, 48–53.
- Ma, Z.; Hu, J.; Wang, X.; Wang, S. NMR Spectroscopic And MS/MS Spectrometric Characterization of a New Lipopeptide Antibiotic Bacillopeptin B1 Produced by a Marine Sediment-Derived Bacillus amyloliquefaciens SH-B74. J. Antibiot. 2014, 67, 175–178.
- Ma, Y.X.; Kong, Q.; Qin, C.; Chen, Y.L.; Chen, Y.J.; Lv, R.H.; Zhou, G.H. Identification of Lipopeptides in Bacillus megaterium by Two-Step Ultrafiltration and LC-ESI-MS/MS. AMB Express 2016, 6, 79.
- Wu, Q.H.; Throckmorton, K.; Maity, M.; Chevrette, M.G.; Braun, D.R.; Rajski, S.R.; Currie, C.R.; Thomas, M.G.; Bugni, T.S. Bacillibactins E and F from a Marine Sponge-Associated Bacillus sp. J. Nat. Prod. 2021, 84, 136–141.
- Shin, D.; Byun, W.S.; Moon, K.; Kwon, Y.; Bae, M.; Um, S.; Lee, S.K.; Oh, D.C. Coculture of Marine Streptomyces sp. With Bacillus sp. Produces a New Piperazic Acid-Bearing Cyclic Peptide. Front. Chem. 2018, 6, 498.
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319.
- Fu, X.T.; Gong, L.F.; Liu, Y.; Lai, Q.L.; Li, G.Y.; Shao, Z.Z. Bacillus pumilus Group Comparative Genomics: Toward Pangenome Features, Diversity, and Marine Environmental Adaptation. Front. Microbiol. 2021, 2, 571212.
- Subramenium, G.A.; Swetha, T.K.; Iyer, P.M.; Balamurugan, K.; Pandian, S.K. 5-Hydroxymethyl-2-Furaldehyde from Marine Bacterium Bacillus Subtilis Inhibits Biofilm and Virulence of Candida albicans. Microbiol. Res. 2018, 207, 19–32.
- Yuan, Y.H.; Yu, Q.H.; Yang, S.; Wen, J.; Guo, Z.H.; Wang, X.L.; Wang, N. Ultrafast Recovery of Uranium from Seawater by Bacillus velezensis Strain UUS-1 with Innate Anti-Biofouling Activity. Adv. Sci. (Weinh.) 2019, 6, 1900961.
- Ibrahim, A.Y.; Youness, E.R.; Mahmoud, M.G.; Asker, M.S.; El-Newary, S.A. Acidic Exopolysaccharide Produced from Marine Bacillus Amyloliquefaciens 3MS 2017 For the Protection and Treatment of Breast Cancer. Breast Cancer 2020, 14, 1178223420902075.
- Zhou, H.; Cong, B.L.; Tian, Y.Q.; He, Y.; Yang, H.H. Characterization of Novel Cyclic Lipopeptides Produced by Bacillus Sp. SY27F. Process Biochem. 2019, 83, 206–213.
- Dan, A.K.; Manna, A.; Ghosh, S.; Sikdar, S.; Sahu, R.; Parhi, P.K.; Parida, S. Molecular Mechanisms of the Lipopeptides from Bacillus subtilis in the Apoptosis of Cancer Cells-A Review on Its Current Status in Different Cancer Cell Lines. Adv. Cancer Biol. Met. 2021, 3, 100019.
- Routhu, S.R.; Nagarjuna Chary, R.; Shaik, A.B.; Prabhakar, S.; Kumar, C.G.; Kamal, A. Induction of Apoptosis in Lung Carcinoma Cells by Antiproliferative Cyclic Lipopeptides from Marine Algicolous Isolate Bacillus atrophaeus Strain AKLSR1. Process Biochem. 2019, 79, 142–154.
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological Control of Plant Pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55.
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Gageostatins A–C, Antimicrobial Linear Lipopeptides from a Marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885.
- Aleti, G.; Sessitsch, A.; Brader, G. Genome mining: Prediction of Lipopeptides and Polyketides from Bacillus and Related Firmicutes. Comput. Struct. Biotechnol. J. 2015, 13, 192–203.
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth-Promoting Bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014.
- Chen, X.H.; Scholz, R.; Borriss, M.; Junge, H.; Mögel, G.; Kunz, S.; Borriss, R. Difficidin and Bacilysin Produced by Plant-Associated Bacillus amyloliquefaciens Are Efficient in Controlling Fire Blight Disease. J. Biotechnol. 2009, 140, 38–44.
- Mi, Y.; Zhang, J.R.; He, S.; Yan, X.J. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Mar. Drugs 2017, 15, 132.
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2007, 16, 115–125.
- Vairagkar, U.; Mirza, Y. Antagonistic Activity of Antimicrobial Metabolites Produced from Seaweed-Associated Bacillus amyloliquefaciens MTCC 10456 Against Malassezia Spp. Probiotics Antimicrob. Proteins 2021, 13, 1228–1237.
- Nair, D.; Vanuopadath, M.; Nair, B.G.; Pai, J.G.; Nair, S.S. Identification and Characterization of a Library of Surfactins and Fengycins from a Marine Endophytic Bacillus Sp. J. Basic Microbiol. 2016, 56, 1159–1172.
- Ma, Z.W.; Zhang, S.Y.; Zhang, S.H.; Wu, G.Y.; Shao, Y.; Mi, Q.F.; Liang, J.Y.; Hu, J.C. Isolation and Characterization of a New yclic Lipopeptide Surfactin from a Marine-Derived Bacillus velezensis SH-B74. J. Antibiot. 2020, 73, 863–867.
- Chen, Y.L.; Liu, S.L.A.; Mou, H.J.; Ma, Y.X.; Li, M.; Hu, X.K. Characterization of Lipopeptide Biosurfactants Produced by Bacillus licheniformis MB01 from Marine Sediments. Front. Microbiol. 2017, 8, 871.
- Ma, Z.W.; Hu, J.C. Production and Characterization of Iturinic Lipopeptides as Antifungal Agents and Biosurfactants Produced by a Marine Pinctada martensii-Derived Bacillus mojavensis B0621A. Appl. Biochem. Biotechnol. 2014, 173, 705–715.
- Ma, Z.W.; Hu, J.C. Plipastatin A1 Produced by a Marine Sediment-Derived Bacillus amyloliquefaciens SH-B74 Contributes to the Control of Gray Mold Disease in Tomato. 3 Biotech 2018, 8, 125.
- Tareq, F.S.; Hasan, C.M.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Surovy, M.Z.; Islam, M.T.; Shin, H.J. Gageopeptins A and B, New Inhibitors of Zoospore Motility of the Phytopathogen Phytophthora Capsici from a Marine-Derived Bacterium Bacillus Sp. 109GGC020. Bioorg. Med. Chem. Lett. 2015, 25, 3325–3329.
- Wiese, J.; Abdelmohsen, U.R.; Motiei, A.; Humeida, U.H.; Imhoff, J.F. Bacicyclin, a New Antibacterial Cyclic Hexapeptide from Bacillus Sp. Strain BC028 Isolated from Mytilus edulis. Bioorg. Med. Chem. Lett. 2018, 28, 558–561.
- Li, W.L.; Xia, J. Recent Advances in Diketopiperazines Biosynthesis. Microbiol. China 2012, 41, 111–121.
- Bhattacharya, D.; Lai, T.K.; Saha, A.; Selvin, J.; Mukherjee, J. Structural Elucidation and Antimicrobial Activity of a Diketopiperazine Isolated from A Bacillus Sp. Associated with the Marine Sponge Spongia officinalis. Nat. Prod. Res. 2021, 35, 2315–2323.
- Wu, S.; Yu, S.Y.; Jiang, W.; Zhang, L.K.; Jin, C.L.; Zhou, X.J. Isolation and Identification of Antidiatom Attachment Active Constituents of a Strain of Epiphytic Bacillus Sponge. Mar. Sci. 2016, 40, 23–32.
- Mohan, G.; Thipparamalai Thangappanpillai, A.K.; Ramasamy, B. Antimicrobial Activities of Secondary Metabolites and Phylogenetic Study of Sponge Endosymbiotic Bacteria, Bacillus Sp. at Agatti Island, Lakshadweep Archipelago. Biotechnol. Rep. 2016, 11, 44–52.
- Yan, Y.H.; Li, Y.Z.; Zhang, Z.W.; Wang, X.H.; Niu, Y.Z.; Zhang, S.H.; Xu, W.L.; Ren, C.G. Advances of peptides for antibacterial applications. Colloid. Surf. B 2021, 202, 111682.
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Gageotetrins A–C, Noncytotoxic Antimicrobial Linear Lipopeptides from a Marine Bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931.
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Non-Cytotoxic Antifungal Agents: Isolation and Structures of Gageopeptides A–D from a Bacillus Strain 109GGC020. J. Agric. Food Chem. 2014, 62, 5565–5572.
- Gulick, A.M. Nonribosomal Peptide Synthetase Biosynthetic Clusters of ESKAPE Pathogens. Nat. Prod. Rep. 2017, 34, 981–1009.
- Zhou, M.J.; Liu, F.W.; Yang, X.Y.; Jin, J.; Dong, X.; Zeng, K.-W.; Liu, D.; Zhang, Y.T.; Ma, M.; Yang, D.H. Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus Sp. PKU-MA00093 and PKU-MA00092. Mar. Drugs 2018, 16, 22.
- Tabbene, O.; Azaiez, S.; Di Grazia, A.; Karkouch, I.; Ben Slimene, I.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F.; et al. Bacillomycin D And Its Combination with Amphotericin B: Promising Antifungal Compounds with Powerful Antibiofilm Activity and Wound-Healing Potency. J. Appl. Microbiol. 2016, 120, 289–300.
- Miyanaga, A. Structure and Function of Polyketide Biosynthetic Enzymes: Various Strategies for Production of Structurally Diverse Polyketides. Biosci. Biotechnol. Biochem. 2017, 81, 2227–2236.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Previously Undescribed Antibacterial Polyketides from Heterotrophic Bacillus amyloliquefaciens Associated with Seaweed Padina gymnospora. Appl. Microbiol. Biotechnol. 2018, 184, 716–732.
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-Heterocyclic Derivatives with Antibacterial Properties from Marine Bacterium Bacillus Subtilis Associated with Seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583.
- Mondol, M.A.; Shahidullah Tareq, F.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, J.S.; Lee, Y.-J.; Shin, H.J. New Antimicrobial Compounds from a Marine-Derived Bacillus sp. J. Antibiot. 2013, 66, 89–95.
- Yan, X.; Zhou, Y.-X.; Tang, X.-X.; Liu, X.-X.; Yi, Z.-W.; Fang, M.-J.; Wu, Z.; Jiang, F.-Q.; Qiu, Y.-K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Mar. Drugs 2016, 14, 195.
- Gao, C.H.; Chen, X.Q.; Yu, L.; Jiang, L.; Pan, D.J.; Jiang, S.; Gan, Y.M.; Liu, Y.H.; Yi, X.X. New 24-Membered Macrolactins Isolated from Marine Bacteria Bacillus siamensis as Potent Fungal Inhibitors against Sugarcane Smut. J. Agric. Food Chem. 2021, 69, 4392–4401.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide Family of Novel Antibacterial 7-O-Methyl-5’-Hydroxy-3’-Heptenoate-Macrolactin from Seaweed-Associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208.
- Wang, W.; Park, K.-H.; Lee, J.; Oh, E.; Park, C.; Kang, E.; Lee, J.; Kang, H. A New Thiopeptide Antibiotic, Micrococcin P3, from a Marine-Derived Strain of the Bacterium Bacillus stratosphericus. Molecules 2020, 25, 4383.
- Zhou, S.-Y.; Hu, Y.-J.; Meng, F.-C.; Qu, S.-Y.; Wang, R.; Andersen, R.J.; Liao, Z.-H.; Chen, M. Bacillamidins A-G from a Marine-Derived Bacillus pumilus. Mar. Drugs 2018, 16, 326.
- Tareq, F.S.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519.
- Kim, K.; Leutou, A.S.; Jeong, H.; Kim, D.; Seong, C.N.; Nam, S.-J.; Lim, K.-M. Anti-Pigmentary Effect of (-)-4-Hydroxysattabacin from the Marine-Derived Bacterium Bacillus Sp. Mar. Drugs 2017, 15, 138.
- Lotfya, W.A.; Mostafab, S.W.; Adel, A.A.; Ghanem, K.M. Production of Di-(2-Ethylhexyl) Phthalate by Bacillus subtilis AD35: Isolation, Purifification, Characterization and Biological Activities. Microb. Pathog. 2018, 124, 89–100.
- Ma, G.-Z.; Fu, H.-R.; Wu, S.-J.; Bao, Z.-H.; Wang, S.-F.; Ge, P.-H. Isolation and Structure Elucidation of Antifungal Metabolites from marine Paenibacillus polymyxa Strain L1–9. Acta Pharmacol. Sin. 2014, 44, 486–496.
More
Information
Subjects:
Psychology, Biological
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
819
Revisions:
2 times
(View History)
Update Date:
08 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




