Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephanie Duguez | -- | 2084 | 2022-09-13 14:58:11 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2084 | 2022-10-04 15:55:11 | | | | |
| 3 | Beatrix Zheng | + 10 word(s) | 2094 | 2022-10-04 15:57:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mccluskey, G.; Donaghy, C.; Morrison, K.E.; Mcconville, J.; Duddy, W.; Duguez, S. The Role of Sphingomyelin in Motor Neuron Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/28185 (accessed on 07 February 2026).
Mccluskey G, Donaghy C, Morrison KE, Mcconville J, Duddy W, Duguez S. The Role of Sphingomyelin in Motor Neuron Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/28185. Accessed February 07, 2026.
Mccluskey, Gavin, Colette Donaghy, Karen E. Morrison, John Mcconville, William Duddy, Stephanie Duguez. "The Role of Sphingomyelin in Motor Neuron Diseases" Encyclopedia, https://encyclopedia.pub/entry/28185 (accessed February 07, 2026).
Mccluskey, G., Donaghy, C., Morrison, K.E., Mcconville, J., Duddy, W., & Duguez, S. (2022, September 30). The Role of Sphingomyelin in Motor Neuron Diseases. In Encyclopedia. https://encyclopedia.pub/entry/28185
Mccluskey, Gavin, et al. "The Role of Sphingomyelin in Motor Neuron Diseases." Encyclopedia. Web. 30 September, 2022.
Copy Citation
Amyotrophic Lateral Sclerosis (ALS), Spinal Bulbar Muscular Atrophy (SBMA), and Spinal Muscular Atrophy (SMA) are motor neuron diseases (MNDs) characterised by progressive motor neuron degeneration, weakness and muscular atrophy. Lipid dysregulation is well recognised in each of these conditions and occurs prior to neurodegeneration. Several lipid markers have been shown to predict prognosis in ALS. Sphingolipids are complex lipids enriched in the central nervous system and are integral to key cellular functions including membrane stability and signalling pathways, as well as being mediators of neuroinflammation and neurodegeneration.
sphingomyelin
ceramide
Amyotrophic Lateral Sclerosis
sphingolipid
motor neuron disease
1. Sphingolipid Synthesis
Sphingolipids (SLs) are a diverse class of lipids with eighteen carbon amino-alcohol backbones, which are synthesized in the ER from non-sphingolipid precursors [1]. They play significant roles in membrane structure and have many bioactive metabolites, which regulate cellular function [1][2]. The basic structure of SLs is ceramide. Ceramide consists of a sphingoid long-chain base and a fatty acid acyl chain connected to an amine bond [3]. The most common mammalian long-chain base is sphingosine (d18:1), an 18 Carbon chain with a trans double bond at positions 4–5 [4]. The structure of sphingosine, ceramide and SM are shown in Figure 1.
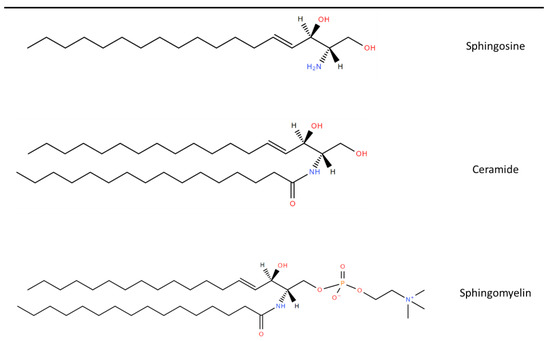
Figure 1. Chemical structure of common Sphingolipids. Sphingosine is the most common long-chain base. A fatty acid acyl chain is connected to the C2 amide group to form Ceramide and then Sphingomyelin is formed by the subsequent addition of a phosphocholine head group. Lipid structures created using LIPID MAPS® tools [5].
2. Role of Sphingolipids in MNDs
Given the roles of SLs in many vital biological processes and their high abundance in the central nervous system as major components of oligodendrocytes and myelin sheaths, SL metabolism is thought to be a key pathway in neurodegeneration and neuroinflammation [6]. Alteration in SL metabolism has been linked to multiple neurodegenerative diseases such as Alzheimer’s Disease and Parkinson’s Disease, as well as neuroinflammatory conditions such as Multiple Sclerosis. These are discussed in detail in other reviews [7][8][9].
Increased levels of SM and ceramide have been found in spinal cord tissue of patients with Amyotrophic Lateral Sclerosis (ALS) and SOD1 mice [10]. A study in the wobbler mouse, which is a model of motor neuron degeneration, identified the mis-sorting of lysosomal SL degradation enzymes with a resultant increase in SL intermediates [11]. Lipid dysregulation in ALS can occur decades before classical symptoms, and lipid biomarkers can be used to identify individuals at risk of developing ALS [12][13]. In keeping with this, increased levels of SLs were identified in spinal cords of ALS mice prior to the onset of clinical signs, and SM was demonstrated to mediate motor neuron death via oxidative stress [10]. A transcriptomic meta-analysis study on spinal cord tissue from SOD1 mice found that cholesterol, ceramides and eicosanoid pathways were altered early in the disease course [14]. This has also been shown in human studies, with SL alteration identified in plasma samples of patients who subsequently developed ALS [15]. Importantly, these studies suggest that alterations in SL occur before motor neuron degeneration and are therefore an upstream process in ALS pathophysiology.
Over 20 risk genes in ALS are involved in lipid raft homeostasis and ceramide metabolic pathways [16]. Mutations or abnormal DNA methylation have been found in genes encoding for enzymes necessary for SL synthesis in patients with ALS and Spinal Muscular Atrophy (SMA), as well as bovine SMA. These are shown in Table 1. In addition, mutations in ASAH1, which result in dysfunctional acid ceramidase, cause a non-5q form of SMA associated with progressive myoclonic epilepsy [17]. Mutations in the SPTLC1 gene are associated with juvenile ALS and Hereditary Sensory and Autonomic Neuropathy type 1 (HSAN1) [18][19]. This gene encodes for a subunit of SPT, the enzyme required for the first step of SL synthesis. C-terminal SPTLC1 variants cause the formation of atypical deoxysphingolipids and result in HSAN1 [20]. The ALS-causing variants map to a transmembrane domain, which interacts with negative regulators of SPT activity and results in unregulated SPT and excess SL synthesis [21]. Epigenomic studies have also shown abnormal DNA methylation in SGMS2, which encodes for SMS2, the enzyme for converting ceramide to SM [22]. The CAV1 gene, which encodes for calveolin 1, has also recently been identified as a risk modifying gene in ALS. Calveolin 1 is found in lipid rafts and ALS variants in CAV1 were shown to disrupt lipid raft formation in patient-derived lymphoblastoid cells [23].
Table 1. Abnormalities of Sphingolipid metabolism in Motor Neuron Diseases and sphingolipidoses.
| Condition | Gene | Affected Enzyme/Protein | Effect on Sphingolipids |
|---|---|---|---|
| Sphingolipid synthesis | |||
| Juvenile ALS [18] HSAN1 [19] |
SPTLC1 | SPT | Atypical deoxysphingolipids, cannot be converted into complex SLs or degraded |
| Bovine SMA [24] | FVT1 | KSR | Reduced ceramide synthesis from de novo pathway |
| ALS type 8 [25] Late onset SMA [26] |
VAPB | VAPB with effect on CERT and FAPP2 | Impaired transfer of ceramide and glucosylceramide from ER to golgi apparatus |
| ALS [22] | SGMS2 | SMS2 | Affects sphingomyelin synthesis |
| Sphingolipid degradation | |||
| SMA-PME [17] Farber’s disease [27] |
ASAH1 | Acid ceramidase | Ceramide accumulation |
| GM1 gangliodosis [28] | GLB1 | β-Galactosidase | GM1 ganglioside accumulation |
GM2 gangliodoses [28]
|
HEXA HEXB |
|
GM2 ganglioside accumulation GM2 ganglioside, glycolipid GA2 and globoside accumulation |
| Fabry’s Disease [29] | GLA | α-Galactosidase A | Globotriaosylceramide accumulation |
| Metachromatic Leukodystrophy [30] | ARSA | Arylsulphatase A | Sulfatides accumulation |
Niemann-Pick Disease [31]
|
SMPD1 NPC1/NPC2 |
Sphingomyelinase | Sphingomyelin accumulation |
| Gaucher’s Disease [32] | GBA | Glucocerebrosidase | Glucosylceramide accumulation |
| Krabbe’s Disease [33] | GALC | Galactosylceramidase | Galactosylceramide accumulation |
HSAN1—hereditary sensory and autonomic neuropathy type 1, SPT—Serine palmitoyltransferase, VAPB—Vesicle associated membrane protein B, CERT—ceramide transfer protein, FAPP2—four phosphate adapter protein 2, SMS2—sphingomyelin synthase 2, SMA-PME—spinal muscular atrophy and progressive myoclonic epilepsy.
Another mechanism of how SLs can affect MNDs is through intercellular communication. Neutral SMase2 affects EV secretion. This has been demonstrated by studies showing that stimulation of SMase2 with TNF alpha increases EV secretion and inhibiting it with 1 PDDC reduces EV secretion [34][35]. EVs are being increasingly investigated in ALS as mediators of intercellular transfer of neurotoxic proteins such as TDP 43, FUS and SOD1 [36][37]. EVs secreted by muscle cells from ALS patients have been shown to be toxic to motor neurons [38].
Further insight into the importance of SL metabolism in neurodegenerative diseases is evident from lysosomal storage disorders. These are a group of over 40 conditions with a combined prevalence of 1 in 7000–8000 live births [39]. These diseases all are the result of impaired lysosomal degradation of various metabolites and the consequent effects on cellular function [40]. Several involve the degradation of SLs and are termed sphingolipidoses. These are a group of autosomal recessive or X-linked conditions with defects in enzymes required for the catabolism of SLs [41]. The cellular impact of the conditions depends on the concentration of the relevant SL and the degree of enzymatic deficiency. The sphingolipidoses and their enzymatic defects and effects on SLs are shown in Table 1. They each have a broad and unique clinical phenotype. However, given that SLs are enriched in the nervous system, these conditions often have the predominant feature of severe progressive neurodegeneration [41][42].
3. Lipidomic Studies in MNDs
The lipid profiles in MNDs have been mainly assessed via metabolomic analysis. Table 2 lists all of the published metabolomic studies that have included lipidomic analysis to date, detailing a range of different SM and ceramides identified. This may in part be explained by the different samples studied and the differing mass spectrometry methodologies for quantifying metabolites. Two studies were performed using spinal cord tissue, nine using plasma, two using serum, and two using CSF samples. Of the 12 studies comparing ALS to controls, all identified changes in SM concentrations, with SM species being increased in 11 studies and decreased in the other. Six studies identified increases in ceramide species, with decreases in some ceramides reported in one of these. A study of only of ALS patients found that multiple SMs were able to predict markers of disease progression such as the ALSFRS-R, manual muscle testing and respiratory function [43]. Another metabolomic study in 28 patients with ALS and 30 controls reported that out of 317 metabolites, 50 were increased and 70 decreased in ALS, although the individual metabolites were not listed [44].
Table 2. Metabolomic studies in patients with ALS showing the changes in lipid metabolites.
| Study | Patients | Sample Type | Quantification Platform | Metabolites Evaluated | Lipid Changes in MND | Prognostic Use |
|---|---|---|---|---|---|---|
| Blasco et al. 2017 [45] | 40 ALS 45 Controls |
CSF | HRMS | 122 lipids | ↑: PC (36:4p), PC (36:4e), SM (d43:2), SM (d34:0) | Higher SM (d43:2) and lower TG (16:0/16:0/18:1) and TG (18:0/16:0/18:1) had slower progression |
| ↓: TG (16:1/18:1/18:2) | ||||||
| Lawton et al. 2012 [46] | 161 ALS 117 Controls |
Plasma | GC/MS and UPLC-MS/MS | 335 lipids, proteins and carbohydrates | ↑: LPC (16:1) and SM (18:0) | Not evaluated |
| Cutler et al. 2002 [10] | 9 ALS 3 Control |
Spinal cord | ES/MS/MS | Sphingolipids, Phospholipids, Cholesterol Esters, and Lipid Peroxides |
↑: Cer (C16:0), Cer (C24:0), SM (C16:0), CE (C16:0) and CE (C18:0) | Not evaluated |
| Goutman et al. 2020 [47] | 125 ALS 71 Controls |
Plasma | UPLC-MS/MS | 899 metabolites | ↑: 8 Cers, 28 DAGs, 5 HEXC, 24 SMs, | Not evaluated |
| ↓: 5 DAGs, 5 SMs | ||||||
| Goutman et al. 2022 [48] | Above cohort of 125 ALS and 71 controls with 2nd cohort 225 ALS, 104 controls | Plasma | UPLC-MS/MS | 640 metabolites | SM most significant sub-pathway LCFA, acyl intermediates and Cers also raised |
SM (d18:1/24:0), SM (d18:1/20:0, d16:1/22:0), SM (d18:1/14:0, d16:1/16:0) and lignoceroylcarnitine (C24) correlated with ALSFRS-R |
| Bjornevik et al. 2019 [15] | 275 ALS 549 Controls |
Plasma | LC/MS | 404 metabolites | ↑: SM (C18:2), PC (C40:7), PC (C38:4), CE (C22:4) | Not evaluated |
| ↓: 12 TAGs, DAG (C36:1), DAG (C36:2), PC (C36:2), 21-deoxycortisol, butyrobetaine |
||||||
| Lawton et al. 2014 [49] | 172 ALS 73 neurological mimics 50 Controls |
plasma | GC/MS and UPLC-MS/MS | 367 metabolites | ↑: SM (d18:1/16:0), 5 FAs, 3-dehydrocarnitine, 1,2-propanediol, Chol, 1-stearoyl-GPI |
1,2-propanediol correlated with ALSFRS-R |
| Chang et al. 2021 [50] | 36 ALS 36 Controls |
plasma | LC–MS/MS | 185 metabolites | ↑: SM (C24:1), SM (C20:2), PC (C44:5), PC (C34:2) | 14 PCs and (OH) SM(C22:1) correlated with ALSFRS-R |
| ↓: (OH) SM(C22:1) (OH) SM(C24:1) 29 other PCs |
||||||
| Fernandez-Eulate et al. 2020 [51] | 20 ALS 20 Controls |
Serum | UPLC-MS | 416 lipids | ↑: SM (39:1), SM (33:1), PE (P-20:1/0:0), PE (O-16:0/0:0), 5 PCs, androsterone, etiocholanolone and 2 FAs |
Not evaluated |
| Blasco et al. 2018 [43] | 74 ALS | Plasma | HPLC-MS/MS | 188 metabolites | Not evaluated—no control participants | SM (C22:3) and SM (C34:1) correlated with disease progression, SM (24:1), SM (C16:1) and (OH) SM (C22:2) correlated with SVC |
| Dodge et al. 2015 [52] | 6 ALS 6 Control |
Spinal cord | LC-MS/MS | Cer, SM and GSLs | ↑: Cer (C18:0), Cer (C24:1), (OH) Cer (C24:0), Cerebroside (C18:0 and C24:1), GlcCer (C18:0 and C24:1), LacCer (18:0), GL3 (C22:1), GM3 (C23:0), GM1 (C18:0) AND SM (C18:0) | Not evaluated |
| Sol et al. 2021 [53] | 23 ALS 10 Controls |
CSF Plasma |
LC-MS/MS | 1018 lipids in plasma and 843 in CSF | ↑: 3 Fas, 2 DAGs, 13 TGs, 17 GPLs, 3 Cer, 1 SM | Fast vs. slow progressors had increased- 1 FA, 4 GLs, 4 GPLs, 2 Cer, 1 GM3, and decreased- 46 GLs, 36 GPLs, 2 Cer, 8 SM, 5 CE |
| ↓: 2 DAGs, 4 GPLs, 3 Cer, 3 GLs | ||||||
| Area-Gomez et al. 2021 [54] | 40 ALS 28 PLS 28 Control |
Serum/Plasma | LC/MS | 532 lipids | ↑: Cer, LacCer, CE | SM declined and Cer increased at follow up |
| ↓: SM, PC, PS |
HRMS—high-resolution mass spectrometry, GC/MS—gas chromatography/mass spectrometry, LC/MS—liquid chromatography/mass spectrometry, LC–MS/MS—liquid chromatography/tandem mass spectrometry, UPLC-MS/MS—ultra-high-performance liquid chromatography/tandem mass spectrometry, ES/MS/MS—electrospray ionization tandem mass spectrometry, CSF—cerebrospinal fluid, ALSFRS-R—Revised ALS Functional Rating Scale, SVC—slow vital capacity, SM—sphingomyelin, TG—triglyceride, LPC—palmitoleoyl-glycerophosphocholine, Cer—ceramide, CE—cholesterol ester, DAG-Diacylglycerol, HEXC—hexosylceramide, LCFA—long chain fatty acid, TAG—Triacylglycerol, PC—phosphatidylcholine, FA—fatty acids, GPI—glycophosphatidylinositol, (OH)SM—hydroxysphingomyelin, PE—phosphatidylethanolamines, PS—phosphatidylserines, GPL—glycerophospholipids, GL—glycerolipid.
One study identified four lipids, including SM C18:2, which were elevated several years before symptom onset [15]. This is of particular relevance with the progress in developing genotype-specific treatments, such as antisense oligonucleotides (ASOs) for patients with SOD1 and C9orf72 mutations and the need for biomarkers to guide the optimal timing for commencing treatment [55][56][57]. The ATLAS trial is currently evaluating Tofersen, an ASO for SOD1, in presymptomatic patients who develop raised neurofilament light chain levels, a marker of neuronal damage that becomes elevated 6–12 months prior to symptoms [58]. Given that lipids including SM and ceramide are altered early in the disease course [10][13], they could be of use in identifying presymptomatic patients for potential treatments. In addition, Blasco et al. have shown how SL biomarkers could be incorporated into pharmaco-metabolomic studies [43]. Baseline and follow-up SL profiles could be used to (1) further validate their use as prognostic markers compared to common clinical measurements of disease progression (such as lung function and ALSFRS-R) and (2) determine if treatments lead to alterations in metabolite levels.
There are little data on the lipidomic profile of other MNDs. There have been no lipidomic studies in Spinal Bulbar Muscular Atrophy (SBMA). SBMA and SMA patients were included as neurological mimics in one study but were combined as part of a group containing other conditions such as cervical myelopathy and multiple sclerosis [49]. In a metabolomic study of patients with SMA, H-nuclear magnetic resonance-based metabolic profiling demonstrated diagnostic and prognostic utility, but individual metabolites were not listed [59]. Another metabolomic study in 108 patients with SMA showed 200 metabolites correlating with the modified Hammersmith functional motor scale, including 12 lipids. Only 1 lipid (SM (C24:1)) was among the top 20 metabolites identified [60].
References
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23.
- Verderio, C.; Gabrielli, M.; Giussani, P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J. Lipid Res. 2018, 59, 1325–1340.
- Pant, D.C.; Aguilera-Albesa, S.; Pujol, A. Ceramide signalling in inherited and multifactorial brain metabolic diseases. Neurobiol. Dis. 2020, 143, 105014.
- Fanani, M.L.; Maggio, B. The many faces (and phases) of ceramide and sphingomyelin I—Single lipids. Biophys Rev. 2017, 9, 589–600.
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, 606.
- Ayub, M.; Jin, H.K.; Bae, J.S. Novelty of Sphingolipids in the Central Nervous System Physiology and Disease: Focusing on the Sphingolipid Hypothesis of Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7353.
- Alessenko, A.V.; Albi, E. Exploring Sphingolipid Implications in Neurodegeneration. Front. Neurol. 2020, 11, 437.
- Signorelli, P.; Conte, C.; Albi, E. The Multiple Roles of Sphingomyelin in Parkinson’s Disease. Biomolecules 2021, 11, 1311.
- Podbielska, M.; Ariga, T.; Pokryszko-Dragan, A. Sphingolipid Players in Multiple Sclerosis: Their Influence on the Initiation and Course of the Disease. Int. J. Mol. Sci. 2022, 23, 5330.
- Cutler, R.G.; Pedersen, W.A.; Camandola, S.; Rothstein, J.D.; Mattson, M.P. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann. Neurol. 2002, 52, 448–457.
- Petit, C.S.; Lee, J.J.; Boland, S.; Swarup, S.; Christiano, R.; Lai, Z.W.; Mejhert, N.; Elliott, S.D.; McFall, D.; Haque, S.; et al. Inhibition of sphingolipid synthesis improves outcomes and survival in GARP mutant wobbler mice, a model of motor neuron degeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 10565.
- Thompson, A.G.; Talbot, K.; Turner, M.R. Higher blood high density lipoprotein and apolipoprotein A1 levels are associated with reduced risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 75–81.
- Mariosa, D.; Hammar, N.; Malmström, H.; Ingre, C.; Jungner, I.; Ye, W.; Fang, F.; Walldius, G. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: A more than 20-year follow-up of the Swedish AMORIS cohort. Ann. Neurol. 2017, 81, 718–728.
- Fernández-Beltrán, L.C.; Godoy-Corchuelo, J.M.; Losa-Fontangordo, M.; Williams, D.; Matias-Guiu, J.; Corrochano, S. A Transcriptomic Meta-Analysis Shows Lipid Metabolism Dysregulation as an Early Pathological Mechanism in the Spinal Cord of SOD1 Mice. Int. J. Mol. Sci. 2021, 22, 9553.
- Bjornevik, K.; Zhang, Z.; O’Reilly, É.; Berry, J.D.; Clish, C.B.; Deik, A.; Jeanfavre, S.; Kato, I.; Kelly, R.S.; Kolonel, L.N.; et al. Prediagnostic plasma metabolomics and the risk of amyotrophic lateral sclerosis. Neurology 2019, 92, e2089–e2100.
- Moll, T.; Marshall, J.N.G.; Soni, N.; Zhang, S.; Cooper-Knock, J.; Shaw, P.J. Membrane lipid raft homeostasis is directly linked to neurodegeneration. Essays Biochem. 2021, 65, 999–1011.
- Zhou, J.; Tawk, M.; Tiziano, F.D.; Veillet, J.; Bayes, M.; Nolent, F.; Garcia, V.; Servidei, S.; Bertini, E.; Castro-Giner, F.; et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy is caused by mutations in ASAH1. Am. J. Hum. Genet. 2012, 91, 5–14.
- Johnson, J.O.; Chia, R.; Miller, D.E.; Li, R.; Kumaran, R.; Abramzon, Y.; Alahmady, N.; Renton, A.E.; Topp, S.D.; Gibbs, J.R.; et al. Association of Variants in the SPTLC1 Gene With Juvenile Amyotrophic Lateral Sclerosis. JAMA Neurol. 2021, 78, 1236–1248.
- Houlden, H.; King, R.; Blake, J.; Groves, M.; Love, S.; Woodward, C.; Hammans, S.; Nicoll, J.; Lennox, G.; O’Donovan, D.G.; et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 2006, 129 Pt 2, 411–425.
- Kölbel, H.; Kraft, F.; Hentschel, A.; Czech, A.; Gangfuss, A.; Mohassel, P.; Nguyen, C.; Stenzel, W.; Schara-Schmidt, U.; Preuße, C.; et al. New Insights into the Neuromyogenic Spectrum of a Gain of Function Mutation in SPTLC1. Genes 2022, 13, 893.
- Mohassel, P.; Donkervoort, S.; Lone, M.A.; Nalls, M.; Gable, K.; Gupta, S.D.; Foley, A.R.; Hu, Y.; Morales Saute, J.A.; Moreira, A.L.; et al. Childhood amyotrophic lateral sclerosis caused by excess sphingolipid synthesis. Nat. Med. 2021, 27, 1197–1204.
- Hop, P.J.; Zwamborn, R.A.J.; Hannon, E.; Shireby, G.L.; Nabais, M.F.; Walker, E.M.; Van Rheenen, W.; Van Vugt, J.J.F.A.; Dekker, A.M.; Westeneng, H.J.; et al. Genome-wide study of DNA methylation shows alterations in metabolic, inflammatory, and cholesterol pathways in ALS. Sci. Transl. Med. 2022, 14, eabj0264.
- Cooper-Knock, J.; Zhang, S.; Kenna, K.P.; Moll, T.; Franklin, J.P.; Allen, S.; Nezhad, H.G.; Iacoangeli, A.; Yacovzada, N.Y.; Eitan, C.; et al. Rare Variant Burden Analysis within Enhancers Identifies CAV1 as an ALS Risk Gene. Cell Rep. 2020, 33, 108456.
- Krebs, S.; Medugorac, I.; Röther, S.; Strässer, K.; Förster, M. A missense mutation in the 3-ketodihydrosphingosine reductase FVT1 as candidate causal mutation for bovine spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 2007, 104, 6746–6751.
- Borgese, N.; Iacomino, N.; Colombo, S.F.; Navone, F. The Link between VAPB Loss of Function and Amyotrophic Lateral Sclerosis. Cells 2021, 10, 1865.
- Nishimura, A.L.; Mitne-Neto, M.; Silva, H.C.; Richieri-Costa, A.; Middleton, S.; Cascio, D.; Kok, F.; Oliveira, J.R.M.; Gillingwater, T.; Webb, J.; et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am. J. Hum. Genet. 2004, 75, 822–831.
- Yu, F.P.S.; Amintas, S.; Levade, T.; Medin, J.A. Acid ceramidase deficiency: Farber disease and SMA-PME. Orphanet. J. Rare Dis. 2018, 13, 121.
- Regier, D.S.; Proia, R.L.; D’Azzo, A.; Tifft, C.J. The GM1 and GM2 Gangliosidoses: Natural History and Progress toward Therapy. Pediatr. Endocrinol. Rev. 2016, 13 (Suppl. S1), 663–673.
- El-Abassi, R.; Singhal, D.; England, J.D. Fabry’s disease. J. Neurol. Sci. 2014, 344, 5–19.
- Biffi, A.; Lucchini, G.; Rovelli, A.; Sessa, M. Metachromatic leukodystrophy: An overview of current and prospective treatments. Bone Marrow Transpl. 2008, 42 (Suppl. S2), S2–S6.
- Vanier, M.T. Niemann-Pick diseases. Handb. Clin. Neurol. 2013, 113, 1717–1721.
- Barth, B.M.; Shanmugavelandy, S.S.; Tacelosky, D.M.; Kester, M.; Morad, S.A.; Cabot, M.C. Gaucher’s disease and cancer: A sphingolipid perspective. Crit. Rev. Oncog. 2013, 18, 221–234.
- Bradbury, A.M.; Bongarzone, E.R.; Sands, M.S. Krabbe disease: New hope for an old disease. Neurosci. Lett. 2021, 752, 135841.
- Choezom, D.; Gross, J.C. Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. J. Cell Sci. 2022, 135, jcs259324.
- Šála, M.; Hollinger, K.R.; Thomas, A.G.; Dash, R.P.; Tallon, C.; Veeravalli, V.; Lovell, L.; Kögler, M.; Hřebabecký, H.; Procházková, E.; et al. Novel Human Neutral Sphingomyelinase 2 Inhibitors as Potential Therapeutics for Alzheimer’s Disease. J. Med. Chem. 2020, 63, 6028–6056.
- Anakor, E.; Milla, V.; Connolly, O.; Martinat, C.; Pradat, P.F.; Dumonceaux, J.; Duddy, W.; Duguez, S. The Neurotoxicity of Vesicles Secreted by ALS Patient Myotubes Is Specific to Exosome-Like and Not Larger Subtypes. Cells 2022, 11, 845.
- Ferrara, D.; Pasetto, L.; Bonetto, V.; Basso, M. Role of Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2018, 12, 574.
- Le Gall, L.; Duddy, W.J.; Martinat, C.; Mariot, V.; Connolly, O.; Milla, V.; Anakor, E.; Ouandaogo, Z.G.; Millecamps, S.; Lainé, J.; et al. Muscle cells of sporadic amyotrophic lateral sclerosis patients secrete neurotoxic vesicles. J. Cachexia Sarcopenia Muscle 2022, 13, 1385–1402.
- Meikle, P.J.; Hopwood, J.J.; Clague, A.E.; Carey, W.F. Prevalence of lysosomal storage disorders. JAMA 1999, 281, 249–254.
- Marques, A.R.A.; Saftig, P. Lysosomal storage disorders—challenges, concepts and avenues for therapy: Beyond rare diseases. J. Cell Sci. 2019, 132, jcs221739.
- Kolter, T.; Sandhoff, K. Sphingolipid metabolism diseases. Biochim. Biophys. Acta 2006, 1758, 2057–2079.
- Sun, A. Lysosomal storage disease overview. Ann. Transl. Med. 2018, 6, 476.
- Blasco, H.; Patin, F.; Descat, A.; Garçon, G.; Corcia, P.; Gelé, P.; Lenglet, T.; Bede, P.; Meininger, V.; Devos, D.; et al. A pharmaco-metabolomics approach in a clinical trial of ALS: Identification of predictive markers of progression. PLoS ONE 2018, 13, e0198116.
- Rozen, S.; Cudkowicz, M.E.; Bogdanov, M.; Matson, W.R.; Kristal, B.S.; Beecher, C.; Harrison, S.; Vouros, P.; Flarakos, J.; Vigneau-Callahan, K.; et al. Metabolomic analysis and signatures in motor neuron disease. Metabolomics 2005, 1, 101–108.
- Blasco, H.; Veyrat-Durebex, C.; Bocca, C.; Patin, F.; Vourc’h, P.; Kouassi Nzoughet, J.; Lenaers, G.; Andres, C.R.; Simard, G.; Corcia, P.; et al. Lipidomics Reveals Cerebrospinal-Fluid Signatures of ALS. Sci. Rep. 2017, 7, 17652.
- Lawton, K.A.; Cudkowicz, M.E.; Brown, M.V.; Alexander, D.; Caffrey, R.; Wulff, J.E.; Bowser, R.; Lawson, R.; Jaffa, M.; Milburn, M.V.; et al. Biochemical alterations associated with ALS. Amyotroph. Lateral Scler. 2012, 13, 110–118.
- Goutman, S.A.; Boss, J.; Guo, K.; Alakwaa, F.M.; Patterson, A.; Kim, S.; Savelieff, M.G.; Hur, J.; Feldman, E.L. Untargeted metabolomics yields insight into ALS disease mechanisms. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1329–1338.
- Goutman, S.A.; Guo, K.; Savelieff, M.G.; Patterson, A.; Sakowski, S.A.; Habra, H.; Karnovsky, A.; Hur, J.; Feldman, E.L. Metabolomics identifies shared lipid pathways in independent amyotrophic lateral sclerosis cohorts. Brain 2022, awac025.
- Lawton, K.A.; Brown, M.V.; Alexander, D.; Li, Z.; Wulff, J.E.; Lawson, R.; Jaffa, M.; Milburn, M.V.; Ryals, J.A.; Bowser, R.; et al. Plasma metabolomic biomarker panel to distinguish patients with amyotrophic lateral sclerosis from disease mimics. Amyotroph Lateral Scler Front. Degener 2014, 15, 362–370.
- Chang, K.H.; Lin, C.N.; Chen, C.M.; Lyu, R.K.; Chu, C.C.; Liao, M.F.; Huang, C.C.; Chang, H.S.; Ro, L.S.; Kuo, H.C. Altered Metabolic Profiles of the Plasma of Patients with Amyotrophic Lateral Sclerosis. Biomedicines 2021, 9, 1944.
- FernÁndez-Eulate, G.; Ruiz-Sanz, J.I.; Riancho, J.; ZufirÍa, M.; GereÑu, G.; FernÁndez-TorrÓn, R.; Poza-Aldea, J.J.; Ondaro, J.; Espinal, J.B.; GonzÁlez-ChinchÓn, G.; et al. A comprehensive serum lipidome profiling of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. Front. Degener 2020, 21, 252–262.
- Dodge, J.C.; Treleaven, C.M.; Pacheco, J.; Cooper, S.; Bao, C.; Abraham, M.; Cromwell, M.; Sardi, S.P.; Chuang, W.L.; Sidman, R.L.; et al. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2015, 112, 8100–8105.
- Sol, J.; Jové, M.; Povedano, M.; Sproviero, W.; Domínguez, R.; Piñol-Ripoll, G.; Romero-Guevara, R.; Hye, A.; Al-Chalabi, A.; Torres, P.; et al. Lipidomic traits of plasma and cerebrospinal fluid in amyotrophic lateral sclerosis correlate with disease progression. Brain Commun. 2021, 3, fcab143.
- Area-Gomez, E.; Larrea, D.; Yun, T.; Xu, Y.; Hupf, J.; Zandkarimi, F.; Chan, R.B.; Mitsumoto, H. Lipidomics study of plasma from patients suggest that ALS and PLS are part of a continuum of motor neuron disorders. Sci. Rep. 2021, 11, 13562.
- Querin, G.; Biferi, M.G.; Pradat, P.F. Biomarkers for C9orf7-ALS in Symptomatic and Pre-symptomatic Patients: State-of-the-art in the New Era of Clinical Trials. J. Neuromuscul. Dis. 2022, 9, 25–37.
- Miller, T.; Cudkowicz, M.; Shaw, P.J.; Andersen, P.M.; Atassi, N.; Bucelli, R.C.; Genge, A.; Glass, J.; Ladha, S.; Ludolph, A.L.; et al. Phase 1-2 Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2020, 383, 109–119.
- Boros, B.D.; Schoch, K.M.; Kreple, C.J.; Miller, T.M. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics 2022.
- Benatar, M.; Wuu, J.; Andersen, P.M.; Bucelli, R.C.; Andrews, J.A.; Otto, M.; Farahany, N.A.; Harrington, E.A.; Chen, W.; Mitchell, A.A.; et al. Design of a Randomized, Placebo-Controlled, Phase 3 Trial of Tofersen Initiated in Clinically Presymptomatic SOD1 Variant Carriers: The ATLAS Study. Neurotherapeutics 2022.
- Saffari, A.; Cannet, C.; Blaschek, A.; Hahn, A.; Hoffmann, G.F.; Johannsen, J.; Kirsten, R.; Kockaya, M.; Kölker, S.; Müller-Felber, W.; et al. (1)H-NMR-based metabolic profiling identifies non-invasive diagnostic and predictive urinary fingerprints in 5q spinal muscular atrophy. Orphanet. J. Rare Dis. 2021, 16, 441.
- Finkel, R.S.; Crawford, T.O.; Swoboda, K.J.; Kaufmann, P.; Juhasz, P.; Li, X.; Guo, Y.; Li, R.H.; Trachtenberg, F.; Forrest, S.J.; et al. Candidate proteins, metabolites and transcripts in the Biomarkers for Spinal Muscular Atrophy (BforSMA) clinical study. PLoS ONE 2012, 7, e35462.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
3 times
(View History)
Update Date:
04 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




