Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Meleti | -- | 1462 | 2022-09-29 12:59:55 | | | |
| 2 | Beatrix Zheng | -179 word(s) | 1283 | 2022-09-30 03:03:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antonelli, R.; Almeida, O.P.D.; Bologna-Molina, R.; Meleti, M. Intraoral Sialadenoma Papilliferum. Encyclopedia. Available online: https://encyclopedia.pub/entry/28050 (accessed on 07 February 2026).
Antonelli R, Almeida OPD, Bologna-Molina R, Meleti M. Intraoral Sialadenoma Papilliferum. Encyclopedia. Available at: https://encyclopedia.pub/entry/28050. Accessed February 07, 2026.
Antonelli, Rita, Oslei Paes De Almeida, Ronell Bologna-Molina, Marco Meleti. "Intraoral Sialadenoma Papilliferum" Encyclopedia, https://encyclopedia.pub/entry/28050 (accessed February 07, 2026).
Antonelli, R., Almeida, O.P.D., Bologna-Molina, R., & Meleti, M. (2022, September 29). Intraoral Sialadenoma Papilliferum. In Encyclopedia. https://encyclopedia.pub/entry/28050
Antonelli, Rita, et al. "Intraoral Sialadenoma Papilliferum." Encyclopedia. Web. 29 September, 2022.
Copy Citation
Sialadenoma papilliferum (SP) is a rare benign epithelial tumour of salivary gland origin, its diagnosis being potentially challenging. It was first described by Abrams and Finck in 1969 as an analog of the cutaneous syringocystadenoma papilliferum.
sialadenoma papilliferum
salivary gland tumors
oral pathology
oral medicine
oral surgery
1. General Introduction
1.1. Age and Sex
Age ranged from 18 to 87 years, with a mean age of 57.2, all in patients older than 30 years, except 2 cases affecting a male of 18 years and a male of 20 years.
The present research has highlighted a predominance of SP among males (39 males, 25 females; M/F ratio: 1.6:1).
1.2. Clinical Features
The palate was the most common involved site, with 48 (75%) cases, of these specifically, 37 (58%) were on the hard palate, 6 (9.5%) on the hard and soft palate junction, 4 (6%) on the soft palate and 1 (1.5%) on unspecified palatal localization.
Other sites included buccal mucosa (6; 9.5%), upper lip mucosa (3; 5%), mandibular retromolar area (2; 3%), floor of the mouth (2; 3%), tongue (2; 3%), and left faucial pillar (1; 1.5%).
SP usually presents as an asymptomatic, a slow growing, and a papillary exophytic lesion. In most cases an erythematous area within an otherwise normal mucosa is present (Figure 1A). Most frequent differential diagnoses is papilloma, on the basis of the keratotic appearance with papillary surface. Other clinical diagnostic hypotheses include palatal fistulas, pyogenic granuloma, soft tissues neoplasms and other benign and malignant minor salivary gland tumors.
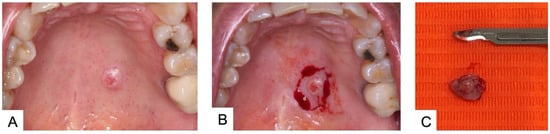
Figure 1. (A) Sialoadenoma papilliferum on the hard palate of a 48-year-old female patient; (B) Surgical excision under local anesthesia; (C) Surgical specimen.
In the cases analyzed in the present research, tumor size ranged from very small (0.3 cm) to large lesions up to 4 cm, with a mean size of 0.79 cm.
Larger cases up to 7 cm can occur in the parotid and again up to now with four cases described including Abram’s case [1][2].
1.3. Histological Features
Histological description of cases evaluated in the present analysis were very similar, the histopathologic pattern of SP being rather characteristic.
The tumor seems to originate from the superficial portion of salivary glands excretory ducts. Papillary processes develop, eventually forming convoluted cleft and spaces. Each papillary projection is lined by consisting of two or three layers of cells, supported by a core of fibrovascular connective tissue. The most superficial portions of the lesion have a squamous epithelial lining; deeper areas show mainly cuboidal to columnar cells, often oncocytic in appearance (Figure 2). As growth progresses, the overlying mucous membrane becomes papillary or verrucous, much like a squamous papilloma.
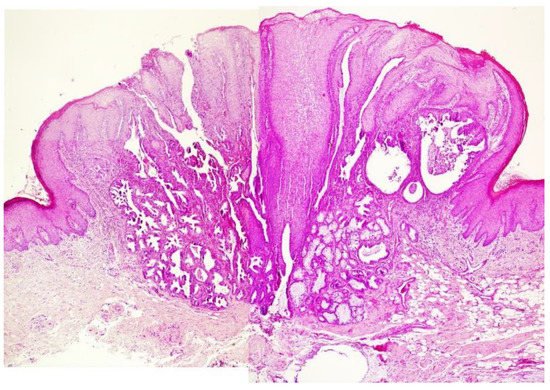
Figure 2. Example of histological features of SP (case different from that presented in Figure 1): exophytic papillary structure covered by stratified squamous epithelium and glandular structures below the mucosa (H&E—50×).
Various research has attempted to identify the cell of origin of SP based on light microscopy, immunohistochemistry (IHC), and electron microscopy (EM) [3]. These methods have yielded variable results with most investigators suggesting excretory duct or excretory duct reserve cell origin [3][4][5][6][7][8][9][10][11][12]. Other authors hypothesized an origin from intercalated duct cells [7][13] or myoepithelial cells [1][3][10].
Fowler and Damm documented that basal cell on the ductal structures were immunoreactive for p63 and p40, a myoepithelial immunophenotype [3]. Variable reactivity in the basal cell layer with smooth muscle actin (SMA) was also identified. In all cases reported in their paper, the luminal cells within the ductal structures were immunoreactive to epithelial membrane antigen (EMA). These results indicate two cell types comprising the convoluted ductal structures of SP: a basal layer of myoepithelial cells (p40+, p63+, and SMA+) and a luminal layer of ductal epithelial cells (EMA+) (Figure 3A,B).
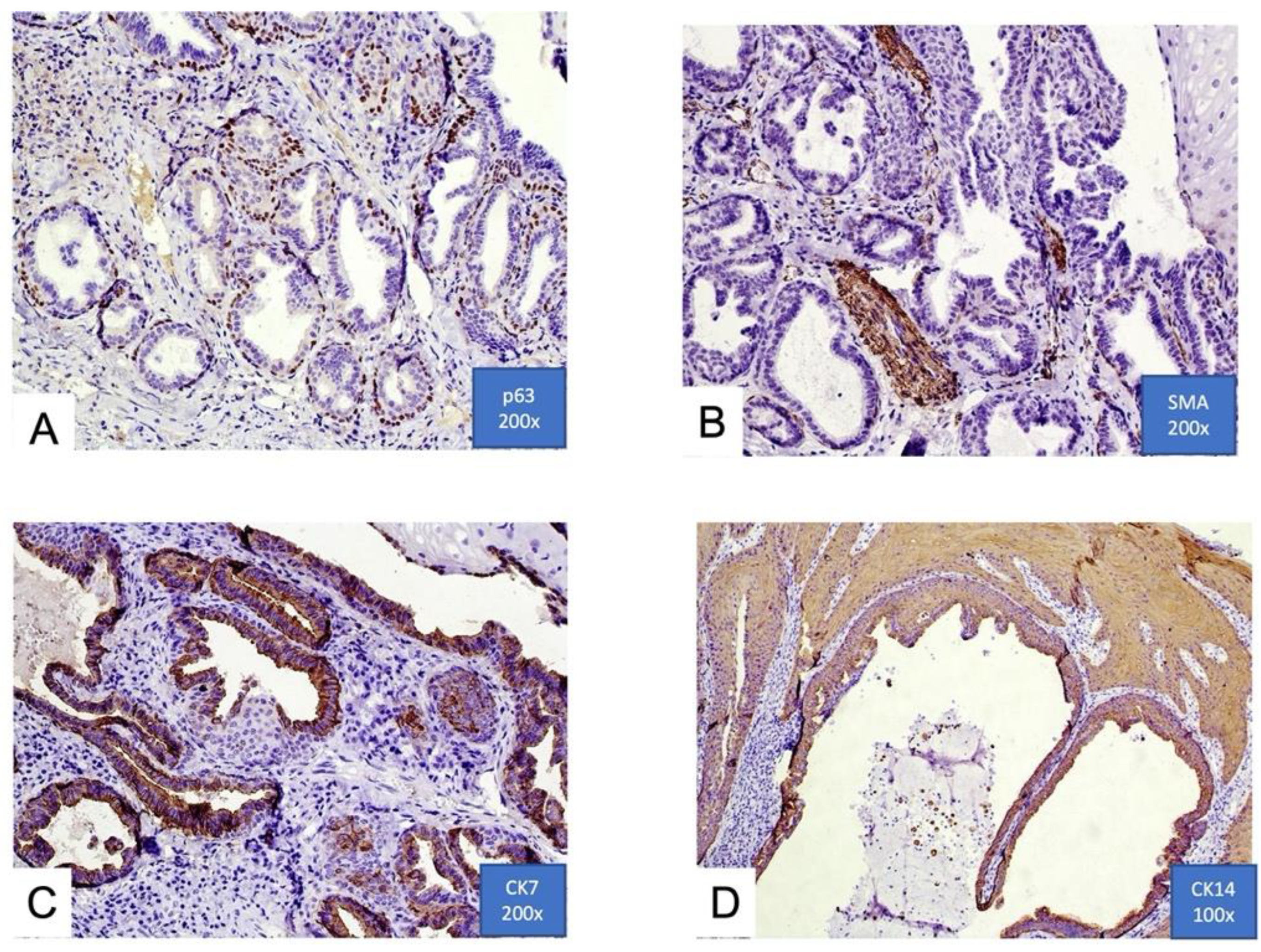
Figure 3. (A,B) Focal immunohistochemical positivity for p63 and smooth muscle actin (SMA). Immunoreactivity for p63 in basal cells of ductal structures suggesting a myoepithelial immunophenotype. Positivity of ductal structures is also evident; (C,D) positive immunohistochemical expression for cytokeratins 7 and 14 (CK7 and CK14) in ductal luminal cells, possibly confirming the epithelial origin (100× and 200×).
A recent immunohistochemical analysis reported by Atarbashi-Moghadam et al. shows positivity for cytokeratins 13, 14, 7, 8, and 19, and it is negative for vimentin and smooth muscle actin. This immunoprofile is similar to excretory ducts of the salivary gland [12] (Figure 3C,D).
1.4. Treatment
Conservative excision seems to be the treatment of choice (Figure 1B,C). Because of the rarity of SP, no clinical protocols have been proposed with regard to the possible duration of follow-up. According to van der Wal and van der Waal, follow-up should be scheduled at regular intervals [14].
1.5. Prognosis
SP is a benign neoplasm with limited growth and limited potential for local aggressiveness, recurrence rate is low, with only 2 cases out of 64 described within 3 years after surgical excision [15][16].
1.6. Possible Malignant Transformation
It is uncertain eventual malignant transformation of SP or on the existence of a malignant variant of the tumor. According to this research four cases with uncertain malignancy have been reported.
Solomon et al., presented a case of a possibly malignant SP [5], even though the diagnosis has been challenged by other authors (Ellis, G.L. and Auclair, P.L., 1991).
Ide et al., described an SP with potentially malignant features, such as rapid and destructive growth, radiographic resorption of the underlying bone, and atypical histological features [17].
Santos et al. also reported a case of SP on the tongue with apparently malignant clinical aspects, which led to the clinical diagnosis of squamous cell carcinoma [18].
Shimoda et al. reported the first case of SP with a definite malignant component [19].
However, Fowler and Damm mentioned that there was insufficient evidence to support this diagnosis and to consider that as a malignancy from preexisting SP [3].
In short, whether SP has a malignant potential therefore remains unanswered.
2. Intraoral Sialadenoma Papilliferum
Salivary gland tumors are a morphologically and clinically diverse group of neoplasms that affect predominantly major salivary glands but also are not uncommon in the minor salivary glands.
The global annual incidence, when all salivary glands tumors are considered, is approximately 1 case per 100,000 per year [13].
Pleomorphic adenoma and mucoepidermoid carcinoma are the two most common benign and malignant minor salivary gland tumors, while SP represents only a small percentage of cases (1.1–1.6%) [4][20].
A usual difficulty in the management of minor salivary gland tumors is the very heterogeneous clinical and radiographic features, as well as the rather wide range of histopathological subtypes.
The exophytic growth pattern of SP is similar to most intraoral salivary gland tumors, which present as submucosal nodular swellings, with or without superficial ulceration. Such a clinic pattern is shared with other lesions, including among others squamous papilloma, verrucous hyperplasia, and exophytic ductal papilloma.
Squamous papilloma and verrucous hyperplasia show only squamous epithelial proliferation and thus can be easily differentiated from SP. Exophytic ductal papilloma displays exophytic papillary ductal epithelial proliferation, but it lacks a ductal proliferation underneath the epithelium. Instead, SP shows as unique histopathologic features an exophytic proliferation of papillary stratified squamous epithelium and a contiguously endophytic salivary ductal proliferation underneath [21].
SP frequently presents as an erythematous lesion, with ulceration or erosion that may suggest a malignant lesion, such as verrucous carcinoma or even sarcomas [22].
From the epidemiological point of view, age and gender are not very helpful in the differential diagnosis, as most salivary gland tumors have no gender predilection and the mean age found in the present research for SP is similar to that reported for other salivary gland tumors (45 years, with a range of 11–74) [23].
SP appears to have a limited growth potential, with an average size at diagnosis of 0.79 cm, facilitating a conservative surgical treatment. There are insufficient data to support the malignant potential of SP, although four cases of supposed malignant transformation were reported.
Research is currently focused to determine the cells of origin of SP, but despite immunohistochemical studies, such question remain unanswered.
In conclusion SP, though rare, should be taken into consideration in the differential diagnosis of intraoral swellings, particularly those located on the palate, and more studies are necessary to better understand its biology.
References
- Abrams, A.M.; Finck, F.M. Sialadenoma papilliferum: A previously un-reported salivary gland tumor. Cancer 1969, 24, 1057–1063.
- Loehn, B.; Sutton, C.; Jastram-Belcher, J.; Harton, A.; Anderson, D.; Walvekar, R.R. Sialadenoma papilliferum of the parotid gland: Case report and review of literature. Head Neck 2013, 35, E74–E76.
- Fowler, C.B.; Damm, D.D. Sialadenoma Papilliferum: Analysis of Seven New Cases and Review of the Literature. Head Neck Pathol. 2018, 12, 193–201.
- Freedman, P.D.; Lumerman, H. Sialoadenoma papilliferum. Oral Surg. 1978, 45, 88–94.
- Solomon, M.P.; Rosen, Y.; Alfonso, A. Intraoral papillary squamous cell tumor of the soft palate with features of sialadenoma papilliferum? Malignant sialadenoma papilliferum. Cancer 1978, 42, 1859–1869.
- Mccoy, J.M.; Eckert, J.R.E.F. Sialoadenoma papilliferum. J. Oral Surg. 1980, 38, 691–693.
- Nasu, M.; Takagi, M.; Ishikawa, G. Sialoadenoma papilliferum. Report of a case. J. Oral Surg. 1981, 39, 367–369.
- Fantasia, J.E.; Nocco, C.E.; Lally, E.T. UItrastructureof sialadenoma papilliferum. Arch. Pathol. Lab. Med. 1986, 110, 523–527.
- Maiorano, E.; Favia, G.; Ricco, R. Sialadenoma papilliferum: An immunohistochemical study of five cases. J. Oral Pathol. Med. 1996, 25, 336–342.
- Ubaidat, M.A.; Robinson, R.A.; Belding, P.J.; Merryman, D.J. Sialadenoma papilliferum of the hard palate: Report of 2 cases and immunohistochemical evaluation. Arch. Pathol. Lab. Med. 2001, 125, 1595–1597.
- Gomes, A.P.; Sobral, A.P.; Loducca, S.V.; de Araujo, V.C. Sialadenoma papilliferum: Immunohistochemical study. Int. J. Oral Maxillofac. Surg. 2004, 33, 621–624.
- Atarbashi-Moghadam, S.; Lotfi, A.; Moshref, M.; Mokhtari, S. Sialadenoma papilliferum of the hard palate: A rare case report. Indian J. Pathol. Microbiol. 2019, 62, 163–164.
- Melo, G.M.; Cervantes, O.; Abrahao, M.; Covolan, L.; Ferreira, E.S.; Baptista, H.A. A brief history of salivary gland surgery. Rev. Colégio Bras. Cir. 2017, 44, 403–412.
- Van der Wal, J.E.; Van der Waal, I. The rare sialadenoma papilliferum: Report of a case and review of the literature. Int. J. Oral Maxillofac. Surg. 1992, 21, 104–106.
- Rennie, J.S.; MacDonald, D.G.; Critchlow, H.A. Sialadenoma papilliferum. A case report and review of the literature. Int. J. Oral Surg. 1984, 13, 452–454.
- Pimentel, M.T.; Lopez Amado, M.; Garcia, S.A. Recurrent sialadenoma papilliferum of the buccal mucosa. J. Laryngol. Otol. 1995, 109, 787–790.
- Ide, F.; Kikuchi, K.; Kusama, K.; Kanazawa, H. Sialadenoma papilliferum with potentially malignant features. J. Clin. Pathol. 2010, 63, 362–364.
- Santos, J.N.; Barros, A.C.; Gurgel, C.A.; Ramalho, L.M.P. Sialadenoma papilliferum of the tongue mimicking a malignant tumor. Braz. J. Otorhinolaryngol. 2013, 79, 404.
- Shimoda, M.; Kameyama, K.; Morinaga, S.; Tanaka, Y.; Hashiguchu, K.; Shimada, M.; Okara, Y. Malignant transformation of sialadenoma papilliferum of the palate: A case report. Virchows Arch. 2004, 445, 641–646.
- Waldron, C.A.; El-Mofty, S.K.; Gnepp, D.R. Tumors of the intraoral minor salivary glands: A demographic and histologic study of 426 cases. Oral Surg. Oral Med. Oral Pathol. 1988, 66, 323–333.
- Hsieh, M.S.; Bishop, J.A.; Yu Fong Chang, J. Sialadenoma Papilliferum. Surg. Pathol. Clin. 2021, 14, 43–51.
- Anuradha, A.; Ram Prasad, V.V.; Kashyap, B.; Srinivas, V. Sialadenoma papilliferum: Clinical misdiagnosis with a histological decree. Case Rep. Dent. 2012, 2012, 356271.
- Vaidya, A.D.; Pantvaidya, G.H.; Metgudmath, R.; Kane, S.V.; D’Cruz, A.K. Minor salivary gland tumors of the oral cavity: A case series with review of literature. J. Cancer Res. Ther. 2012, 8 (Suppl. S1), S111–S115.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
692
Revisions:
2 times
(View History)
Update Date:
30 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




