Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marko Jukic | -- | 2658 | 2022-09-29 08:46:15 | | | |
| 2 | Camila Xu | Meta information modification | 2658 | 2022-09-29 09:41:54 | | | | |
| 3 | Camila Xu | Meta information modification | 2658 | 2022-09-29 09:42:32 | | | | |
| 4 | Marko Jukic | + 102 word(s) | 2760 | 2022-10-04 11:17:56 | | | | |
| 5 | Camila Xu | -10 word(s) | 2750 | 2022-10-08 03:57:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Broz, M.; Kolarič, A.; Jukič, M.; Bren, U. Neuropilin Related Pathological Conditions. Encyclopedia. Available online: https://encyclopedia.pub/entry/27960 (accessed on 07 February 2026).
Broz M, Kolarič A, Jukič M, Bren U. Neuropilin Related Pathological Conditions. Encyclopedia. Available at: https://encyclopedia.pub/entry/27960. Accessed February 07, 2026.
Broz, Matic, Anja Kolarič, Marko Jukič, Urban Bren. "Neuropilin Related Pathological Conditions" Encyclopedia, https://encyclopedia.pub/entry/27960 (accessed February 07, 2026).
Broz, M., Kolarič, A., Jukič, M., & Bren, U. (2022, September 29). Neuropilin Related Pathological Conditions. In Encyclopedia. https://encyclopedia.pub/entry/27960
Broz, Matic, et al. "Neuropilin Related Pathological Conditions." Encyclopedia. Web. 29 September, 2022.
Copy Citation
Neuropilin 1 (NRP1) represents one of the two homologous neuropilins (NRP, splice variants of neuropilin 2 are the other) found in all vertebrates. It forms a transmembrane glycoprotein distributed in many human body tissues as a (co)receptor for a variety of different ligands.
neuropilins
computer-aided drug design
in silico drug design
1. Introduction
Neuropilins (NRPs) represent transmembrane glycoprotein receptors important for the proper functioning of diverse biological processes due to their broad tissue distribution. They are mainly involved in neuronal development and axon guidance, angiogenesis [1], immune functions [2], and, consequently, also in the regulation of several pathological processes such as cancer, cardiovascular diseases [3][4], and viral infections [5]. NRPs lack direct signalling capabilities and act as coreceptors associating with other receptors to transduce a signal, primarily through various receptor tyrosine kinases [3]. There are two NRP types, NRP1 and NRP2, that share 44% sequence identity and exhibit a common domain structure. Their extracellular regions consist of 5 domains (Figure 1): a1/a2 domain, b1/b2 domain, and c (MAM) domain. The a and b domains bind particular endogenous ligands that trigger further signalling and provoke specific intracellular effects (Figure 1). In contrast, the MAM domain was initially thought to mediate NRP oligomerisation, but it more likely participates in the positioning of domains for their interactions with partner receptors by shielding them from the membrane [6]. The extracellular region is connected through a transmembrane (TM) domain to the short intracellular PSD-95/Dlg/ZO-1 (PDZ) binding domain, which lacks catalytic activity [1][3]. The mostly identical domain composition of NRP1 and NRP2 facilitates the involvement of both coreceptors in similar biological processes, yet they are still different enough to allow for distinct biological functions [7].
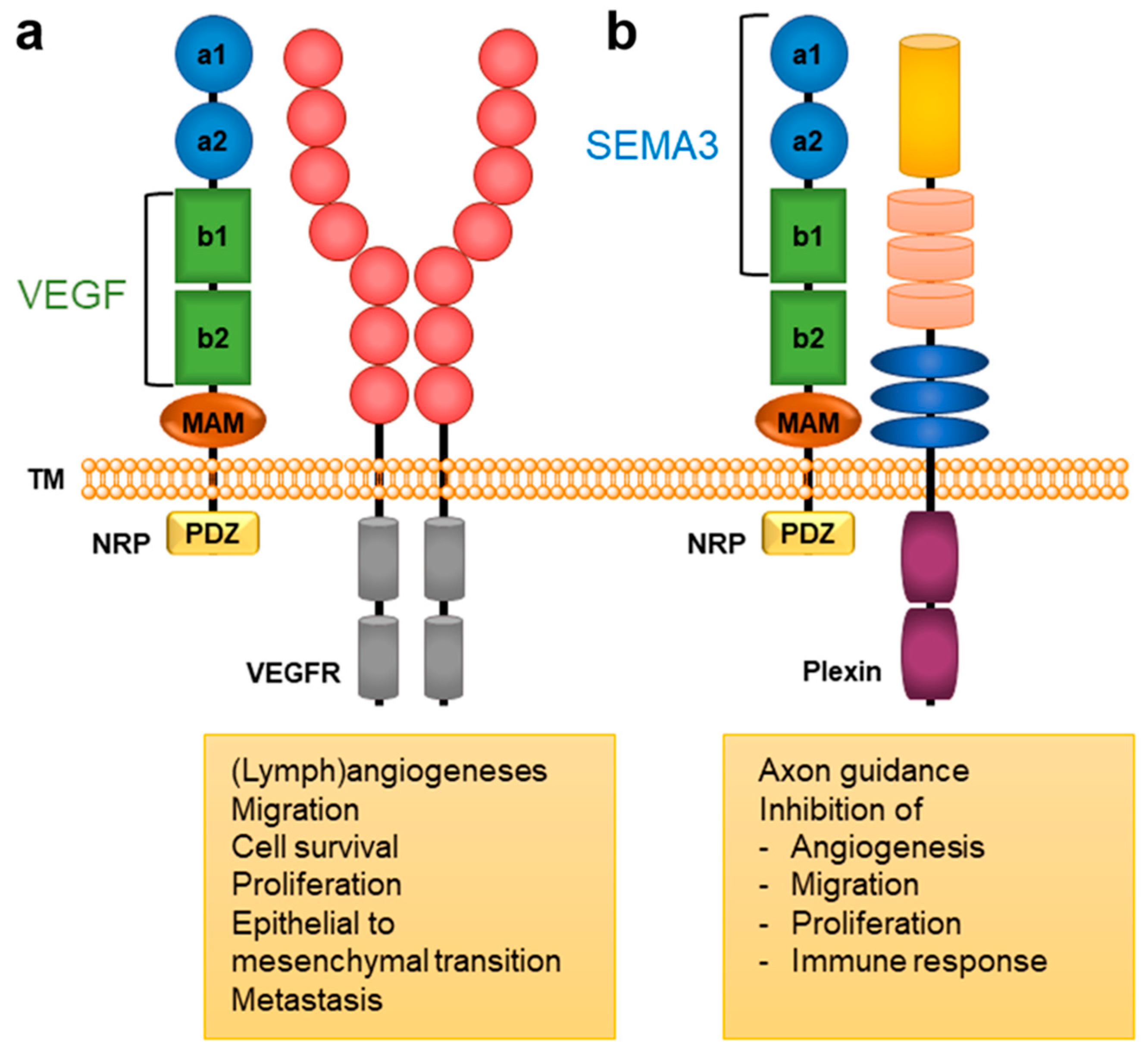
Figure 1. A general structural composition of NRP1 and NRP2 domains and the main NRP-mediated biological responses. The a1/a2 domain, presented in blue circles, is homologous to CUB (for complement C1r/C1s, Uegf, Bmp1); the b1/b2 domain, presented in green squares, is homologous to blood coagulation factor V/VIII domains; and the c domain, presented as an orange ellipse is homologous to meprin, A5, and μ-phosphatase (MAM). The intracellular PDZ domain is represented as a yellow square. Endogenous ligands of the VEGF family bind to the b1/b2 domains, while SEMA3s bind to the a1/a2/b1 domains [1][3]. (a) VEGFs form a complex with NRP and VEGFR that activates signalling pathways involved in angiogenesis associated with cancer [3][8]. (b) SEMA3s form a complex with NRP and plexin to activate signalling pathways that regulate axonal guidance and the immune, respiratory, and cardiovascular system as well as tumour cell responses [3][8][9].
Extracellular domains of NRPs have defined, although not necessarily overlapping, binding sites that can accommodate various endogenous ligands and can interact with diverse receptors. NRPs are well known for their binding of class 3 semaphorins (SEMA3) [10][11] and selected members of the vascular endothelial growth factor (VEGF) family [12] that evoke different biological functions (Table 1). SEMA3s represent signalling proteins of a large and diverse semaphorins family, containing SEMA3A-3G subgroups that are involved not only in the guidance of axons and neural development [13] but also play important roles in immune, respiratory, and cardiovascular systems, as well as in pathological disorders, especially in tumour vasculature [3][8]. They bind with their C-terminal region to the a1/a2 and b1 domains of NRP [3], and since their binding is not sufficient for signal transduction, NRPs need to associate with the SEMA3 main receptor Plexin to form SEMA3-NRP-Plexin complex and to transduce the signal [14][15][16][17]. The members of the SEMA3 class exhibit different preferences for binding to NRP1 and NRP2 (Table 1), which results in various, more specific biological functions.
Table 1. The main groups and subgroups of the most important endogenous ligands binding to both NRP receptors. x indicates binding to both NRPs.
| Endogenous Ligand | Preferences for NRP Binding | Reference | |
|---|---|---|---|
| NRP1 | NRP2 | ||
| SEMA | SEMA3A SEMA3B SEMA3C SEMA3D SEMA3F |
SEMA3B SEMA3C SEMA3D SEMA3F SEMA3G |
[2][9] |
| VEGF | VEGF-A VEGF-A165 VEGF-A189 VEGF-B VEGF-C VEGF-D VEGF-E PIGF |
VEGF-A VEGF-A145 VEGF-A165 VEGF-C VEGF-D PIGF |
[3][9] |
| FGF | FGF-1 FGF-2 FGF-4 FGF-7 |
FGF-2 | [18] |
| HGF | x | x | [19] |
| PDGF | PDGF-BB PDGF-C PDGF-D |
PDGF-BB | [3][20] |
| TGF-β | TGF-β1 | TGF-β1 | [21][22] |
| miRNAs | x | x | [2][23] |
Vascular endothelial growth factor (VEGF) represents a family of signalling proteins involved in the development of blood vessels, including pathological angiogenesis as in cancer, vascular branching, and maturation, along with cardiovascular development [3][24]. The VEGF family consists of growth factors VEGF-A-D, as well as placenta growth factor (PlGF), parapoxvirus VEGF-E, and snake venom VEGF-F [25]. They primarily stimulate cellular responses by binding to their VEGF receptors (VEGFR). However, the binding of a VEGF to a coreceptor NRP forms a VEGF-NRP-VEGFR complex that results in enhanced VEGF signalling [24]. VEGFs bind to the b1/b2 domains of the NRP receptor, with the b1 domain being essential for the binding, while the b2 domain is required to ensure optimal binding [1]. The binding of VEGF ligands to b1 proceeds through the VEGF C-terminus sequence, containing a [R/K]XX[R/K] motif, called the C-end rule (CendR) [26]. Although the SEMA3 ligands also bind to the b1 domain, their binding site differs from one of the VEGF ligands [1]. As for SEMA3, there exists a distinct preference between NRP1 and NRP2 for different VEGF ligands (Table 1), which then perform specific endogenous tasks.
NRPs have also been identified as binding partners of other growth factors (Table 1), which demonstrates their versatility in regulating various signalling pathways. Thereby, NRPs can interact with the Fibroblast Growth Factor (FGF), the Hepatocyte Growth Factor (HGF), the Platelet-Derived Growth Factor (PDGF), the Transforming Growth Factor beta (TGF-β), and their respective receptors [3][9][21]. Moreover, NRPs have been reported to act as a receptor for extracellular microRNAs (miRNAs), which facilitates their internalisation into cells resulting in several physiological and pathological conditions. Thus, miRNAs have been associated with tumour progression, epithelial to mesenchymal transformation, metastasis and disease prognosis [2].
NRPs play an essential role in angiogenesis and lymphogenesis in endothelial cells through the binding of VEGF family members. The main pathway by which NRPs promote angiogenesis is through the formation of the NRP/VEGF/VEGFR complex, in which NRPs act as co-receptors with VEGFRs and enhance VEGF-induced activation of intracellular signaling pathways that consequently influence cell adhesion, migration, and permeability during angiogenesis under both physiological and pathological conditions [27][28][29]. NRP1 is mainly expressed in vascular endothelial tissue, whereas NRP2 is mainly expressed in lymphoid epithelium [30]. Although VEGF and its receptor, VEGFR govern angiogenesis, some studies have provided evidence that NRP1 and NRP2 can also promote blood vessel growth through alternative pathways [31][32].
Class 3 of NRPs endogenous ligands semaphorins also play an important role in vascular development, mainly by inhibiting angiogenesis. The semaphorins SEMA3A, SEMA3B, SEMA3D, SEMA3E, and SEMA3F interfere with VEGF-induced angiogenesis to promote their antiangiogenic effects [33].
Due to NRPs interacting with a broad range of endogenous ligands and triggering diverse physiological as well as pathological mechanisms, the modulation of their endogenous ligand binding exhibits a high potential for drug development. Therefore, small peptide ligands mimicking endogenous ligands have already been developed. Unfortunately, they lack metabolic stability and display a low bioavailability [24]. Moreover, the inhibition with monoclonal antibodies was also pursued, but significant side effects were observed [34]. Consequently, peptidomimetics, as well as small molecules, are gaining particular interest. Despite their limited size, they can interfere with the endogenous ligands binding to NRPs and have been reported to inhibit their signalling and biological functions [24].
2. NRP Binding of Endogenous Ligands
Despite NRP2 being an equivalently important target as NRP1, the latter has been more studied and better characterised. Although NRPs bind a large set of diverse endogenous ligands, little is known about the details of individual ligand interactions with its binding domain on the NRP receptor. The most studied and explored is the binding of VEGF-A165 to the b1 domain of NRP1, which serves as a basis for developing NRP small molecule antagonists (Figure 2). Due to the high structural similarity of both receptors, some of the NRP1 antagonists are able to extend their inhibitory activity on the NRP2-related biological signalling and functions. VEGF-A165 binds to the b1 domain of NRP1 with the C-terminal CendR motif, which has a terminal arginine residue. CendR facilitates the binding into a highly conserved b1 arginine binding pocket, consisting of amino acid residues Tyr297, Trp301, Thr316, Asp320, Ser346, Thr349, Tyr353 and Trp411 that were all recognised in a mutational analysis as crucial for a high VEGF-A165 affinity. The guanidine group forms a salt bridge with Asp320, and the free carboxylate interacts through hydrogen bonds with Ser346, Thr349, and Tyr353. Tyr297 and Tyr353 also participate in cation-π interactions with the CendR arginine side chains (Figure 2) [35]. In an additional exploration of the binding site region with synthetic ligands mimicking the terminal arginine, a hydration profile was analysed, thus revealing a conserved water molecule identified as important for increasing the ligand affinity by forming a hydrogen bond network between Trp301, Glu348, and the ligands [36].
The fact that the protein ligands binding to the b1 domain of NRP1 share a common C-terminal arginine motif is also evident from the recently solved crystal structure of SARS-CoV-2 CendR bound to NRP-1 [37]. The comparison of VEGF-A165 and SARS-CoV-2 CendR revealed almost identical binding modes, which share the interactions with the same key amino acid residues Tyr297, Trp301, Thr316, Asp320, Ser346, Thr349 and Tyr353 as depicted in Figure 2b. These residues seem to contribute to the binding affinity of all CendR-containing ligands; therefore, an interruption of interactions with these residues is deemed an attractive therapeutic approach.
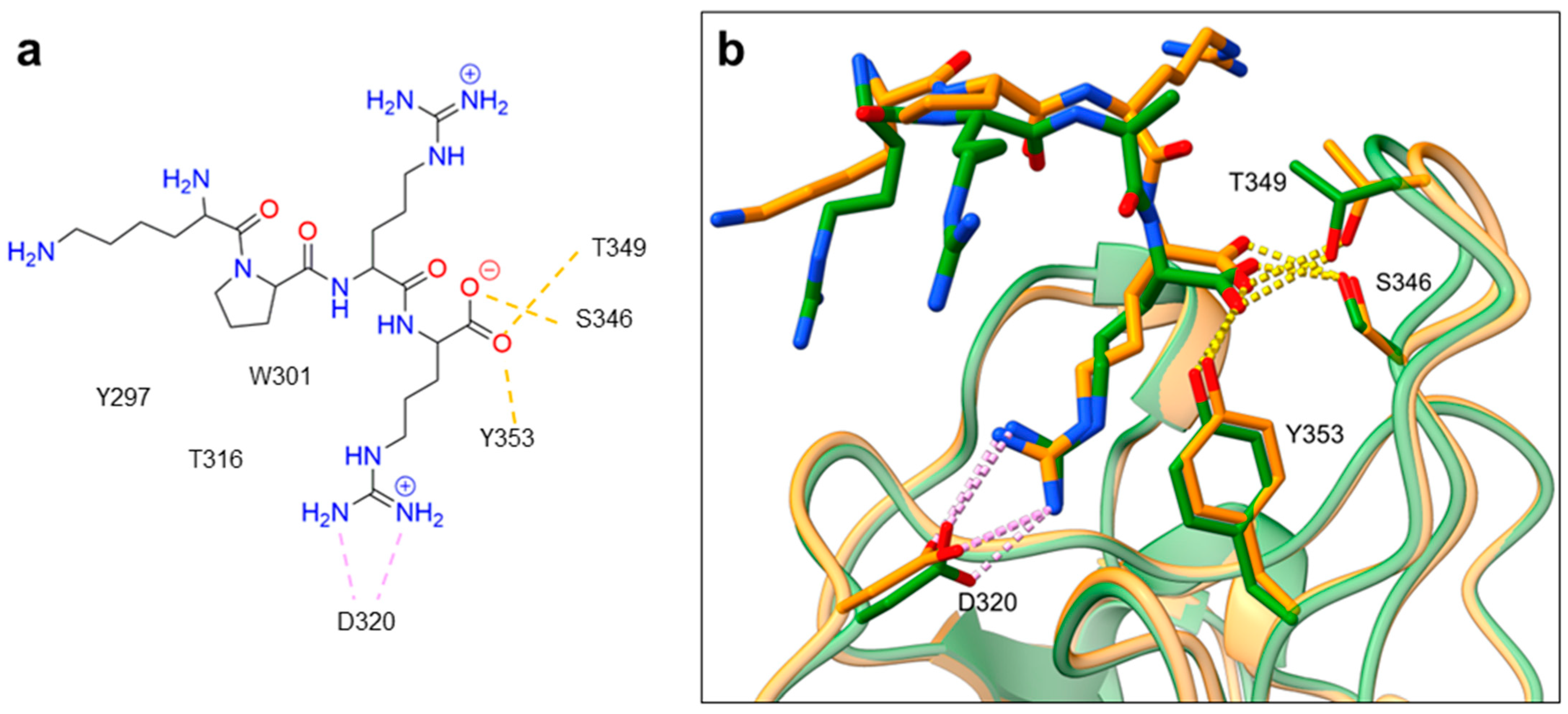

Figure 2. (a) A 2D representation of the VEGF-A165 CendR (KPRR) motif. Hydrogen bonding and salt bridge interactions, crucial for the high affinity of the VEGF-A165 terminal arginine with its NRP1 binding pocket, are depicted as yellow and light pink dots, respectively. (b) Superposition of NRP1 crystal structure complexes with VEGF-A165 (PDB ID: 4DEQ) [38] and SARS-CoV-2 (PDB ID: 7JJC) [37] CendR terminal residues bound into the NRP1 arginine binding pocket, yielding the comparison of the ligand-binding modes. VEGF-A165-NRP-1 complex is presented in orange cartoon and SARS-CoV-2 CendR-NRP-1 complex in green cartoon. NRP1 amino acid residues, significant for forming hydrogen bonds with the terminal arginine, are depicted as sticks of corresponding colours. Hydrogen bonding and salt bridge interactions are shown as yellow and light pink dots, respectively.
3. Neuropilin-Related Pathology
3.1. Pain
Endogenous VEGF family ligands have been found instrumental in the pathophysiology of chronic pain in several diseases, including cancer, neuropathic pain, osteoarthritis, rheumatoid arthritis, migraine, and diabetic complications. Although mainly involved in the development and maintenance of pain, the best-studied VEGF-A165 shows not only pro- but also anti-nociceptive effects leading to analgesia [39][40]. These effects are due to the binding of different VEGF-A165 isoforms to their VEGFR receptors. The VEGF-A165a isoform is pronociceptive and leads to pain, while VEGF-A165b is considered anti-nociceptive, and the ratio between the two isoforms represents the key factor determining the effect on sensory neurons and pain levels [40][41]. VEGF-A binds to VEGFR and the coreceptor NRP1, which associates NRP1 with pain. While VEGF-A165a binds to VEGFR and NRP1 receptors, VEGF-A165b does not contain a CendR motif and, therefore, cannot bind to the NRP1 b1 domain [39][40]. Consequently, it appears that NRP1 is only involved in the activation of the pain pathway, making the VEGF-A165a/NRP1 complex an interesting target for reducing chronic pain.
3.2. Viral Entry
NRP receptors have been found to contribute to the infectivity of many viruses by enhancing their host cell entry (Table 2). This mainly involves viruses that contain the CendR motif, through which they bind to the b1 domain of the NRP1 receptor and this promotes the host cell infection. Among them, Epstein–Barr (EBV) [42] and Human T-cell lymphotropic virus type 1 (HTLV-1) [43][44] represent the best-studied ones. Recently, it was identified that the SARS-CoV-2 virus, the causative agent of the latest COVID-19 pandemic, also contains the CendR motif, which proposed NRP1 as its additional entry point into human cells [37][45].
Table 2. NRP involvement in the entry and/or infectivity of viruses.
Human T-cell lymphotropic virus type 1 (HTLV-1) represents the main cause of T-cell lymphoma and leukaemia. Cell-to-cell contact forms the major route of HTLV-1 infection, with NRP1 being essential for the viral entry. NRP1 is highly expressed in dendritic, T-cells, and endothelial cells, which represent the main targets of the HTLV-1 infection [43]. HTLV-1 enters host cells via a three-step process. First, the viral surface unit attaches to the heparin/heparan sulfate proteoglycan (HSPG) on the host cell surface. Then the HSPG/virus surface complex interacts with the b1 domain of NRP1, which triggers conformational changes of the viral surface that enable its interaction with glucose transporter GLUT-1, yielding HTLV-1 fusion and cell entry [50].
Epstein–Barr virus (EBV) represents human herpesvirus 4 (HVV4) implicated in malignancies of lymphoid or epithelial origin. Glycoprotein B (gB) in the virus envelope forms a critical factor for the infection of B and epithelial cells, whereby NRP1 was demonstrated as the main attachment point of gB, facilitating viral entry and the infection of nasopharyngeal epithelial cells, while its connection to B cells remains unknown. Upon EBV binding to nasopharyngeal epithelial cells, furin cleaves gB, exposing a CendR motif that binds to NRP1 and promotes viral fusion and internalisation into host cells. Moreover, the binding of EBV to NRP1 also activates receptor tyrosine kinase (RTK) signalling, which consequently promotes EBV infection of nasopharyngeal epithelial cells, but the detailed mechanism remains unknown [42]. With both viruses being highly involved in carcinogenic processes, it is assumed that they compete with endogenous VEGF ligands for the binding to NRP1 and might trigger cell signalling to promote the formation of tumour vessels in cancer tissues [51][52].
3.3. Cardiovascular Diseases
NRPs are involved in angiogenesis and cardiovascular diseases. On one hand, the knockout of NRP1 from cardiomyocytes and vascular smooth muscle cells causes cardiomyopathy, aggravated ischemia-induced heart failure, and hereditary haemorrhagic telangiectasia arteriovenous malformations, thus revealing its cardioprotective role [53][54]. On the other hand, NRP1 mediates the activation of human cardiac fibroblasts [55]. NRPs significantly contribute towards cardiovascular disease and the latter represent a serious comorbidity in COVID-19 patients [56][57][58][59][60].
3.4. Diabetes
The role of NRP in diabetes pathology was reviewed in 2002 by Mamluk et al. [61]. Especially after the outbreak of COVID-19 disease, NRP has been studied in more detail as a viral co-receptor and via involvement in co-morbidity [57]. Its involvement can be observed in diabetic nephropathy and the presence of NRP1 inhibitor proof-of-concept peptide compounds is of great interest [62][63]. However, the association between NRP1 and SARS-CoV-2 infection can be summarized in two of the most described scenarios [64][65].
Patients with diabetic nephropathy represent a group at higher risk for COVID-19 disease severity. NRP1 is found in the kidney, particularly in podocyte cells, where it is important for proper podocyte function, such as adhesion to extracellular matrix proteins, cytoskeletal reorganisation, and apoptosis. Its role is therefore important in diabetic nephropathy, in which it has been demonstrated that suppression of NRP1 expression may be responsible for podocyte damage and loss, leading to deterioration of renal function. It is speculated that the high expression of NRP1 in the kidney of diabetic patients facilitates the invasion of SARS-CoV-2 into this tissue, while the interaction of both processes leads to depletion of NRP1, which then exacerbates the pathogenesis of diabetic nephropathy.
3.5. Cancer
Cancer remains the second most common cause of death worldwide, responsible for almost 10 million deaths in 2020 alone [66]. While cancer, in most cases, takes years to develop into a life-threatening disease, it is usually discovered only after it has metastasised to other organs. Therefore, the metastatic potential of cancer cells remains one of the main prognostic factors for the overall survival of cancer patients. NRP1 overexpression in cancer cells has been associated with tumour aggressiveness, enhanced cell proliferation, and metastasis (Table 3).
Moreover, the other member of the neuropilin family, NRP2, also contributes to the cancer progression. For example, it was shown that NRP2 is expressed during macrophage differentiation, promotes efferocytosis, facilitates tumour growth [67] and promotes mobilisation [68]. In contrast, its deletion downregulates tumour-promoting genes, increases secondary necrosis within tumours and impairs apoptosis [67]. NRP2, but not NRP1, is expressed in cytokine-induced killer cells, which are responsible for the controlled apoptosis [69] of precancerous cells.
Table 3. Scientific literature collection on NRP1 and NRP2 involvement in different cancer types.
| Cancer Type | NRP1 | NRP2 | Reference |
|---|---|---|---|
| Leukaemia | x | [70][71][72][73][74][75] | |
| Breast cancer | x | x | [76][77][78][79][80][81][82][83][84][85][86] |
| Carcinoma | x | x | [87][88][89][90][91][92][93][94][95][96][97][98][99][100] |
| Colon & Colorectal cancer | x | x | [101][102][103][104][105][106][107][108][109][110] |
| Gastric cancer | x | [111][112][113][114][115][116][117][118][119][120][121][122] | |
| Lung cancer | x | x | [123][124][125][126][127][128][129][130][131][132][133] |
| Pancreatic cancer | x | [134][135][136][137][138] | |
| Prostate cancer | x | x | [139][140][141] |
| Melanoma | x | x | [142][143][144][145] |
| Glioma | x | [146][147][148][149][150][151][152] | |
| Liver cancer | x | [153] | |
| Mammary stem cells cancer | x | [154][155] | |
| Esophageal cancer | x | [156][157] | |
| Stem cell cancer | x | [158][159] | |
| Thyroid cancer | x | [160][161] | |
| Multiple myeloma | x | [162] | |
| Lymphoma | x | [163] | |
| Bladder cancer | x | [164][165] | |
| Tongue cancer | x | [166] | |
| Cervical cancer | x | [167][168] | |
| Gallbladder cancer | x | [169] | |
| Endometrium cancer | x | x | [170][171] |
References
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226.
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted role of neuropilins in the immune system: Potential targets for immunotherapy. Front. Immunol. 2017, 8, 1228.
- Niland, S.; Eble, J.A. Neuropilin: Handyman and power broker in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1223, 31–67.
- Carmeliet, P.; Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 2005, 436, 193–200.
- Mayi, B.S.; Leibowitz, J.A.; Woods, A.T.; Ammon, K.A.; Liu, A.E.; Raja, A. The role of Neuropilin-1 in COVID-19. PLoS Pathog. 2021, 17, e1009153.
- Yelland, T.; Djordjevic, S. Crystal structure of the neuropilin-1 MAM domain: Completing the neuropilin-1 ectodomain picture. Structure 2016, 24, 2008–2015.
- Nakamura, F.; Goshima, Y. Structural and functional relation of neuropilins. Neuropilin: From Nervous System to Vascular and Tumor Biol. Adv. Exp. Med. Bio 2002, 515, 55–69.
- Nasarre, P.; Gemmill, R.M.; Drabkin, H.A. The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. Onco Targets Ther. 2014, 7, 1663.
- Prud’homme, G.J.; Glinka, Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget 2002, 3, 921.
- He, Z.; Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 1997, 90, 739–751.
- Kolodkin, A.L.; Levengood, D.V.; Rowe, E.G.; Tai, Y.T.; Giger, R.J.; Ginty, D.D. Neuropilin is a semaphorin III receptor. Cell 1997, 90, 753–762.
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745.
- Zhao, L.; Chen, H.; Lu, L.; Wang, L.; Zhang, X.; Guo, X. New insights into the role of coreceptor neuropilins in tumour angiogenesis and lymphangiogenesis and targeted therapy strategies. J. Drug Target. 2021, 29, 155–167.
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 1999, 99, 59–69.
- Tamagnone, L.; Artigiani, S.; Chen, H.; He, Z.; Ming, G.L.; Song, H.J.; Chedotal, A.; Winberg, M.L.; Goodman, C.S.; Poo, M.; et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 1999, 99, 71–80.
- Meyer, L.A.; Fritz, J.; Pierdant-Mancera, M.; Bagnard, D. Current drug design to target the Semaphorin/Neuropilin/Plexin complexes. Cell Adhes. Migr. 2016, 10, 700–708.
- Toledano, S.; Nir-Zvi, I.; Engelman, R.; Kessler, O.; Neufeld, G. Class-3 semaphorins and their receptors: Potent multifunctional modulators of tumor progression. Int. J. Mol. Sci. 2019, 20, 556.
- West, D.C.; Rees, C.G.; Duchesne, L.; Patey, S.J.; Terry, C.J.; Turnbull, J.E.; Delehedde, M.; Heegaard, C.W.; Allain, F.; Vanpouille, C.; et al. Interactions of multiple heparin binding growth factors with neuropilin-1 and potentiation of the activity of fibroblast growth factor-2. J. Biol. Chem. 2005, 280, 13457–13464.
- Sulpice, E.; Plouet, J.; Bergé, M.; Allanic, D.; Tobelem, G.; Merkulova-Rainon, T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2008, 111, 2036–2045.
- Pellet-Many, C.; Mehta, V.; Fields, L.; Mahmoud, M.; Lowe, V.; Evans, I.; Ruivo, J.; Zachary, I. Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialisation following arterial injury. Cardiovasc. Res. 2015, 108, 288–298.
- Harman, J.L.; Sayers, J.; Chapman, C.; Pellet-Many, C. Emerging Roles for Neuropilin-2 in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 5154.
- Wittmann, P.; Grubinger, M.; Gröger, C.; Huber, H.; Sieghart, W.; Peck-Radosavljevic, M.; Mikulits, W. Neuropilin-2 induced by transforming growth factor-β augments migration of hepatocellular carcinoma cells. BMC Cancer 2015, 15, 909.
- Do, Y.; Cho, J.G.; Park, J.Y.; Oh, S.; Park, D.; Yoo, K.H.; Lee, M.S.; Kwon, B.S.; Kim, J.; Yang, Y. MiR-146a Regulates Migration and Invasion by Targeting NRP2 in Circulating-Tumor Cell Mimicking Suspension Cells. Genes 2020, 12, 45.
- Peng, K.; Bai, Y.; Zhu, Q.; Hu, B.; Xu, Y. Targeting VEGF–neuropilin interactions: A promising antitumor strategy. Drug Discov. 2019, 24, 656–664.
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625.
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2019, 106, 16157–16162.
- Miao, H.Q.; Klagsbrun, M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000, 19, 29–37.
- Lampropoulou, A.; Ruhrberg, C. Neuropilin regulation of angiogenesis. Biochem. Soc. Trans. 2014, 42, 1623–1628.
- Staton, C.A.; Kumar, I.; Reed, M.W.R.; Brown, N.J. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 2007, 212, 237–248.
- Mei, B.; Chen, J.; Yang, N.; Peng, Y. The regulatory mechanism and biological significance of the Snail-miR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis. 2020, 11, 241.
- Hu, C.; Jiang, X. Role of NRP-1 in VEGF-VEGFR2-independent tumorigenesis. Target. Oncol. 2016, 11, 501–505.
- Alghamdi, A.A.; Benwell, C.J.; Atkinson, S.J.; Lambert, J.; Johnson, R.T.; Robinson, S.D. NRP2 as an emerging angiogenic player; promoting endothelial cell adhesion and migration by regulating recycling of α5 integrin. Front. Cell Dev. Biol. 2020, 8, 395.
- Iragavarapu-Charyulu, V.; Wojcikiewicz, E.; Urdaneta, A. Semaphorins in angiogenesis and autoimmune diseases: Therapeutic targets? Front. Immunol. 2020, 11, 346.
- Caunt, M.; Mak, J.; Liang, W.C.; Stawicki, S.; Pan, Q.; Tong, R.K.; Plowman, G.; Kowalski, J.; Ho, C.; Reslan, H.B.; et al. Supplemental Data Blocking Neuropilin-2 Function Inhibits Tumor Cell Metastasis. Cancer Cell 2008, 13, 331–342.
- Jarvis, A.; Allerston, C.K.; Jia, H.; Herzog, B.; Garza-Garcia, A.; Winfield, N.; Ellard, K.; Aqil, R.; Lynch, R.; Chapman, C.; et al. Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction. J. Med. Chem. 2010, 53, 2215–2226.
- Mota, F.; Fotinou, C.; Rana, R.R.; Chan, A.; Yelland, T.; Arooz, M.T.; O’Leary, A.P.; Hutton, J.; Frankel, P.; Zachary, I.; et al. Architecture and hydration of the arginine-binding site of neuropilin-1. FEBS J. 2018, 285, 1290–1304.
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865.
- Parker, M.W.; Xu, P.; Li, X.; Vander Kooi, C.W. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 2012, 287, 11082–11089.
- Llorián-Salvador, M.; González-Rodríguez, S. Painful understanding of VEGF. Front. Pharmacol. 2018, 9, 1267.
- Perez-Miller, S.; Patek, M.; Moutal, A.; Duran, P.; Cabel, C.R.; Thorne, C.A.; Khanna, R. Novel compounds targeting neuropilin receptor 1 with potential to interfere with SARS-CoV-2 virus entry. ACS Chem. Neurosci. 2021, 12, 1299–1312.
- Hulse, R.P. Role of VEGF-A in chronic pain. Oncotarget 2017, 8, 10775.
- Wang, H.B.; Zhang, H.; Zhang, J.P.; Li, Y.; Zhao, B.; Feng, G.K.; Zeng, M.S. Neuropilin 1 is an entry factor that promotes EBV infection of nasopharyngeal epithelial cells. Nat. Commun. 2015, 6, 6240.
- Ghez, D.; Lepelletier, Y.; Lambert, S.; Fourneau, J.M.; Blot, V.; Janvier, S.; Hermine, O. Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry. J. Virol. 2006, 80, 6844.
- Lambert, S.; Bouttier, M.; Vassy, R.; Seigneuret, M.; Petrow-Sadowski, C.; Janvier, S.; Pique, C. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood 2009, 113, 5176–5185.
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860.
- Lane, R.K.; Guo, H.; Fisher, A.D.; Diep, J.; Lai, Z.; Chen, Y.; Kaiser, W.J. Necroptosis-based CRISPR knockout screen reveals Neuropilin-1 as a critical host factor for early stages of murine cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 20109–20116.
- Wang, H.C.; Huang, P.N.; Hung, H.C.; Tseng, S.N.; Chang, C.C.; Tsai, Y.R.; Hsu, J.T. Effect of a Neuropilin-1-Derived Virus Receptor Trap on Enterovirus A71 infection in vitro. Antimicrob. Agents Chemother. 2020, 65, e00695-20.
- Raaben, M.; Jae, L.T.; Herbert, A.S.; Kuehne, A.I.; Stubbs, S.H.; Chou, Y.Y.; Whelan, S.P. NRP2 and CD63 are host factors for Lujo virus cell entry. Cell Host Microbe 2017, 22, 688–696.
- Martinez-Martin, N.; Marcandalli, J.; Huang, C.S.; Arthur, C.P.; Perotti, M.; Foglierini, M.; Ciferri, C. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 2018, 174, 1158–1171.
- Kusunoki, H.; Tanaka, T.; Kohno, T.; Matsuhashi, K.; Hosoda, K.; Wakamatsu, K.; Hamaguchi, I. A novel neuropilin-1–binding sequence in the human T-cell lymphotropic virus type 1 envelope glycoprotein. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 541–548.
- Gu, Y.Y.; Luo, B.; Li, C.Y.; Huang, L.S.; Chen, G.; Feng, Z.B.; Peng, Z.G. Expression and clinical significance of neuropilin-1 in Epstein-Barr virus-associated lymphomas. Cancer Biomark. 2019, 25, 259–273.
- Hwang, J.Y.; Sun, Y.; Carroll, C.R.; Usherwood, E.J. Neuropilin-1 regulates the secondary CD8 T cell response to virus infection. mSphere 2019, 4, e00221-19.
- Wang, Y.; Cao, Y.; Yamada, S.; Thirunavukkarasu, M.; Nin, V.; Joshi, M.; Rishi, M.T.; Bhattacharya, S.; Camacho-Pereira, J.; Sharma, A.K.; et al. Cardiomyopathy and Worsened Ischemic Heart Failure in SM22-α Cre-Mediated Neuropilin-1 Null Mice: Dysregulation of PGC1α and Mitochondrial Homeostasis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1401–1412.
- Kilari, S.; Wang, Y.; Singh, A.; Graham, R.P.; Iyer, V.; Thompson, S.M.; Torbenson, M.S.; Mukhopadhyay, D.; Misra, S. Neuropilin-1 deficiency in vascular smooth muscle cells is associated with hereditary hemorrhagic telangiectasia arteriovenous malformations. JCI Insight 2022, 7, e155565.
- Matilla, L.; Arrieta, V.; Jover, E.; Garcia-Peña, A.; Martinez-Martinez, E.; Sadaba, R.; Alvarez, V.; Navarro, A.; Fernandez-Celis, A.; Gainza, A.; et al. Soluble St2 Induces Cardiac Fibroblast Activation and Collagen Synthesis via Neuropilin-1. Cells 2020, 9, 1667.
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538.
- Sahni, S.; Gupta, G.; Sarda, R.; Pandey, S.; Pandey, R.M.; Sinha, S. Impact of metabolic and cardiovascular disease on COVID-19 mortality: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 102308.
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100.
- Nicin, L.; Abplanalp, W.T.; Mellentin, H.; Kattih, B.; Tombor, L.; John, D.; Schmitto, J.D.; Heineke, J.; Emrich, F.; Arsalan, M.; et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur. Heart J. 2020, 41, 1804–1806.
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610.
- Klagsbrun, M.; Takashima, S.; Mamluk, R. The role of neuropilin in vascular and tumor biology. In Neuropilin: From Nervous System to Vascular and Tumor Biology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 33–48.
- Mourad, D.; Azar, N.S.; Azar, S.T. Diabetic nephropathy and COVID-19: The potential role of immune actors. Int. J. Mol. Sci. 2021, 22, 7762.
- Wang, J.; Wang, S.; Li, M.; Wu, D.; Liu, F.; Yang, R.; Ji, S.; Ji, A.; Li, Y. The neuropilin-1 inhibitor, ATWLPPR peptide, prevents experimental diabetes-induced retinal injury by preserving vascular integrity and decreasing oxidative stress. PLoS ONE 2015, 10, e0142571.
- Loeffler, I.; Rüster, C.; Franke, S.; Liebisch, M.; Wolf, G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2013, 305, F911–F918.
- Bondeva, T.; Wolf, G. Role of Neuropilin-1 in Diabetic Nephropathy. J. Clin. Med. 2015, 4, 1293–1311.
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/ (accessed on 24 June 2022).
- Roy, S.; Bag, A.K.; Dutta, S.; Polavaram, N.S.; Islam, R.; Schellenburg, S.; Banwait, J.; Guda, C.; Ran, S.; Hollingsworth, M.A.; et al. Macrophage-Derived Neuropilin-2 Exhibits Novel Tumor-Promoting Functions. Cancer Res. 2018, 78, 5600–5617.
- Diaz-Vera, J.; Palmer, S.; Hernandez-Fernaud, J.R.; Dornier, E.; Mitchell, L.E.; Macpherson, I.; Edwards, J.; Zanivan, S.; Norman, J.C. A Proteomic Approach to Identify Endosomal Cargoes Controlling Cancer Invasiveness. J. Cell Sci. 2017, 78, 5600–5617.
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10.
- Piechnik, A.; Dmoszynska, A.; Omiotek, M.; Mlak, R.; Kowal, M.; Stilgenbauer, S.; Bullinger, L.; Giannopoulos, K. The VEGF Receptor, Neuropilin-1, Represents a Promising Novel Target for Chronic Lymphocytic Leukemia Patients. Int. J. Cancer 2013, 133, 1489–1496.
- Lu, L.; Zhang, L.; Xiao, Z.; Lu, S.; Yang, R.; Han, Z.C. Neuropilin-1 in Acute Myeloid Leukemia: Expression and Role in Proliferation and Migration of Leukemia Cells. Leuk. Lymphoma 2008, 49, 331–338.
- Wang, L.; Zhang, W.; Ding, Y.; Xiu, B.; Li, P.; Dong, Y.; Zhu, Q.; Liang, A. Up-regulation of VEGF and its receptor in refractory leukemia cells. Int. J. Clin. Exp. Pathol. 2015, 8, 5282–5290.
- Palodetto, B.; da Silva Santos Duarte, A.; Rodrigues Lopes, M.; Adolfo Corrocher, F.; Roversi, F.M.; Soares Niemann, F.; Priscila Vieira Ferro, K.; Leda Figueiredo Longhini, A.; Melo Campos, P.; Favaro, P.; et al. SEMA3A Partially Reverses VEGF Effects through Binding to Neuropilin-1. Stem Cell Res. 2017, 22, 70–78.
- Yang, Z.-G.; Wen, R.-T.; Qi, K.; Li, J.; Zheng, G.-X.; Wang, Y.-F.; Hong, Y.-G.; Zhang, Y.-M. The Neuropilin-1 Ligand, SEMA3A, Acts as a Tumor Suppressor in the Pathogenesis of Acute Leukemia. Anat. Rec. 2018, 302, 1127–1135.
- Karjalainen, K.; Jaalouk, D.E.; Bueso-Ramos, C.E.; Zurita, A.J.; Kuniyasu, A.; Eckhardt, B.L.; Marini, F.C.; Lichtiger, B.; O’Brien, S.; Kantarjian, H.M.; et al. Targeting neuropilin-1 in human leukemia and lymphoma. Blood 2011, 117, 920–927.
- Arpel, A.; Gamper, C.; Spenlé, C.; Fernandez, A.; Jacob, L.; Baumlin, N.; Laquerriere, P.; Orend, G.; Crémel, G.; Bagnard, D. Inhibition of Primary Breast Tumor Growth and Metastasis Using a Neuropilin-1 Transmembrane Domain Interfering Peptide. Oncotarget 2016, 7, 54723–54732.
- Naik, A.; Al-Zeheimi, N.; Bakheit, C.S.; Al Riyami, M.; Al Jarrah, A.; Al Moundhri, M.S.; Al Habsi, Z.; Basheer, M.; Adham, S.A. Neuropilin-1 Associated Molecules in the Blood Distinguish Poor Prognosis Breast Cancer: A Cross-Sectional Study. Sci. Rep. 2017, 7, 3301.
- Hellec, C.; Diawara, M.; Carpentier, M.; Denys, A.; Allain, F. The pro-Tumoral Activity of Heparan Sulfate 3-O-Sulfotransferase 3B (HS3ST3B) in Breast Cancer MDA-MB-231 Cells Is Dependent on the Expression of Neuropilin-1. Molecules 2018, 23, 2718.
- Seifi-Alan, M.; Shams, R.; Bandehpour, M.; Mirfakhraie, R.; Ghafouri-Fard, S. Neuropilin-1 Expression Is Associated with Lymph Node Metastasis in Breast Cancer Tissues. Cancer Manag. Res. 2018, 10, 1969–1974.
- Zhang, L.; Chen, Y.; Wang, H.; Zheng, X.; Li, C.; Han, Z. Mir-376A Inhibits Breast Cancer Cell Progression by Targeting Neuropilin-1 NR. Onco Targets Ther. 2018, 11, 5293–5302.
- Kiso, M.; Tanaka, S.; Saji, S.; Toi, M.; Sato, F. Long Isoform of VEGF Stimulates Cell Migration of Breast Cancer by Filopodia Formation via NRP1/ARHGAP17/cdc42 Regulatory Network. Int. J. Cancer 2018, 143, 2905–2918.
- Zhang, L.; Chen, Y.; Li, C.; Liu, J.; Ren, H.; Li, L.; Zheng, X.; Wang, H.; Han, Z. RNA Binding Protein pum2 Promotes the Stemness of Breast Cancer Cells via Competitively Binding to Neuropilin-1 (NRP-1) Mrna with Mir-376a. Biomed. Pharm. 2019, 114, 108772.
- Al-Zeheimi, N.; Naik, A.; Bakheit, C.S.; Al Riyami, M.; Al Ajarrah, A.; Al Badi, S.; Al Baimani, K.; Malik, K.; Al Habsi, Z.; Al Moundhri, M.S.; et al. Neoadjuvant Chemotherapy Alters Neuropilin-1, PLGF, and SNAI1 Expression Levels and Predicts Breast Cancer Patients Response. Front. Oncol. 2019, 25, 323.
- Wang, H.; Zhang, Y.N.; Xu, D.Q.; Huang, J.G.; Lv, D.; Shi, X.Y.; Liu, J.Y.; Ren, H.W.; Han, Z.X. Neuropilin1, a Novel Independent Prognostic Factor and Therapeutic Target in Triple-Negative Breast Cancer. Neoplasma 2021, 67, 1335–1345.
- Zhu, Q.; Li, J.; Wu, Q.; Cheng, Y.; Zheng, H.; Zhan, T.; Wang, H.; Yang, Y.; Wang, H.; Liu, Y.; et al. LINC-OIP5 in the Breast Cancer Cells Regulates Angiogenesis of Human Umbilical Vein Endothelial Cells through Yap1/Notch/NRP1 Signaling Circuit at a Tumor Microenvironment. Biol. Res. 2020, 53, 5.
- Zhao, M.; Zhang, M.; Tao, Z.; Cao, J.; Wang, L.; Hu, X. Mir-331-3p Suppresses Cell Proliferation in TNBC Cells by Downregulating NRP2. Technol. Cancer Res. Treat. 2020, 19, 153303382090582.
- Zhang, Y.; Liu, P.; Jiang, Y.; Dou, X.; Yan, J.; Ma, C.; Fan, Q.; Wang, W.; Su, F.; Tang, H.; et al. High Expression of Neuropilin-1 Associates with Unfavorable Clinicopathological Features in Hepatocellular Carcinoma. Pathol. Oncol. Res. 2015, 22, 367–375.
- Fung, T.M.; Ng, K.Y.; Tong, M.; Chen, J.-N.; Chai, S.; Chan, K.-T.; Law, S.; Lee, N.P.; Choi, M.Y.; Li, B.; et al. Neuropilin-2 Promotes Tumourigenicity and Metastasis in Oesophageal Squamous Cell Carcinoma through Erk-MAPK-ETV4-MMP-E-Cadherin Deregulation. J. Pathol. 2016, 239, 309–319.
- Dong, X.; Guo, W.; Zhang, S.; Wu, T.; Sun, Z.; Yan, S.; Zheng, S. Elevated Expression of Neuropilin-2 Associated with Unfavorable Prognosis in Hepatocellular Carcinoma. Onco Targets Ther. 2017, 10, 3827–3833.
- Lin, J.; Zhang, Y.; Wu, J.; Li, L.; Chen, N.; Ni, P.; Song, L.; Liu, X. Neuropilin 1 (NRP1) Is a Novel Tumor Marker in Hepatocellular Carcinoma. Clin. Chim. Acta 2018, 485, 158–165.
- Stasikowska-Kanicka, O.; Wągrowska-Danilewicz, M.; Danilewicz, M. Immunohistochemical Study on Neuropilin 1 (NRP1) Immunoexpression in Oral Squamous Cell Carcinoma. Folia Histochem. Cytobiol. 2018, 56, 98–105.
- Huang, Z.; Cheng, C.; Xiong, H.; Wang, Y.; Chen, K.K.; Yang, J.; Xiao, B.; Zhang, R.; Li, S.; Sang, Y. NRP1 promotes cell migration and invasion and serves as a therapeutic target in nasopharyngeal carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 2460–2469.
- Adil, A.A.; Bommanabonia, A.K.; Vaithy, A.; Kumar, S.; Waseem, M.; Jamal, S.; Ahmed, N. Differential Expression of Helios, Neuropilin-1 and Foxp3 in Head and Neck Squamous Cell Carcinoma (HNSCC) Patients. 3 Biotech. 2019, 9, 178.
- Giannelli, G.; Santoro, A.; Kelley, R.K.; Gane, E.; Paradis, V.; Cleverly, A.; Smith, C.; Estrem, S.T.; Man, M.; Wang, S.; et al. Correction: Biomarkers and Overall Survival in Patients with Advanced Hepatocellular Carcinoma Treated with TGF-ΒRI Inhibitor Galunisertib. PLoS ONE 2021, 16, e0253671.
- Zheng, Y.; Wang, C.; Song, A.; Jiang, F.; Zhou, J.; Li, G.; Zhang, W.; Ye, J.; Ding, X.; Zhang, W.; et al. CMTM6 promotes cell proliferation and invasion in oral squamous cell carcinoma by interacting with NRP1. Am. J. Cancer Res. 2020, 10, 1691–1709.
- Wu, Y.-N.; He, L.-H.; Bai, Z.-T.; Li, X. NRP1 Is a Prognostic Factor and Promotes the Growth and Migration of Cells in Intrahepatic Cholangiocarcinoma. Cancer Manag. Res. 2020, 12, 7021–7032.
- Morin, E.; Lindskog, C.; Johansson, M.; Egevad, L.; Sandström, P.; Harmenberg, U.; Claesson-Welsh, L.; Sjöberg, E. Perivascular Neuropilin-1 Expression Is an Independent Marker of Improved Survival in Renal Cell Carcinoma. J. Pathol. 2020, 250, 387–396.
- Xu, J.-C.; Chen, T.-Y.; Liao, L.-T.; Chen, T.; Li, Q.-L.; Xu, J.-X.; Hu, J.-W.; Zhou, P.-H.; Zhang, Y.-Q. NETO2 Promotes Esophageal Cancer Progression by Inducing Proliferation and Metastasis via PI3K/Akt and Erk Pathway. Int. J. Biol. Sci. 2021, 17, 259–270.
- Dumond, A.; Brachet, E.; Durivault, J.; Vial, V.; Puszko, A.K.; Lepelletier, Y.; Montemagno, C.; Pagnuzzi-Boncompagni, M.; Hermine, O.; Garbay, C.; et al. Neuropilin 1 and Neuropilin 2 Gene Invalidation or Pharmacological Inhibition Reveals Their Relevance for the Treatment of Metastatic Renal Cell Carcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 33.
- Lee, S.W.; Park, K.C.; Kim, J.G.; Moon, S.J.; Kang, S.B.; Lee, D.S.; Sul, H.J.; Ji, J.S.; Jeong, H.Y. Dysregulation of MicroRNA-196B-5p and MicroRNA-375 in Gastric Cancer. J. Gastric Cancer 2016, 16, 221–229.
- Lian, H.; Liu, C.; Li, M.; Hu, Y.; Shi, N.; Yu, H.; Liu, H. Mir-486-5p Attenuates Tumor Growth and Lymphangiogenesis by Targeting Neuropilin-2 in Colorectal Carcinoma. Onco Targets Ther. 2016, 9, 2865–2871.
- Chen, Z.-P.; Wei, J.-C.; Wang, Q.; Yang, P.; Li, W.-L.; He, F.; Chen, H.C.; Hu, H.; Zhong, J.-B.; Cao, J. Long Non-Coding RNA 00152 Functions as a Competing Endogenous RNA to Regulate NRP1 Expression by Sponging with MIRNA-206 in Colorectal Cancer. Int. J. Oncol. 2018, 53, 1227–1236.
- Tomida, C.; Yamagishi, N.; Nagano, H.; Uchida, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. VEGF Pathway-Targeting Drugs Induce Evasive Adaptation by Activation of Neuropilin-1/CMET in Colon Cancer Cells. Int. J. Oncol. 2018, 52, 1350–1362.
- Liu, A.; Liu, L.; Lu, H. LncRNA Xist Facilitates Proliferation and Epithelial–Mesenchymal Transition of Colorectal Cancer Cells through Targeting Mir-486-5p and Promoting Neuropilin-2. J. Cell Physiol. 2019, 234, 13747–13761.
- Bollard, J.; Patte, C.; Radkova, K.; Massoma, P.; Chardon, L.; Valantin, J.; Gadot, N.; Goddard, I.; Vercherat, C.; Hervieu, V.; et al. Neuropilin-2 Contributes to Tumor Progression in Preclinical Models of Small Intestinal Neuroendocrine Tumors. J. Pathol. 2019, 249, 343–355.
- Huang, X.; Ye, Q.; Chen, M.; Li, A.; Mi, W.; Fang, Y.; Zaytseva, Y.Y.; O’Connor, K.L.; Vander Kooi, C.W.; Liu, S.; et al. N-Glycosylation-Defective Splice Variants of Neuropilin-1 Promote Metastasis by Activating Endosomal Signals. Nat. Commun. 2019, 10, 3708.
- Ding, G.Y.; Xu, W.G.; Guo, S.; Zhan, Q.Q.; Yang, X.; Jia, C.L. Effect of RNA interference targeting neuropilin-2 gene on proliferation and apoptosis of colon cancer cell line HCT-8. Zhonghua Yi Xue Za Zhi 2020, 100, 3879–3883.
- De Vlaeminck, Y.; Bonelli, S.; Awad, R.M.; Dewilde, M.; Rizzolio, S.; Lecocq, Q.; Bolli, E.; Santos, A.R.; Laoui, D.; Schoonooghe, S.; et al. Targeting Neuropilin-1 with Nanobodies Reduces Colorectal Carcinoma Development. Cancers 2020, 12, 3582.
- Kang, S.; Lee, S.; Park, S. IRGD Peptide as a Tumor-Penetrating Enhancer for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 1906.
- Akagi, M.; Kawaguchi, M.; Liu, W.; McCarty, M.F.; Takeda, A.; Fan, F.; Stoeltzing, O.; Parikh, A.A.; Jung, Y.D.; Bucana, C.D.; et al. Induction of Neuropilin-1 and Vascular Endothelial Growth Factor by Epidermal Growth Factor in Human Gastric Cancer Cells. Br. J. Cancer 2003, 88, 796–802.
- Li, L.; Jiang, X.; Zhang, Q.; Dong, X.; Gao, Y.; He, Y.; Qiao, H.; Xie, F.; Xie, X.; Sun, X. Neuropilin-1 Is Associated with Clinicopathology of Gastric Cancer and Contributes to Cell Proliferation and Migration as Multifunctional Coreceptors. J. Exp. Clin. Cancer Res. 2016, 35, 16.
- Zhang, L.; Xing, Y.; Gao, Q.; Sun, X.; Zhang, D.; Cao, G. Combination of NRP1-Mediated Irgd with 5-Fluorouracil Suppresses Proliferation, Migration and Invasion of Gastric Cancer Cells. Biomed. Pharmacother. 2017, 93, 1136–1143.
- Wang, X.; Hu, H.; Liu, H. RNA Binding Protein lin28b Confers Gastric Cancer Cells Stemness via Directly Binding to NRP-1. Biomed. Pharmacother. 2018, 104, 383–389.
- Wang, G.; Shi, B.; Fu, Y.; Zhao, S.; Qu, K.; Guo, Q.; Li, K.; She, J. Hypomethylated Gene NRP1 Is Co-Expressed with PDGFRB and Associated with Poor Overall Survival in Gastric Cancer Patients. Biomed. Pharmacother. 2019, 111, 1334–1341.
- Zhuo, Y.J.; Shi, Y.; Wu, T. NRP-1 and KDR Polymorphisms Are Associated with Survival Time in Patients with Advanced Gastric Cancer. Oncol. Lett. 2019, 18, 4629–4638.
- Pang, W.; Zhai, M.; Wang, Y.; Li, Z. Long Noncoding RNA SNHG16 Silencing Inhibits the Aggressiveness of Gastric Cancer via Upregulation of MicroRNA-628-3P and Consequent Decrease of nrp1. Cancer Manag. Res. 2019, 11, 7263–7277.
- Hang, C.; Yan, H.S.; Gong, C.; Gao, H.; Mao, Q.H.; Zhu, J.X. MicroRNA-9 Inhibits Gastric Cancer Cell Proliferation and Migration by Targeting Neuropilin-1. Exp. Ther. Med. 2019, 18, 2524–2530.
- Wu, C.; Zeng, M.-H.; Liao, G.; Qian, K.; Li, H. Neuropilin-1 Interacts with Fibronectin-1 to Promote Epithelial–Mesenchymal Transition Progress in Gastric Cancer. Onco Targets Ther. 2020, 13, 10677–10687.
- Wei, Y.; Guo, S.; Tang, J.; Wen, J.; Wang, H.; Hu, X.; Gu, Q. MicroRNA-19B-3P Suppresses Gastric Cancer Development by Negatively Regulating Neuropilin-1. Cancer Cell Int. 2020, 25, 193.
- Cao, H.; Li, Y.; Huang, L.; Bai, B.; Xu, Z. Clinicopathological Significance of Neuropilin 1 Expression in Gastric Cancer: A Meta-Analysis. Dis. Markers 2020, 2020, 4763492.
- Seo, H.S.; Hyeon, J.; Song, I.H.; Lee, H.H. Relationship between Neuropilin-1 Expression and Prognosis, According to Gastric Cancer Histology. J. Mol. Histol. 2020, 51, 199–208.
- Dong, J.C.; Gao, H.; Zuo, S.Y.; Zhang, H.Q.; Zhao, G.; Sun, S.L.; Han, H.L.; Jin, L.L.; Shao, L.H.; Wei, W.; et al. Neuropilin 1 Expression Correlates with the Radio-Resistance of Human Non-Small-Cell Lung Cancer Cells. J. Cell Mol. Med. 2015, 19, 2286–2295.
- Gemmill, R.M.; Nasarre, P.; Nair-Menon, J.; Cappuzzo, F.; Landi, L.; D’Incecco, A.; Uramoto, H.; Yoshida, T.; Haura, E.B.; Armeson, K.; et al. The Neuropilin 2 Isoform nrp2b Uniquely Supports TGFΒ-Mediated Progression in Lung Cancer. Sci. Signal. 2017, 10, eaag0528.
- Hu, C.; Zhu, P.; Xia, Y.; Hui, K.; Wang, M.; Jiang, X. Role of the NRP-1-Mediated VEGFR2-Independent Pathway on Radiation Sensitivity of Non-Small Cell Lung Cancer Cells. J. Cancer Res. Clin. Oncol. 2018, 144, 1329–1337.
- Jimenez-Hernandez, L.; Vazquez-Santillan, K.; Castro-Oropeza, R.; Martinez-Ruiz, G.; Muñoz -Galindo, L.; Gonzalez-Torres, C.; Cortes-Gonzalez, C.; Victoria-Acosta, G.; Melendez-Zajgla, J.; Maldonado, V. NRP1-Positive Lung Cancer Cells Possess Tumor-Initiating Properties. Oncol. Rep. 2017, 39, 349–357.
- Xiong, K.; Shao, L.H.; Zhang, H.Q.; Jin, L.; Wei, W.; Dong, Z.; Zhu, Y.Q.; Wu, N.; Jin, S.Z.; Xue, L.X. MicroRNA-9 Functions as a Tumor Suppressor and Enhances Radio-Sensitivity in Radio-Resistant A549 Cells by Targeting Neuropilin 1. Oncol. Lett. 2017, 15, 2863–2870.
- Ding, Z.; Du, W.; Lei, Z.; Zhang, Y.; Zhu, J.; Zeng, Y.; Wang, S.; Zheng, Y.; Liu, Z.; Huang, J.A. Neuropilin 1 Modulates Tgf-β1-Induced Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. Int. J. Oncol. 2019, 15, 2863–2870.
- Ding, Z.; Zhu, J.; Zeng, Y.; Du, W.; Zhang, Y.; Tang, H.; Zheng, Y.; Qin, H.; Liu, Z.; Huang, J. The Regulation of Neuropilin 1 Expression by Mir-338-3p Promotes Non-Small Cell Lung Cancer via Changes in EGFR Signaling. Mol. Carcinog. 2019, 58, 1019–1032.
- Dong, Z.; Zhang, H.; Gong, X.; Wei, W.; Lv, Y.; Chen, Z.; Wang, R.; Yi, J.; Shen, Y.; Jin, S. The Role of the Tumor Microenvironment in Neuropilin 1-Induced Radiation Resistance in Lung Cancer Cells. J. Cancer 2019, 10, 4017–4030.
- Kim, Y.-J.; Baek, D.-S.; Lee, S.; Park, D.; Kang, H.N.; Cho, B.C.; Kim, Y.-S. Dual-Targeting of EGFR and Neuropilin-1 Attenuates Resistance to EGFR-Targeted Antibody Therapy in KRAS-Mutant Non-Small Cell Lung Cancer. Cancer Lett. 2019, 466, 23–34.
- Ding, M.; Liu, L.; Hu, C.; Liu, Y.; Qiao, Y.; Jiang, X. Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance. Chin. J. Cancer Res. 2014, 26, 669–677.
- Dimou, A.; Nasarre, C.; Peterson, Y.K.; Pagano, R.; Gooz, M.; Nasarre, P.; Drabkin, H.A.; Armeson, K.E.; Gibney, B.C.; Gemmill, R.M.; et al. Neuropilin-2B Facilitates Resistance to Tyrosine Kinase Inhibitors in Non–Small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 2021, 162, 463–473.
- Matkar, P.N.; Singh, K.K.; Rudenko, D.; Kim, Y.J.; Kuliszewski, M.A.; Prud’homme, G.J.; Hedley, D.W.; Leong-Poi, H. Novel Regulatory Role of Neuropilin-1 in Endothelial-to-Mesenchymal Transition and Fibrosis in Pancreatic Ductal Adenocarcinoma. Oncotarget 2016, 7, 69489–69506.
- Liu, X.; Lin, P.; Perrett, I.; Lin, J.; Liao, Y.-P.; Chang, C.H.; Jiang, J.; Wu, N.; Donahue, T.; Wainberg, Z.; et al. Tumor-Penetrating Peptide Enhances Transcytosis of Silicasome-Based Chemotherapy for Pancreatic Cancer. J. Clin. Investig. 2017, 127, 2007–2018.
- Morin, E.; Sjöberg, E.; Tjomsland, V.; Testini, C.; Lindskog, C.; Franklin, O.; Sund, M.; Öhlund, D.; Kiflemariam, S.; Sjöblom, T.; et al. VEGF Receptor-2/Neuropilin 1 Trans.-Complex Formation between Endothelial and Tumor Cells Is an Independent Predictor of Pancreatic Cancer Survival. J. Pathol. 2018, 246, 311–322.
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861.
- Ma, L.; Zhai, B.; Zhu, H.; Li, W.; Jiang, W.; Lei, L.; Zhang, S.; Qiao, H.; Jiang, X.; Sun, X. The Mir-141/Neuropilin-1 Axis Is Associated with the Clinicopathology and Contributes to the Growth and Metastasis of Pancreatic Cancer. Cancer Cell Int. 2019, 19, 248.
- Li, X.; Fan, S.; Pan, X.; Xiaokaiti, Y.; Duan, J.; Shi, Y.; Pan, Y.; Tie, L.; Wang, X.; Li, Y.; et al. Nordihydroguaiaretic Acid Impairs Prostate Cancer Cell Migration and Tumor Metastasis by Suppressing Neuropilin 1. Oncotarget 2016, 7, 86225–86238.
- Tse, B.W.; Volpert, M.; Ratther, E.; Stylianou, N.; Nouri, M.; McGowan, K.; Lehman, M.L.; McPherson, S.J.; Roshan-Moniri, M.; Butler, M.S.; et al. Neuropilin-1 Is Upregulated in the Adaptive Response of Prostate Tumors to Androgen-Targeted Therapies and Is Prognostic of Metastatic Progression and Patient Mortality. Oncogene 2017, 36, 3417–3427.
- Borkowetz, A.; Froehner, M.; Rauner, M.; Conrad, S.; Erdmann, K.; Mayr, T.; Datta, K.; Hofbauer, L.C.; Baretton, G.B.; Wirth, M.; et al. Neuropilin-2 Is an Independent Prognostic Factor for Shorter Cancer-Specific Survival in Patients with Acinar Adenocarcinoma of the Prostate. Int. J. Cancer 2019, 146, 2619–2627.
- Moriarty, W.F.; Kim, E.; Gerber, S.A.; Hammers, H.; Alani, R.M. Neuropilin-2 Promotes Melanoma Growth and Progression in Vivo. Melanoma Res. 2016, 26, 321–328.
- Dou, X.; Yan, J.; Zhang, Y.; Liu, P.; Jiang, Y.; Lv, S.; Zeng, F.; Chen, X.; Wang, S.; Zhang, H.; et al. SPECT Imaging of Neuropilin Receptor Type-1 Expression with 131i-Labeled Monoclonal Antibody. Int. J. Oncol. 2016, 49, 961–970.
- Eisenstein, A.; Panova, I.P.; Chung, H.J.; Goldberg, L.J.; Zhang, Q.; Lazova, R.; Bhawan, J.; Busam, K.J.; Symanowski, J.T.; Alani, R.M.; et al. Quantitative Assessment of Neuropilin-2 as a Simple and Sensitive Diagnostic Assay for SPITZOID Melanocytic Lesions. Melanoma Res. 2018, 28, 71–75.
- Rizzolio, S.; Cagnoni, G.; Battistini, C.; Bonelli, S.; Isella, C.; Van Ginderachter, J.A.; Bernards, R.; Di Nicolantonio, F.; Giordano, S.; Tamagnone, L. Neuropilin-1 Upregulation Elicits Adaptive Resistance to Oncogene-Targeted Therapies. J. Clin. Investig. 2018, 128, 3976–3990.
- Zhang, G.; Chen, L.; Sun, K.; Khan, A.A.; Yan, J.; Liu, H.; Lu, A.; Gu, N. Neuropilin-1 (NRP-1)/GIPC1 Pathway Mediates Glioma Progression. Tumor Biol. 2016, 37, 13777–13788.
- Kwiatkowski, S.C.; Guerrero, P.A.; Hirota, S.; Chen, Z.; Morales, J.E.; Aghi, M.; McCarty, J.H. Neuropilin-1 Modulates TGFΒ Signaling to Drive Glioblastoma Growth and Recurrence after Anti-Angiogenic Therapy. PLoS ONE 2017, 12, e0185065. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caponegro, M.D.; Moffitt, R.A.; Tsirka, S.E. Expression of Neuropilin-1 Is Linked to Glioma Associated Microglia and Macrophages and Correlates with Unfavorable Prognosis in High Grade Gliomas. Oncotarget 2018, 9, 35655–35665. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyauchi, J.T.; Caponegro, M.D.; Chen, D.; Choi, M.K.; Li, M.; Tsirka, S.E. Deletion of Neuropilin 1 from Microglia or Bone Marrow–Derived Macrophages Slows Glioma Progression. Cancer Res. 2017, 78, 685–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.; Chen, L.; Khan, A.A.; Li, B.; Gu, B.; Lin, F.; Su, X.; Yan, J. MIRNA-124-3p/Neuropilin-1(NRP-1) Axis Plays an Important Role in Mediating Glioblastoma Growth and Angiogenesis. Int. J. Cancer 2018, 143, 635–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, C.; Almasoud, A.; Pellegrini-Moïse, N.; Pinel, S.; Barberi-Heyob, M.; Chastagner, P.; Boura, C. PO-013 a Novel Peptidomimetic Targeting NRP1 Increases Radiosensitivity of Medulloblastoma Stem Cells. ESMO Open 2018, 3, A232. [Google Scholar] [CrossRef][Green Version]
- Gong, C.; Valduga, J.; Chateau, A.; Richard, M.; Pellegrini-Moïse, N.; Barberi-Heyob, M.; Chastagner, P.; Boura, C. Stimulation of Medulloblastoma Stem Cells Differentiation by a Peptidomimetic Targeting Neuropilin-1. Oncotarget 2018, 9, 15312–15325. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.C.; Shen, H.X.; Chen, C.; Ma, L.; Li, W.Z.; Wang, L.; Geng, Z.M. Neuropilin-1 Promotes Primary Liver Cancer Progression by Potentiating the Activity of Hepatic Stellate Cells. Oncol. Lett. 2017, 15, 2245–2251.
- Grun, D.; Adhikary, G.; Eckert, R.L. VEGF-A Acts via Neuropilin-1 to Enhance Epidermal Cancer Stem Cell Survival and Formation of Aggressive and Highly Vascularised Tumors. Oncogene 2016, 35, 4379–4387.
- Liu, W.; Wu, T.; Dong, X.; Zeng, Y.A. Neuropilin-1 Is Upregulated by Wnt/β-Catenin Signaling and Is Important for Mammary Stem Cells. Sci. Rep. 2017, 7, 10941.
- Tang, Y.H.; Rockstroh, A.; Sokolowski, K.A.; Lynam, L.R.; Lehman, M.; Thompson, E.W.; Gregory, P.A.; Nelson, C.C.; Volpert, M.; Hollier, B.G. Neuropilin-1 is over-expressed in claudin-low breast cancer and promotes tumor progression through acquisition of stem cell characteristics and RAS/MAPK pathway activation. Breast Cancer Res. 2022, 24, 8.
- Shi, F.; Shang, L.; Yang, L.-Y.; Jiang, Y.-Y.; Wang, X.-M.; Hao, J.-J.; Zhang, Y.; Huang, D.-K.; Cai, Y.; Xu, X.; et al. Neuropilin-1 Contributes to Esophageal Squamous Cancer Progression via Promoting p65-Dependent Cell Proliferation. Oncogene 2018, 37, 935–943.
- Grun, D.; Adhikary, G.; Eckert, R.L. NRP-1 Interacts with GIPC1 and SYX to Activate p38 MAPK Signaling and Cancer Stem Cell Survival. Mol. Carcinog. 2018, 58, 488–499.
- Grun, D.; Adhikary, G.; Eckert, R.L. NRP-1 Interacts with GIPC1 and α6/Β4-Integrins to Increase yap1/∆np63α-Dependent Epidermal Cancer Stem Cell Survival. Oncogene 2019, 37, 4711–4722.
- Tu, D.-G.; Chang, W.-W.; Jan, M.-S.; Tu, C.-W.; Lu, Y.-C.; Tai, C.-K. Promotion of Metastasis of Thyroid Cancer Cells via NRP-2-Mediated Induction. Oncol. Lett. 2016, 12, 4224–4230.
- Lee, G.; Kang, Y.E.; Oh, C.; Liu, L.; Jin, Y.; Lim, M.A.; Won, H.-R.; Chang, J.W.; Koo, B.S. Neuropilin-2 Promotes Growth and Progression of Papillary Thyroid Cancer Cells. Auris Nasus Larynx 2020, 47, 870–880.
- Karczmarczyk, A.; Bilska, S.; Korpysz, M.; Purkot, J.; Grząśko, N.; Hus, M.; Giannopoulos, K. Expression and Clinical Significance of Neuropilin-1 in Patients with Multiple Myeloma. Anticancer Res. 2020, 40, 5437–5443.
- Gu, Y.Y.; Luo, B.; Li, C.Y.; Huang, L.S.; Chen, G.; Feng, Z.B.; Peng, Z.G. Expression and clinical significance of neuropilin-1 in Epstein-Barr virus-associated lymphomas. Cancer Biomark. 2019, 25, 259–273.
- Keck, B.; Wach, S.; Taubert, H.; Zeiler, S.; Ott, O.J.; Kunath, F.; Hartmann, A.; Bertz, S.; Weiss, C.; Hönscheid, P.; et al. Neuropilin-2 and Its Ligand VEGF-C Predict Treatment Response after Transurethral Resection and Radiochemotherapy in Bladder Cancer Patients. Int. J. Cancer 2014, 136, 443–451.
- Schulz, A.; Gorodetska, I.; Behrendt, R.; Fuessel, S.; Erdmann, K.; Foerster, S.; Datta, K.; Mayr, T.; Dubrovska, A.; Muders, M.H. Linking NRP2 with EMT and Chemoradioresistance in Bladder Cancer. Front. Oncol. 2020, 9, 1461.
- Ong, H.S.; Gokavarapu, S.; Xu, Q.; Tian, Z.; Li, J.; Ji, T.; Zhang, C.P. Cytoplasmic Neuropilin 2 Is Associated with Metastasis and a Poor Prognosis in Early Tongue Cancer Patients. Int. J. Oral Maxillofac. Surg. 2017, 46, 1205–1219.
- Fujii, T.; Shimada, K.; Asano, A.; Tatsumi, Y.; Yamaguchi, N.; Yamazaki, M.; Konishi, N. MicroRNA-331-3p Suppresses Cervical Cancer Cell Proliferation and E6/E7 Expression by Targeting NRP2. Int. J. Mol. Sci. 2016, 17, 1351.
- Yang, L.; Liu, L.; Zhu, Y.; Wang, B.-B.; Chen, Y.-N.; Zhang, F.; Zhang, X.-A.; Ren, C.-C. Neuropilin-1 Is Associated with the Prognosis of Cervical Cancer in Henan Chinese Population. Onco Targets Ther. 2019, 12, 2911–2920.
- Chen, C.; Zhang, R.; Ma, L.; Li, Q.; Zhao, Y.; Zhang, G.; Zhang, D.; Li, W.; Cao, S.; Wang, L.; et al. Neuropilin-1 Is up-Regulated by Cancer-Associated Fibroblast-Secreted Il-8 and Associated with Cell Proliferation of Gallbladder Cancer. J. Cell Mol. Med. 2020, 24, 12608–12618.
- Oplawski, M.; Dziobek, K.; Grabarek, B.; Zmarzły, N.; Dąbruś, D.; Januszyk, P.; Brus, R.; Tomala, B.; Boroń, D. Expression of NRP-1 and NRP-2 in Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 254–260.
- Huang, N.; Xu, L.; Qiu, Y.; Zhan, J.; Chen, X. Down-Regulated Mir-124-3p Enhanced the Migration and Epithelial-Stromal Transformation of Endometrial Stromal Cells Extracted from Eutopic Endometrium in Subjects with Adenomyosis by up-Regulating Neuropilin 1. Tissue Cell 2021, 69, 101474.
- Miyauchi, J.T.; Chen, D.; Choi, M.; Nissen, J.C.; Shroyer, K.R.; Djordevic, S.; Zachary, I.C.; Selwood, D.; Tsirka, S.E. Ablation of Neuropilin 1 from glioma-associated microglia and macrophages slows tumor progression. Oncotarget 2016, 7, 9801–9814.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
5 times
(View History)
Update Date:
08 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




