Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ioana Buculei | -- | 4923 | 2022-09-28 19:11:37 | | | |
| 2 | Catherine Yang | Meta information modification | 4923 | 2022-09-29 02:51:18 | | | | |
| 3 | Catherine Yang | Meta information modification | 4923 | 2022-10-04 04:33:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matei, D.; Buculei, I.; Luca, C.; Corciova, C.; Andritoi, D.; Fuior, R.; Iordan, D.; Onu, I. Non-Pharmacological Options in Atherosclerosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/27916 (accessed on 07 February 2026).
Matei D, Buculei I, Luca C, Corciova C, Andritoi D, Fuior R, et al. Non-Pharmacological Options in Atherosclerosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/27916. Accessed February 07, 2026.
Matei, Daniela, Ioana Buculei, Catalina Luca, Calin-Petru Corciova, Doru Andritoi, Robert Fuior, Daniel-Andrei Iordan, Ilie Onu. "Non-Pharmacological Options in Atherosclerosis" Encyclopedia, https://encyclopedia.pub/entry/27916 (accessed February 07, 2026).
Matei, D., Buculei, I., Luca, C., Corciova, C., Andritoi, D., Fuior, R., Iordan, D., & Onu, I. (2022, September 28). Non-Pharmacological Options in Atherosclerosis. In Encyclopedia. https://encyclopedia.pub/entry/27916
Matei, Daniela, et al. "Non-Pharmacological Options in Atherosclerosis." Encyclopedia. Web. 28 September, 2022.
Copy Citation
Atherosclerosis remains the leading cause of mortality and morbidity worldwide characterized by the deposition of lipids and fibrous elements in the form of atheroma plaques in vascular areas which are hemodynamically overloaded. Atherosclerotic cardiovascular disease is the leading cause of death and disability. Prolonged survival with chronic disease explains why the prevalence, burden, and costs of the disease remain high. Given these issues, aggressive treatment should be started at the first indication and continued over several years, along with the reduction in disease risk factors such as visceral adiposity, dyslipidemia, hypertension, and diabetes.
endothelial dysfunction
sympathetic nervous system
caloric restriction
fasting
diet
1. Caloric Restriction
Caloric restriction (CR) is a concept involving dietary interventions with chronic or periodic reduced energy intake, without malnutrition. CR prolongs a healthy lifespan in a variety of animal species and humans, but the most important limitation for humans is its long-term sustainability. CR generates cellular and metabolic adaptations that delay aging processes, prolong the maximum lifespan and consistently improve insulin resistance. Insulin resistance is associated with cardiovascular disease, but there are not enough data at present to show that increased insulin sensitivity reduces the progression of atherosclerosis [1][2].
CR is considered the most effective and reproducible dietary intervention, and is known to affect the aging process and increase healthy lifespan in primates and rodents. CR involves a 20–40% reduction in dietary requirements compared to normal intake; exceeding a 40% reduction can be considered a severe intervention, resulting in both beneficial and detrimental effects. However, there is no common agreement regarding how severe CR needs to be to have measurable benefits in different organs and systems [3][4].
CR improves risk factors for cardiometabolic disease, risks that include metabolic syndrome, atherogenic dyslipidemia (elevated triglycerides and fasting glucose, reduced high-density lipoprotein (HDL)-cholesterol), as well as high blood pressure and diabetes, and a large waist circumference. Visceral and subcutaneous adiposity leading to prediabetes and type 2 diabetes are seen as the most impotent risk factors in cardiometabolic diseases. CR can also enhance risk factors by imposing a negative energy balance-in both animal models and humans [2][5][6].
The Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) was a two-year multicenter RCT to assess CR in healthy non-obese individuals for anti-aging adaptations in resting metabolic rate and core body temperature. CR reduced body weight, BMI, and blood pressure and improved plasma cholesterol concentrations to 300 kcal/day. At this resting metabolic rate, there was no significant influence on baseline body temperature. CALERIE answered a number of questions on CR in metabolically normal individuals by pointing out that there are effects on emerging measures of cardiometabolic risk and that these effects were modified by beginning BMI strata and gender [7][8].
It is well established that CR delays aging processes, especially in primates. The mechanisms by which CR exerts its effects are not fully elucidated, but chronic CR is associated with improved insulin resistance, delaying physiological changes, and age-associated diseases. The molecular etiology of insulin resistance directly contributes to the development of atherosclerotic cardiovascular disease through the inhibition of nitric oxide production and stimulation of the Mitogen-Activated Protein Kinase (MAPK) pathway [9]. It has been shown that by using pharmacological or non-pharmacological approaches, such as CR, insulin resistance can be improved, with a positive impact on specific risk factors for cardiovascular disease in humans. Furthermore, it has been hypothesized that improving insulin sensitivity will lead to a reduction in cardiovascular events, which ultimately attenuates the progression of atherosclerosis. This hypothesis does not refer to the direct relationship between atherosclerosis and improved insulin sensitivity, but rather to the beneficial effects of improved insulin sensitivity on other risk factors in cardiovascular disease [9].
CR is considered the most cost-effective intervention to reduce body weight and control risk factors for cardiovascular disease. To achieve weight loss and metabolic benefits, it is necessary to induce a negative energy balance by reducing caloric intake in obese subjects. This negative energy balance will have a favorable impact on the risk factors for cardiovascular diseases, particularly atherosclerosis [10]. CR reduces body weight, waist circumference by decreasing visceral fat, insulin levels and improves insulin sensitivity, and serum lipids, which will induce a reduction in proinflammatory adipokines, IL-6, IL-12, IL-18, and TNFα, etc., as well as an increase in anti-inflammatory adipokines, adiponectin and omentin, and lead to a significant reduction in oxidative stress [11][12]. Weight loss due to CR improves flow-mediated dilatation, which, in turn, significantly improves endothelial function in vitro in overweight adults [11].
CR has a complex molecular mechanism involving a reduction in insulin (IGF-1 pathway) and insulin-like signaling, mammalian target of rapamycin (mTOR) signaling kinase pathway, and an increase in the energy balance modulator sirtuin. From the same complex mechanism, a decrease is achieved in pro-inflammatory mediators, ROS production, and growth factors [3]. Sirtuins are responsible for the beneficial and longevity-promoting effects of CR in many animal species. Sirtuins have been shown to play an important role in physiological adaptation to CR with two distinct characteristics: resistance to oxidative stress and metabolic reprogramming to oxidative metabolism (required to access the highest possible energy from fuel sources) [13]. Sirtuins (especially sirtuin 1) are involved in many physiological effects, such as inflammation, aging, mitochondrial biogenesis apoptosis, and circadian clock control [14].
In this context, CR is an important clinical tool that reduces insulin signaling, and inflammation (NF-κB signaling and COX-2 expression-decreases pro-inflammatory adipokine levels), reduces angiogenic mediators, and pro-angiogenic leptin, and increases anti-angiogenic adiponectin levels [15][16]. CR has anti-inflammatory effects that contribute to endothelial function, which is directly related to the restoration of vascular tone, preservation of vascular wall integrity, regulation of angiogenesis, and hemostasis. It appears that the anti-inflammatory effect is not the only effect that CR exerts on the endothelium, in addition to the regulation of fibrinolysis, angiogenic factors, and the integrity of the basement membrane and extracellular matrix proteins [17]. CR influences angiogenesis by consistently decreasing circulating levels of the plasminogen activator inhibitor-1 (PAI-1), and matrix metalloproteinases (MMPs). It is shown that (PAI-1), u-PA, t-PA, and MMPs are deeply involved in angiogenesis [18]. CR leads to the increased expression of eNOS and the transcriptional factor nuclear erythroid factor 2-related factor (Nrf2), which activates the VEGF-dependent metabolic pathways and produces antioxidant stress proteins [4].
2. Fasting
Along with CR, fasting is proving to be an effective strategy to optimize health, reduce weight, and delay aging. Results from well-controlled investigations in animal and human experimental models have validated the method as being effective, but in certain situations, human subject compliance makes this infeasible. Fasting is defined as the absence of food or caloric drinks for periods ranging from 12 h to 16 h or, even more severely, 22 h. During the allowed hours, 2–3 meals are still eaten at one-hour intervals, and whole foods are still consumed. Fasting is associated with ketogenesis and promotes changes in metabolic pathways and cellular processes, such as lipolysis, autophagy, and stress resistance [19][20].
In 2015, Longo VD et al. concluded that intermittent fasting is less restrictive than traditional 40% CR, as daily caloric intake is similar to a short, strict CR. The authors highlighted the positive effects of intermittent fasting on cardiovascular health [21]. The most feasible form of fasting is intermittent fasting, including alternative fasting or fasting 2 days a week, for example. The most widely discussed forms of intermittent fasting are time-restricted feeding, in which individuals fast for 16 h–20 h a day; the 5:2 approach, meaning 2 non-consecutive days of energy restriction per week; or alternative days with total fasting.
In a clinical study of middle-aged individuals, Stekovic S. et al. demonstrated that 4-week intermittent fasting improved cardiovascular parameters, inflammatory markers, triiodothyronine and LDL levels, and significantly reduced trunk fat mass [22]. Whether intermittent fasting directly affects cardiovascular markers, or whether cardiovascular risks decrease secondary to weight loss, is debatable. However, a 3–24 week fast reduced the body weight of individuals enrolled in the studies, and the best results were obtained in individuals who had a low-calorie meal each day of fasting [23][24].
Klempel MC et al. compared alternative fasting and periodic fasting and demonstrated that alternative fasting is much more effective in reducing body weight [23]. However, both fasting strategies showed positive effects in reducing triglyceride levels, decreasing body weight [23][25].
Several studies show that weight loss from intermittent fasting is associated with decreased systolic and diastolic blood pressure, suggesting that both strategies prevent progression from prehypertension to hypertension [25]. Intermittent fasting has a positive effect on individuals with prediabetes by lowering blood glucose, but no changes were observed in healthy individuals. The reported evidence showed benefits in individuals who fasted on alternate days, and the insulin levels considerably decreased. Strong arguments have been made in favor of lowering insulin concentrations in studies in which subjects had severe CR, and lower insulin levels were associated with the severity of energy restriction [23][25].
3. Diet
Cardiovascular diseases are known to be the leading cause of death in developed countries due to the consumption of processed, high-carbohydrate, and high-fat foods. However, it has been observed that the incidence of cardiovascular diseases is much lower in Mediterranean countries, and this has mainly been attributed to dietary habits. In patients with coronary heart disease, following a Mediterranean diet is more effective in reducing the progression of atherosclerosis than following a low-fat diet. It appears that the consumption of extra virgin olive oil is a key element that could prevent the progression of atherosclerosis, as it is predominantly consumed in the Mediterranean diet [26][27].
The Mediterranean diet is rich in fish, vegetables, whole grains, fruit, nuts, and extra-virgin olive oil. In a systematic review on the effect of lifestyle and dietary changes in patients with coronary heart disease, Iestra JA et al. demonstrated that the mortality rate decreased in subjects who consumed extra-virgin olive oil compared to those who consumed other fats [28]. This could be explained by the improved endothelial function, reduced serum cholesterol and lower blood pressure found in individuals following the Mediterranean diet.
Fasting, the Mediterranean diet, and CR can be considered useful clinical tools in the management of atherosclerotic cardiovascular disease by managing conventional risk factors. These non-pharmacological options are positively associated with increased endothelial function, anti-inflammatory effect, and reductions in low oxidative stress, thus reducing the risk of cardiovascular disease (Figure 1).
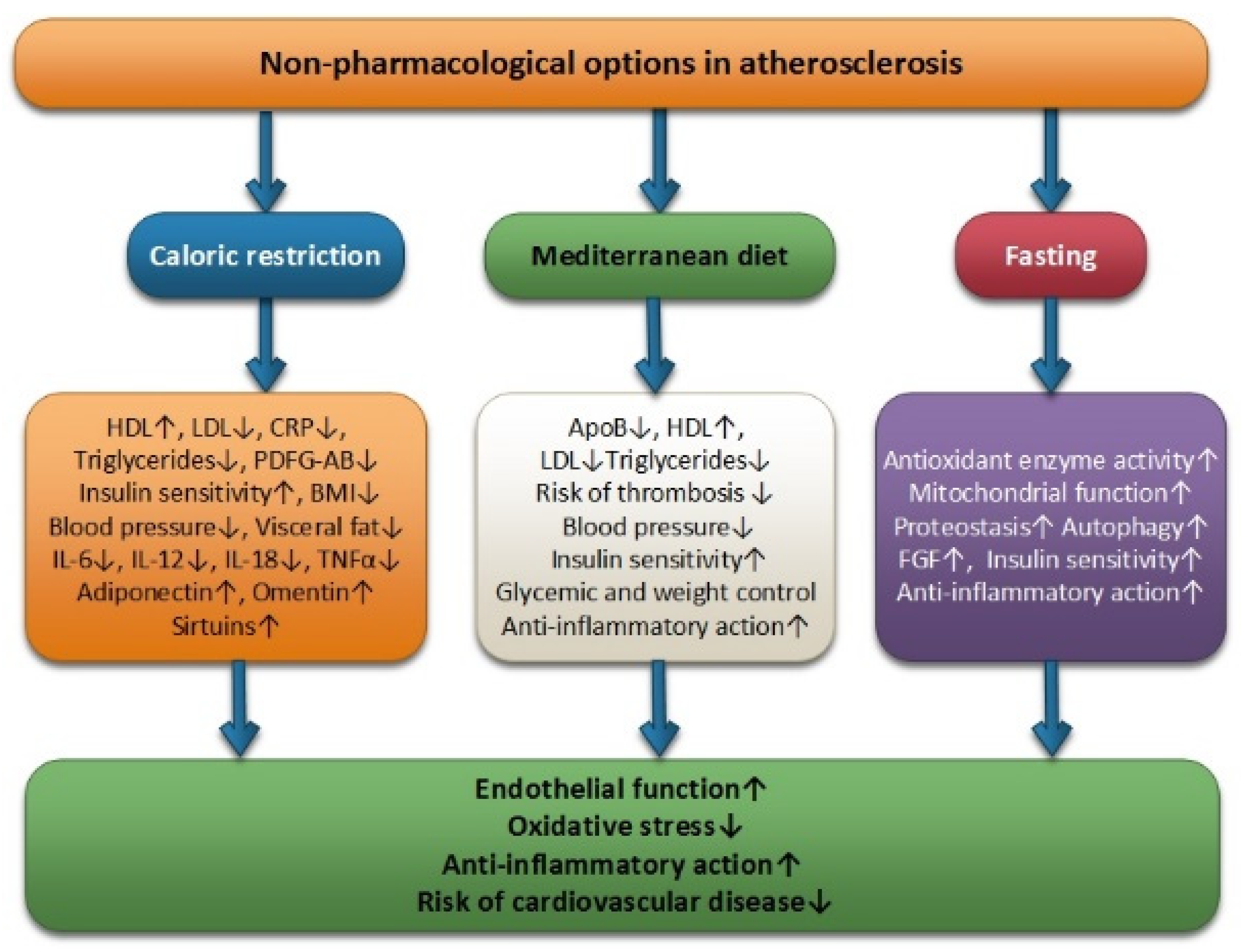
Figure 1. Non-pharmacological options in atherosclerosis: caloric restriction, Mediterranean diet and fasting.
4. Physical Exercise
The protective impact that physical exercise (PE) has on the cardiovascular system has been studied in recent years with the intention of explaining the mechanisms involved; the increase in heat shock proteins, antioxidant enzymes and regulators of cardiac myocyte proliferation concentration seem to be the molecular and biochemical shifts that are involved [29]. Figure 2 shows the main mechanisms involved in the positive impact of PE on endothelial dysfunction.
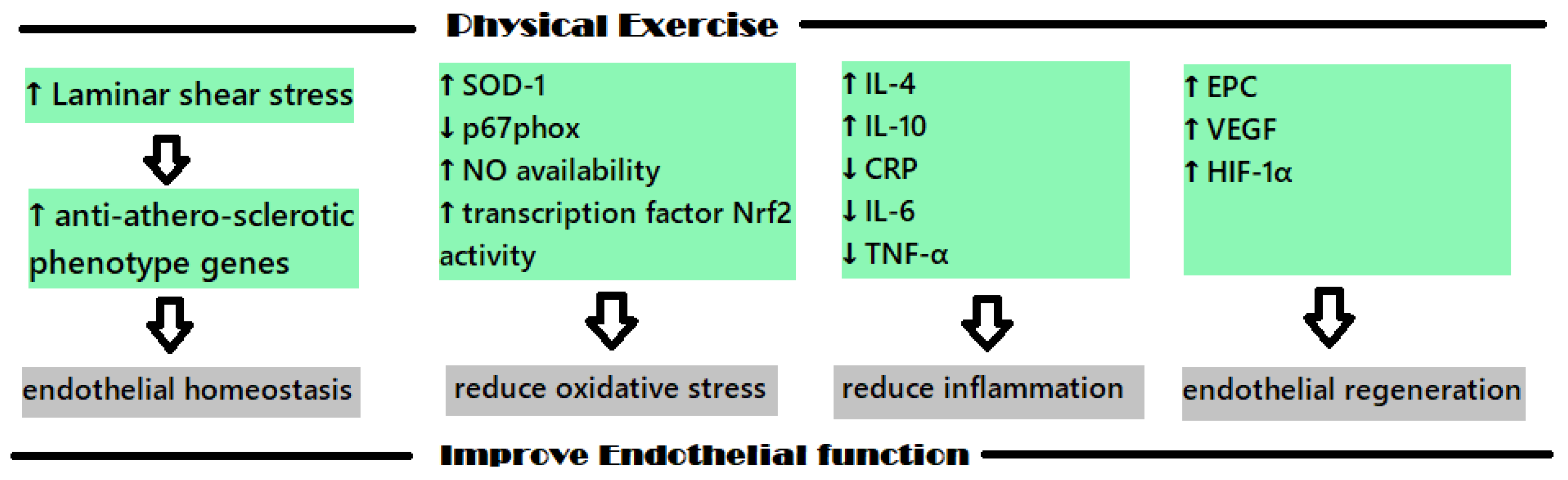
Figure 2. The main mechanisms involved in the protective cardiovascular effects of PE.
The endothelium is a monolayer of endothelial cells that acts as an interface between the blood flow and the intima of blood vessels. As the endothelial cells are organized in to align with the direction of blood flow, they are directly affected by the physical forces induced by this flow. When sustain laminar shear stress (LSS) acts in vitro, an upregulation of the anti-athero-sclerotic phenotype genes is determined at the physiological levels, which seems to also manifest in vivo. These types of genes were identified using the GeneCalling method, and some representatives are as follows: intracellular adhesion molecule-1 (ICAM-1), eNOS, Cu/Zn superoxide dismutase (SOD), thrombomodulin, aldehyde dehydrogenase 6, SMAD6, heme oxygenase-1, cyclooxygenase 2, transforming growth factor (TGF)-b1 [30]. These genes that are related to LSS are associated with inflammation, oxidative stress and metabolism, apoptosis, cellular growth and differentiation, and seem to play an important role in the preservation of the endothelial homeostasis [30].
For example, the transcription factor “nuclear factor (erythroid-derived 2)-like 2 (Nrf2)” plays an important role in PE’s positive effect on the endothelium dysfunction because it is linked to the organism’s fight against oxidative stress [31]. Nrf2 regulates the expressions of some antioxidants, such as NQO-1, glutathione-S-transferase, glutathione peroxidase, and HO-1 when located in the nucleus; however, in the absence of oxidative free radicals, it usually remains dormant in the cytoplasm of the cells [31][32].
During PE, a short-term inflammatory response appears, which is followed by a long-term anti-inflammatory adaptative response. This short-term inflammatory response correlates with an increase in the number of leukocytes, oxidants and C-reactive protein (CRP) level. When exercise is methodically performed, a decrease in the pro-inflammatory molecules level is noticed, and an increase in anti-inflammatory molecules levels is also noticed; substances such as IL-4 and IL-10 are produced and CRP, IL-6 and TNF-α levels decrease [31]. Regarding IL-6, this cytokine has both pro-inflammatory and anti-inflammatory effects; the anti-inflammatory effect is present when this molecule is secreted in the muscles and is related to the inhibitory effect is has on TNF-α, IL-10, IL-1ra and IL-1β [31].
Endothelial precursor cells (EPC) are important cells that are implicated in the regeneration of the endothelium and, depending on their number, they are positively associated with vascular function. These cells originate in the bone marrow and are circulating precursors of endothelial cells, but their circulating levels are usually small and, when injuries appear, the body has to mobilize them in higher levels with the aim of supporting endothelial repair [33]. Studies conducted in this field have revealed that physical effort can act as an impulse for the mobilization of EPC from bone marrow [34].
Ribeiro et al. conducted a study with the aim of assessing the impact that different intensity resistance exercise, performed one time, has on EPC levels over 24 h. All participants in this study were females (n = 38). Along with the determination of EPC levels, the underlying mechanisms for EPC mobilization by effort was assessed using vascular endothelial growth factor (VEGF), the angiogenic factors stromal-cell-derived factor 1 (SDF-1α), erythropoietin (EPO) and hypoxia-inducible factor 1-alpha (HIF-1α) [35]. After exercise, the number of EPCs increased, with the greatest increase occurring 6 h after effort was performed. This was also corelated with increasing levels of VEGF and HIF-1α. It was also observed that higher-intensity exercises were associated with a greater increase in EPC levels [35].
In a review published in 2021, Ferentinos et al. studied the impact of different forms of exercise on EPC levels in patients suffering from cardiovascular and metabolic diseases. Thirty-six trials were included in this study. The authors concluded that the diseases and type of exercise that was performed influenced EPC mobilization and that the regularly performed PE has an impact on the magnitude of EPC circulating levels [36]. For example, in patients suffering from chronic heart failure, after moderate- to high-intensity PE training, EPC levels showed an acute increase, and in patients suffering from ischemic or revascularized coronary artery disease maximal exercise tests, an acute increase in EPC levels was observed [36].
With regard to cardiovascular diseases, PE seems to play an important part in the prevention of this type of disease, and also in reducing the mortality risk. It has also been shown that inactivity and a sedentary lifestyle are related to a higher risk of CVD and mortality. PE causes an increase in cardiac output and blood flow [37]. The beneficial effects of PE seem to have greater impact in patients who have cardiovascular risk factors or suffer from cardiovascular diseases [31].
PE’s impact on the structure and function of the carotid was studied by Königstein, et al. In this study, 2893 adolescents and young adults were included, with ages between 14 and 28 years old; 49.6% of the participants were females. The efforts performed by each participant were assessed with the help of a questionnaire. Carotid intima-media thickness (cIMT) and stiffness (cS), which are early markers of atherosclerosis and vascular aging, were measured with the help of real-time B-mode ultrasound sequences with semi-automated edge-detection and automatic electrocardiogram-gated quality [38]. Atherosclerotic risk was assessed using the cumulative index for atherosclerotic risk (CV-R), which included the determination of mean blood pressure, HbA1c, triglycerides, body mass index and total/HDL-cholesterol ratio. The study results show a better cardiovascular profile for participants that declared high levels of exercise, but not for cS and cIMT. This can be due to the fact that the changes occurring in this age group are subtle [38].
Tomoto et al. studied the same theory in 70 patients suffering from amnestic mild cognitive impairment. Patients that participated in moderate-to-vigorous-intensity aerobic exercise training (AET) two years prior to the study, and patients that suffered from uncontrolled diabetes or hypertension or were obese, were excluded from the study. The participants were included in a 12-month program of moderate-to-vigorous AET or stretching-and-toning (SAT) interventions [39]. Forty-eight of the 77 participants completed one year of training, and the results showed that AET decreased carotid-stiffness index and cerebral blood flow (CBF) pulsatility, increased normalized CBF and improved peak oxygen uptake (VO2peak) [39].
The effects of PE on vascular function and other health measurements in pediatric patient survivors of oncology cerebral insults were studied by Long et al. In this 48-week study, 13 survivors aged between 16 and 23 were included. The exercise intervention lasted 24 weeks and different variables were assessed at the beginning of the study and at 24 and 48 weeks. The endothelial function was assessed using flow-mediated dilation (FMD). Other measurements were also performed: body composition, blood pressure, heart rate, muscular strength, aerobic capacity, anthropometry, muscular endurance and physical activity levels using accelerometers [40]. The results at baseline were compared with those obtained after 28 weeks of exercise intervention and FMD of the brachial artery suffered an increase, with shows that exercise has a positive impact on vascular function [40].
New studies are emerging because one of the main pathophysiological mechanisms of COVID-19 was shown to be endothelial dysfunction. One of these studies was conducted by Ambrosino et al. in 2022, and assessed the existing connection between cardiopulmonary exercise performance and endothelial dysfunction in patients suffering from COVID-19 [41]. The results of this study show that the disruptions that occurred in the endothelial barrier of the systemic and pulmonary circulation played an important role in the reduction in cardiopulmonary exercise testing performance, and new therapies (pharmacological and rehabilitation) that target the endothelial functions should be found [41].
In addition to the exercise’s important role in the normal maintenance of the vascular endothelium, it also has important anti-inflammatory and antioxidant roles. Physical exercise is an efficient clinical tool that limits chronic inflammation by increasing anti-inflammatory cytokines levels and limits pro-inflammatory cytokine by reducing oxidative stress [42].
The recommendations regarding moderate-intensity exercises are exercise for 30 min/day 5 days/week for most people. For people with diseases such as autonomic disorders, this training should be conducted under expert supervision.
5. Vagus Nerve Stimulation
From the previously discussed subjects, it appears that the pathophysiological mechanisms involved in the formation of atherosclerosis are excessive sympathetic stimulation, the inflammatory process, oxidative stress and endothelial dysfunction. Finding a way to simultaneously combat these factors would be ideal for the prevention of atherosclerosis. Vagus nerve stimulation could meet these criteria. Several non-pharmacological methods to activate the vagus nerve exist, such as the invasive and non-invasive electrical stimulation of the vagus nerve, and non-invasive stimulation of vagus nerve during different types of deep breathing or during heart rate variability biofeedback (HRVB) training.
Vagus nerve stimulation (VNS) can modulate the myocardial redox state to reduce oxidative stress [43]. VNS reduced protein oxidation in one study in mice with a myocardial infarction [44]. Using the cholinergic anti-inflammatory pathway, VNS could reduce cytokines levels with attenuation of the inflammatory process. This inhibitory pathway may be of interest to control the neural reflex of inflammation, with great potential in combating inflammatory diseases [45][46]. Several clinical studies have used implanted nerve stimulators to activate the vagus nerve, which have reported encouraging results in relieving chronic inflammation. In experimental models, it has been reported that interruption of the neuronal signal in the vagus nerve aggravates systemic inflammation [47][48][49][50].
The vagus nerve activity inhibits sympathetic activity [51]. The vagus nerve also induced increases in vasoactive intestinal peptide, which then increases coronary blood flow [52]. Hypoxia due to excessive sympathetic stimulation, oxidative stress and inflammation contributes to the formation of a vicious cycle that can be successfully combated by VNS [53].
VNS can be subcutaneous (sVNS) or transcutaneaous (tVNS). sVNS was approved for the treatment of refractory epilepsy and depression [54]. sVNS used bipolar electrodes tunneled under the skin, wrapped around the left vagus nerve in the neck, and connected to a pulse generator that is surgically implanted in the chest wall or axilla [55][56]. Less invasive VNS methods were developed, using electrode placement inside the internal jugular vein at spinal level C5–C7 (referred to as transvenous VNS) [57]. There is no evidence that transvenous VNS can decrease cytokine levels to date [57].
Invasive electrical stimulation of the vagus nerve is difficult andha s many risks, such as trauma to the vagus nerve, which can easily occur during surgical isolation, and suspension of the nerve on the electrode. The physiology of the nerve is significantly affected by compression maneuvers and stretching during manipulation. This causes physical stress on the nerve, which interferes with nerve function [58]. The electrodes used to stimulate the vagus nerve can be finely made of platinum-iridium, silver and tungsten-titanium. The most important characteristic of the electros is the integrity of the surface in contact with the vagus nerve and a stable load delivery to a high-quality, constant current voltage source converter [59][60].
Another important component of direct contact is that a non-toxic, efficient and stable interface must be created between the stimulation electrode and the nerve. The integrity of the electrode can be affected by various factors, such as long-term exposure to buffered saline, so it is mandatory to measure the electrical impedance at the interface during electrical stimulation [61].
The most common clinical use of VNS involves the surgical implantation of a commercially available programmable pulse generator device (NCP System; Cyberonics, Inc., Houston, TX, USA) [62]. The generator is subcutaneously implanted in the left upper chest or left axillary margin. The electrode lead is attached to the left middle cervical vagus nerve through a second incision in the left neck area. The leads wire is passed through a subcutaneous tunnel and attached to the pulse generator. Various surgical complications may occur, including wound infection and hoarseness as a result of temporary or permanent paralysis of the left vocal cord, which can be found in approximately 1% of patients.
To set the parameters of the stimulation generator and program the mode of operation, a laptop is needed. The transmission/programming system will be connected to this laptop, which will be placed on the patient’s skin, above the device. The parameters that can be set are as follows: charge current (electrical stimulus intensity, measured in milliamps (mA)), pulse width (electrical pulse duration, measured in microseconds), pulse frequency (measured in Hertz (Hz)), and on/off duty cycle (stimulus on and off time, measured in seconds or minutes). The initial settings for the four parameters can each be adjusted to optimize efficacy (for seizure control or other symptom control, depending on the indication) and tolerability [62]. The generator runs continuously, but patients can temporarily turn off VNS by holding a magnet over the device, and VNS can be turned on and off by the programmer. The lifetime of the pulse generator battery depends on the stimulus parameters and could be permanently replaced or removed by a simple surgical procedure.
A VNS device system (CardioFit System; BioControl Medical Ltd., Yehud, Israel) has been developed for the treatment of heart failure [63]. This programmable device is implanted in the right chest wall. This is connected to the right cervical vagus using a cuff designed to preferentially activate vagal efferent fibers (intended to affect cardiac function). The pacemaker detects the heart rate and stops at a predetermined bradycardia threshold. Current preclinical phase II studies suggest that chronic right cervical VNS is safe and effective for treating heart failure [64]. A similar VNS system (FitNeS System; BioControl Medical Ltd., Yehud, Israel) has been designed, with a cuff electrode that preferentially activates afferent fibers, which aims to minimize the typical VNS side effects related to efferent fiber stimulation. Left cervical VNS using this device was described in five patients with epilepsy, who showed some benefits and did not experience the side effects that are typical of VNS [65].
The least invasive tVNS methods use superficial stimulation of the vagus nerve through the skin, which can be applied to the anterolateral surface of the neck (cervical tVNS) or to the cymba conchae on the ear (auricle tVNS) [54]. Studies on the reduction in inflammation caused by tVNS are just beginning, but some are promising [66][67]. A recent study in 26 migraine patients receiving cervical tVNS (n = 14) or sham stimulation were performed over 2 months. Stimulation was self-administered bilaterally, twice daily for 120 s. Before and after 2 months of serum IL-1β, IL-6, IL-10, and TNFα were measured. After the 2-month period, serum IL-1β was higher in the sham control condition as compared to the tVNS group, and only mild relief was observed in migraine patients [66]. In another study, 20 healthy males and females were randomized to receive either nVNS or sham stimulation at 8.30 a.m., 12 p.m., and 6 p.m. An initial blood sample was taken at 8 a.m., and another blood sample was withdrawn 90 min and 24 h after the first stimulation session. The study highlights a significant decrease in the IL-1β, TNF, IL-8, macrophage inflammatory protein [MIP]-1α, and monocyte chemoattractant protein [MCP]-1 levels, which was observed in the nVNS group that was non-lipopolysaccharide (LPS)-stimulated after 24 h. The nVNS group showed a significant percent increase in LPS-stimulated IL-10 levels at the 24 h timepoint in comparison with the sham stimulation [67].
These studies revealed mixed results, but less invasive tVNS was allowed, which will improve the ability to test for the causal effects of VNS on inflammation. Given the novelty of VNS in humans for anti-inflammatory effects, there is no standardized method of treatment (administration methodology, duration, current levels) so the development of new guidelines for researchers and practitioners interested in vagus nerve modulation for inflammation control is needed. Future research using larger samples are needed to provide stronger evidence regarding the effect of tVNS on inflammation.
Furthermore, tVNS contributes to reduced activity in limbic brain regions [68], and was more recently found to increase activity in the anterior cingulate and the left prefrontal cortex [69]. These studies may suggest that tVNS can increase executive control and emotional regulation. Higher executive function can modulate risk factors such as smoking, unhealthy diets and sedentary behaviors, and can moderate the intention–behavior link between physical activity and dietary behavior [70].
Transcutaneous electrical nerve stimulation devices can also be used to deliver tVNS by placing contact electrodes in the cymba cup region. Patients can self-administer tVNS, which can be applied unilaterally or bilaterally (depending on the device system used), but there is no established clinical paradigm for how tVNS should be administered (i.e., stimulation parameters, duration and treatment frequency in each stimulation session, duration of treatment, etc.).
Another type of tVNS device (gammaCore; electroCore LLC, Basking Ridge, NJ, USA) has European approval for the prophylactic and acute treatment of cluster headache, migraine, persistent hemicranias and medication overuse headache. Therapy using gammaCore is delivered through a hand-held device with two flat stimulation contact surfaces that transmit a proprietary electrical signal in the vicinity of the vagus nerve. The device is placed on the neck over the vagus nerve in an area in which the subject’s pulse can be located. [71]. The intensity of the stimulation is controlled by the patient, and the application of the stimulation lasts for 90 s. Patients may experience headache relief when this is used as needed, but the device can be used several times a day to prevent headaches.
VNS studies have exponentially increased in recent years [72][73]. Expanding knowledge of the mechanisms of neural control of vascular inflammation, balance autonomic nervous system activities, reduce ROS/RNS will be important for the treatment of cardiovascular diseases such as atherosclerosis.
Another non-invasive method that stimulates the vagus nerve is paced vagal breathing, which is practiced during different types of physical activities, meditation, yoga, or during heartrate variability biofeedback (HRVB) training.
There is increasing evidence that yoga appears to have beneficial effects on the cardiovascular system through downregulation of the hypothalamic–pituitary–adrenal axis (HPA axis) and the SNS by increasing vagal activity and by improving baroreceptor sensitivity [74]. Yoga may restore the autonomic balance between SNS and PNS, by posterior hypothalamus inhibition, which generates a decrease in SNS activity [75].
HRVB is a non-invasive therapy training that aims to increase heartrate oscillations through real-time feedback and slow breathing training [76]. HRVB has a positive effect on psychological symptoms and increases wellbeing [77][78]. Lehrer et al. recently performed a systematic and meta-analytic review on the efficacy of HRVB and/or paced breathing (six breaths/min) and conclude that HRVB improves emotional and physical health and performance [79].
HRVB may have regulatory effects on the autonomic nervous system function. By enhancing vagal activity and reducing SNS activity, HRVB could represent a promising method for the management of a wide range of chronic diseases.
Evidence of biofeedback’s effects on inflammation is still limited. In a study by Lehrer et al. (2010), 11 healthy adults received an administration of endotoxin, and then the patients were randomly assigned to biofeedback training or control conditions. HRV parameters and serum cytokines (IL-6, IL-8, TNFα) were measured. Biofeedback training resulted in increases in time-domain measures of HRV, although no group differences were observed in cytokine levels [80]. Another study tested the effect of biofeedback training on airway inflammation. After 3 months, increases in resting RSA and reductions in eNO were observed in the biofeedback group, but not in controls [81]. HRVB significantly decreased systolic blood pressure and improved baroreflex sensitivity [82]. HRVB was associated with a reduced systolic blood pressure in response to exercise [83]. Thus, HRVB is a relatively simple, non-invasive technique that could be implemented in cardiac rehabilitation programs. Autonomic nervous system activity improvements represent one of the essential mechanisms through which HRV-biofeedback influences cardiovascular outcomes.
References
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; De Cabo, R. A time to fast. Science 2018, 362, 770–775.
- Fontana, L.; Meyer, T.E.; Klein, S.; Holloszy, J.O. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6659–6663.
- Hursting, S.D.; Dunlap, S.M.; Ford, N.A.; Hursting, M.J.; Lashinger, L.M. Calorie restriction and cancer prevention: A mechanistic perspective. Cancer Metab. 2013, 1, 10.
- Ungvari, Z.; Parrado-Fernandez, C.; Csiszar, A.; de Cabo, R. Mechanisms underlying caloric restriction and lifespan regulation: Implications for vascular aging. Circ. Res. 2008, 102, 519–528.
- Meyer, T.E.; Kovács, S.J.; Ehsani, A.A.; Klein, S.; Holloszy, J.O.; Fontana, L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 2006, 47, 398–402.
- Kraus, W.E.; Bhapkar, M.; Huffman, K.M.; Pieper, C.F.; Das, S.K.; Redman, L.M.; Villareal, D.T.; Rochon, J.; Roberts, S.B.; CALERIE Investigators; et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): Exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 673–683.
- Rochon, J.; Bales, C.W.; Ravussin, E.; Redman, L.M.; Holloszy, J.O.; Racette, S.B.; Roberts, S.B.; Das, S.K.; Romashkan, S.; Galan, K.M.; et al. Design and conduct of the CALERIE study: Comprehensive assessment of the long-term effects of reducing intake of energy. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 97–108.
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-year randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. J. Gerontol. Ser. A 2015, 70, 1097–1104.
- Di Pino, A.; DeFronzo, R.A. Insulin resistance and atherosclerosis: Implications for insulin-sensitizing agents. Endocr. Rev. 2019, 40, 1447–1467.
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U.; Stratmann, B. Effects of an energy-restricted diet rich in plant-derived α-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br. J. Nutr. 2014, 112, 1315–1322.
- Joris, P.J.; Zeegers, M.P.; Mensink, R.P. Weight loss improves fasting flow-mediated vasodilation in adults: A meta-analysis of intervention studies. Atherosclerosis 2015, 239, 21–30.
- Raitakari, M.; Ilvonen, T.; Ahotupa, M.; Lehtimäki, T.; Harmoinen, A.; Suominen, P.; Elo, J.; Hartiala, J.; Raitakari, O.T. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: Role of plasma glucose. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 124–128.
- Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. 2013, 27, 2072–2085.
- Wątroba, M.; Szukiewicz, D. The role of sirtuins in aging and age-related diseases. Adv. Med. Sci. 2016, 61, 52–62.
- Cullberg, K.B.; Christiansen, T.; Paulsen, S.K.; Bruun, J.M.; Pedersen, S.B.; Richelsen, B. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity 2013, 21, 454–460.
- Higami, Y.; Barger, J.L.; Page, G.P.; Allison, D.B.; Smith, S.R.; Prolla, T.A.; Weindruch, R. Energy restriction lowers the expression of genes linked to inflammation, the cytoskeleton, the extracellular matrix, and angiogenesis in mouse adipose tissue. J. Nutr. 2006, 136, 343–352.
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660.
- Avogaro, A.; de Kreutzenberg, S.V. Mechanisms of endothelial dysfunction in obesity. Clin. Chim. Acta 2005, 360, 9–26.
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192.
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118.
- Longo, V.D.; Antebi, A.; Bartke, A.; Barzilai, N.; Brown-Borg, H.M.; Caruso, C.; Curiel, T.J.; de Cabo, R.; Franceschi, C.; Gems, D.; et al. Interventions to slow aging in humans: Are we ready? Aging Cell 2015, 14, 497–510.
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019, 30, 462–476.
- Klempel, M.C.; Kroeger, C.M.; Varady, K.A. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism 2013, 62, 137–143.
- Eshghinia, S.; Mohammadzadeh, F. The effects of modified alternate-day fasting diet on weight loss and CAD risk factors in overweight and obese women. J. Diabetes Metab. Disord. 2013, 12, 4.
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity 2014, 22, 2524–2531.
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727.
- Knoops, K.T.B.; Fidanza, F.; Alberti-Fidanza, A.; Kromhout, D.; Van Staveren, W.A. Comparison of three different dietary scores in relation to 10-year mortality in elderly European subjects: The HALE project. Eur. J. Clin. Nutr. 2006, 60, 746–755.
- Iestra, J.A.; Kromhout, D.M.P.H.P.; Van der Schouw, Y.T.; Grobbee, D.E.; Boshuizen, H.C.; Van Staveren, W.A. Effect size estimates of lifestyle and dietary changes on all-cause mortality in coronary artery disease patients: A systematic review. Circulation 2005, 112, 924–934.
- Weeks, K.L.; Gao, X.; Du, X.-J.; Boey, E.J.; Matsumoto, A.; Bernardo, B.C.; Kiriazis, H.; Cemerlang, N.; Tan, J.W.; Tham, Y.K.; et al. Phosphoinositide 3-kinase p110α is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ. Heart Fail. 2012, 5, 523–534.
- Leung, F.P.; Yung, L.M.; Laher, I.; Yao, X.; Chen, Z.Y.; Huang, Y.U. Exercise, vascular wall and cardiovascular diseases. Sports Med. 2008, 38, 1009–1024.
- Golbidi, S.; Laher, I. Exercise and the aging endothelium. J. Diabetes Res. 2013, 2013, 789607.
- Lee, S.; Park, Y.; Zuidema, M.Y.; Hannink, M.; Zhang, C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J. Cardiol. 2011, 3, 18–24.
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899.
- Volaklis, K.A.; Tokmakidis, S.P.; Halle, M. Acute and chronic effects of exercise on circulating endothelial progenitor cells in healthy and diseased patients. Clin. Res. Cardiol. 2013, 102, 249–257.
- Ribeiro, F.; Ribeiro, I.P.; Gonçalves, A.C.; Alves, A.J.; Melo, E.; Fernandes, R.; Costa, R.; Ribeiro, A.B.S.; Duarte, J.A.; Carreira, I.M.; et al. Effects of resistance exercise on endothelial progenitor cell mobilization in women. Sci. Rep. 2017, 7, 17880.
- Ferentinos, P.; Tsakirides, C.; Swainson, M.; Davison, A.; James, M.S.; Ispoglou, T. The impact of different forms of exercise on circulating endothelial progenitor cells in cardiovascular and metabolic disease. Eur. J. Appl. Physiol. 2022, 122, 815–860.
- Ross, M.D.; Malone, E.; Florida-James, G. Vascular ageing and exercise: Focus on cellular reparative processes. Oxidative Med. Cell. Longev. 2016, 2016, 3583956.
- Königstein, K.; Büschges, J.C.; Sarganas, G.; Krug, S.; Neuhauser, H.; Schmidt-Trucksäss, A. Exercise and Carotid Properties in the Young—The KiGGS-2 Study. Front. Cardiovasc. Med. 2021, 8, 767025.
- Tomoto, T.; Liu, J.; Tseng, B.Y.; Pasha, E.P.; Cardim, D.; Tarumi, T.; Hynan, L.S.; Cullum, C.M.; Zhang, R. One-year aerobic exercise reduced carotid arterial stiffness and increased cerebral blood flow in amnestic mild cognitive impairment. J. Alzheimer’s Dis. 2021, 80, 841–853.
- Long, T.M.; Rath, S.R.; Wallman, K.E.; Howie, E.K.; Straker, L.M.; Bullock, A.; Walwyn, T.S.; Gottardo, N.; Cole, C.H.; Choong, C.S.; et al. Exercise training improves vascular function and secondary health measures in survivors of pediatric oncology related cerebral insult. PLoS ONE 2018, 13, e0201449.
- Ambrosino, P.; Parrella, P.; Formisano, R.; Perrotta, G.; D’anna, S.E.; Mosella, M.; Papa, A.; Maniscalco, M. Cardiopulmonary Exercise Performance and Endothelial Function in Convalescent COVID-19 Patients. J. Clin. Med. 2022, 11, 1452.
- Matei, D.; Luca, C.; Onu, I.; Matei, P.; Iordan, D.A.; Buculei, I. Effects of Exercise Training on the Autonomic Nervous System with a Focus on Anti-Inflammatory and Antioxidants Effects. Antioxidants 2022, 11, 350.
- Tsutsumi, T.; Ide, T.; Yamato, M.; Kudou, W.; Andou, M.; Hirooka, Y.; Utsumi, H.; Tsutsui, H.; Sunagawa, K. Modulation of the myocardial redox state by vagal nerve stimulation after experimental myocardial infarction. Cardiovasc. Res. 2008, 77, 713–721.
- Bezerra, O.C.; França, C.M.; Rocha, J.A.; Neves, G.A.; Souza, P.R.M.; Teixeira Gomes, M.; Malfitano, C.; Loleiro, T.C.A.; Dourado, P.M.; Llesuy, S.; et al. Cholinergic stimulation improves oxidative stress and inflammation in experimental myocardial infarction. Sci. Rep. 2017, 7, 13687.
- Tracey, K.J. Approaching the next revolution? Revolutionary integration of neural and immune pathogen sensing and response. Cold Spring Harb. Perspect. Biol. 2015, 7, a016360.
- Eberhardson, M.; Hedin, C.R.H.; Carlson, M.; Tarnawski, L.; Levine, Y.A.; Olofsson, P.S. Towards improved control of inflammatory bowel disease. Scand. J. Immunol. 2019, 89, e12745.
- Koopman, F.A.; Chavan, S.S.; Miljko, S.; Grazio, S.; Sokolovic, S.; Schuurman, P.R.; Mehta, A.D.; Levine, Y.A.; Faltys, M.; Zitnik, R.; et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2016, 113, 8284–8289.
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462.
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017, 282, 46–63.
- Levine, Y.A.; Koopman, F.A.; Faltys, M.; Caravaca, A.; Bendele, A.; Zitnik, R.; Vervoordeldonk, M.J.; Tak, P.P. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS ONE 2014, 9, e104530.
- Saku, K.; Kishi, T.; Sakamoto, K.; Hosokawa, K.; Sakamoto, T.; Murayama, Y.; Kakino, T.; Ikeda, M.; Ide, T.; Sunagawa, K. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol. Rep. 2014, 2, e12136.
- Feliciano, L.; Henning, R.J. Vagal nerve stimulation releases vasoactive intestinal peptide which significantly increases coronary artery blood flow. Cardiovasc. Res. 1998, 40, 45–55.
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24.
- Wang, Y.; Zhan, G.; Cai, Z.; Jiao, B.; Zhao, Y.; Li, S.; Luo, A. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci. Biobehav. Rev. 2021, 127, 37–53.
- Bonaz, B.; Sinniger, V.; Hoffmann, D.; Clarençon, D.; Mathieu, N.; Dantzer, C.; Vercueil, L.; Picq, C.; Trocmé, C.; Faure, P.; et al. Chronic vagus nerve stimulation in Crohn’s disease: A 6-month follow-up pilot study. Neurogastroenterol. Motil. 2016, 28, 948–953.
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790.
- Kox, M.; van Eijk, L.T.; Verhaak, T.; Frenzel, T.; Kiers, H.D.; Gerretsen, J.; van der Hoeven, J.G.; Kornet, L.; Scheiner, A.; Pickkers, P. Transvenous vagus nerve stimulation does not modulate the innate immune response during experimental human endotoxemia: A randomized controlled study. Arthritis Res. Ther. 2015, 17, 150.
- Thompson, S.; Chesher, D. Lot-to-lot variation. Clin. Biochem. Rev. 2018, 39, 51–60.
- Olofsson, P.S.; Levine, Y.A.; Caravaca, A.; Chavan, S.S.; Pavlov, V.A.; Faltys, M.; Tracey, K.J. Single-pulse and unidirectional electrical activation of the cervical vagus nerve reduces TNF in endotoxemia. Bioelectron. Med. 2015, 2, 37–42.
- Caravaca, A.S.; Tsaava, T.; Goldman, L.; Silverman, H.; Riggott, G.; Chavan, S.S.; Bouton, C.; Tracey, K.J.; DeSimone, R.; Boyden, E.S.; et al. A novel flexible cuff-like microelectrode for dual purpose, acute and chronic electrical interfacing with the mouse cervical vagus nerve. J. Neural Eng. 2017, 14, 066005.
- Howlader, M.M.R.; Ul Alam, A.; Sharma, R.P.; Deen, M.J. Materials analyses and electrochemical impedance of implantable metal electrodes. Phys. Chem. Chem. Phys. 2015, 17, 10135–10145.
- Terry, R.S.; Tarver, W.B.; Zabara, J. The implantable neurocybernetic prosthesis system. Pacing Clin. Electrophysiol. 1991, 14, 86–93.
- De Ferrari, G.M.; Schwartz, P.J. Vagus nerve stimulation: From pre-clinical to clinical application: Challenges and future directions. Heart Fail. Rev. 2011, 16, 195–203.
- De Ferrari, G.M.; Crijns, H.J.G.M.; Borggrefe, M.; Milasinovic, G.; Smid, J.; Zabel, M.; Gavazzi, A.; Sanzo, A.; Dennert, R.; Kuschyk, J.; et al. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 2011, 32, 847–855.
- Ben-Menachem, E.; Rydenhag, B.; Silander, H. Preliminary experience with a new system for vagus nerve stimulation for the treatment of refractory focal onset seizures. Epilepsy Behav. 2013, 29, 416–419.
- Chaudhry, S.R.; Lendvai, I.S.; Muhammad, S.; Westhofen, P.; Kruppenbacher, J.; Scheef, L.; Boecker, H.; Scheele, D.; Hurlemann, R.; Kinfe, T.M. Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): A proof-of-concept study. Brain Stimul. 2019, 12, 643–651.
- Lerman, I.; Hauger, R.; Sorkin, L.; Proudfoot, J.; Davis, B.; Huang, A.; Lam, K.; Simon, B.; Baker, D.G. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: A randomized, blinded, healthy control pilot trial. Neuromodulation 2016, 19, 283–291.
- Kraus, T.; Hösl, K.; Kiess, O.; Schanze, A.; Kornhuber, J.; Forster, C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural Transm. 2007, 114, 1485–1493.
- Badran, B.W.; Dowdle, L.T.; Mithoefer, O.J.; LaBate, N.T.; Coatsworth, J.; Brown, J.C.; DeVries, W.H.; Austelle, C.W.; McTeague, L.M.; George, M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018, 11, 492–500.
- Hall, P.A.; Fong, G.T.; Epp, L.J.; Elias, L.J. Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychol. Health 2008, 23, 309–326.
- Kraus, T.; Kiess, O.; Hosl, K.; Terekhin, P.; Kornhuber, J.; Forster, C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal—A pilot study. Brain Stimul. 2013, 6, 798–804.
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus nerve stimulation and the cardiovascular system. Cold Spring Harb. Perspect. Med. 2020, 10, a034173.
- Fitchett, A.; Mastitskaya, S.; Aristovich, K. Selective neuromodulation of the vagus nerve. Front. Neurosci. 2021, 15, 685872.
- Ramos-Jiménez, A.; Hernández-Torres, R.P.; Wall-Medrano, A. Hatha yoga program determinants on cardiovascular health in physically active adult women. J. Yoga Phys. Ther. 2011, 1, 103.
- Mehrotra, R.; Phadke, A.V.; Kharche, J.S.; Pranita, A.; Joshi, A.R. Effect of yoga on anxiety score and resting heart rate in young healthy individuals. Natl. J. Integr. Res. Med. (NJIRM) 2012, 3, 142–146.
- Lehrer, P.M.; Gevirtz, R. Heart rate variability biofeedback: How and why does it work? Front. Psychol. 2014, 5, 756.
- Jimenez Morgan, S.; Molina Mora, J.A. Effect of heart rate variability biofeedback on sport performance, a systematic review. Appl. Psychophysiol. Biofeedback 2017, 42, 235–245.
- Yu, L.-C.; Lin, I.-M.; Fan, S.-Y.; Chien, C.-L.; Lin, T.-H. One-year cardiovascular prognosis of the randomized, controlled, shortterm heart rate variability biofeedback among patients with coronary artery disease. Int. J. Behav. Med. 2018, 25, 271–282.
- Lehrer, P.; Kaur, K.; Sharma, A.; Shah, K.; Huseby, R.; Bhavsar, J.; Sgobba, P.; Zhang, Y. Heart rate variability biofeedback improves emotional and physical health and performance: A systematic review and meta analysis. Appl. Psychophysiol. Biofeedback 2020, 45, 109–129.
- Lehrer, P.; Karavidas, M.K.; Lu, S.-E.; Coyle, S.M.; Oikawa, L.O.; Macor, M.; Calvano, S.E.; Lowry, S.F. Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: An exploratory study. Appl. Psychophysiol. Biofeedback 2010, 35, 303–315.
- Lehrer, P.M.; Irvin, C.G.; Lu, S.-E.; Scardella, A.; Roehmheld-Hamm, B.; Aviles-Velez, M.; Graves, J.; Vaschillo, E.G.; Vaschillo, B.; Hoyte, F.; et al. Heart rate variability biofeedback does not substitute for asthma steroid controller medication. Appl. Psychophysiol. Biofeedback 2018, 43, 57–73.
- Lin, G.; Xiang, Q.; Fu, X.; Wang, S.; Wang, S.; Chen, S.; Shao, L.; Zhao, Y.; Wang, T. Heart rate variability biofeedback decreases blood pressure in prehypertensive subjects by improving autonomic function and baroreflex. J. Altern. Complement. Med. 2012, 18, 143–152.
- Jones, C.U.; Sangthong, B.; Pachirat, O. Slow breathing training reduces resting blood pressure and the pressure responses to exercise. Physiol. Res. 2015, 64, 673–682.
More
Information
Subjects:
Others; Cardiac & Cardiovascular Systems; Sport Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
3 times
(View History)
Update Date:
04 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




