
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theo Kirkland | -- | 4902 | 2022-09-28 18:33:25 | | | |
| 2 | Amina Yu | -8 word(s) | 4894 | 2022-09-30 10:41:47 | | | | |
| 3 | Amina Yu | Meta information modification | 4894 | 2022-10-08 03:23:57 | | |
Video Upload Options
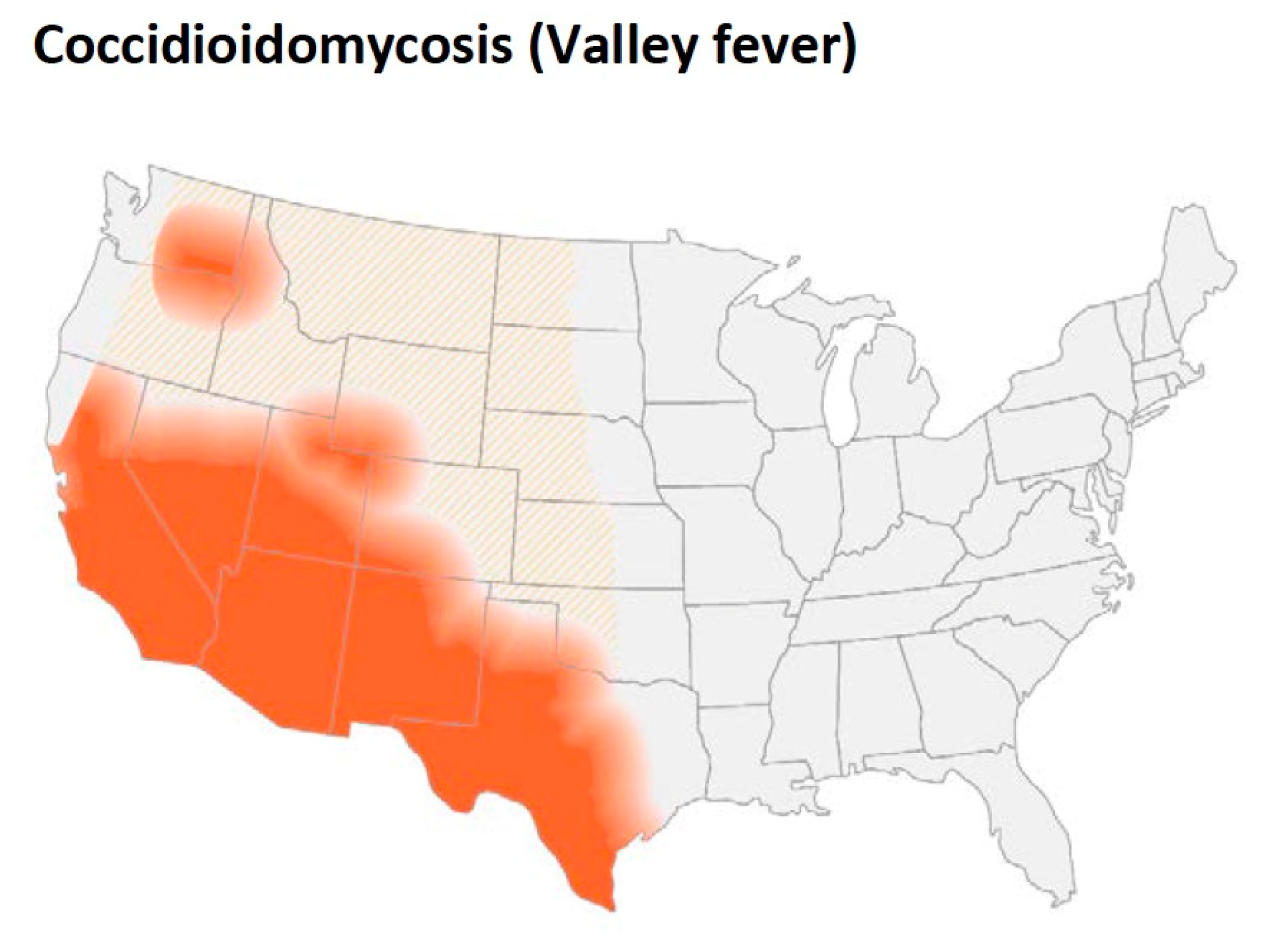
Coccidioidomycosis is an important pathogenic fungal infection in much of the desert and semi-arid regions of the Western hemisphere. The incidence of the disease is increasing in both California and Arizona. Although not all infections result in clinical illness, the infection frequently causes serious disease in immunocompetent people and even self-limited coccidioidomycosis can be a prolonged illness requiring anti-fungal therapy. Coccidioidomycosis is a common cause of community-acquired pneumonia in the endemic area that is frequently misdiagnosed, and the cost of the disease is substantial. In patients where the infection is not self-limited, the disease can spread to skin, bone, and the central nervous system among many other organs. Although disseminated disease is unusual, it occurs in both immunocompetent and immunocompromised people, is frequently difficult to treat, and can be fatal. Prolonged treatment is usually required, and meningitis requires life-long therapy.
1. Coccidioides Spp.
1.1. Taxonomy
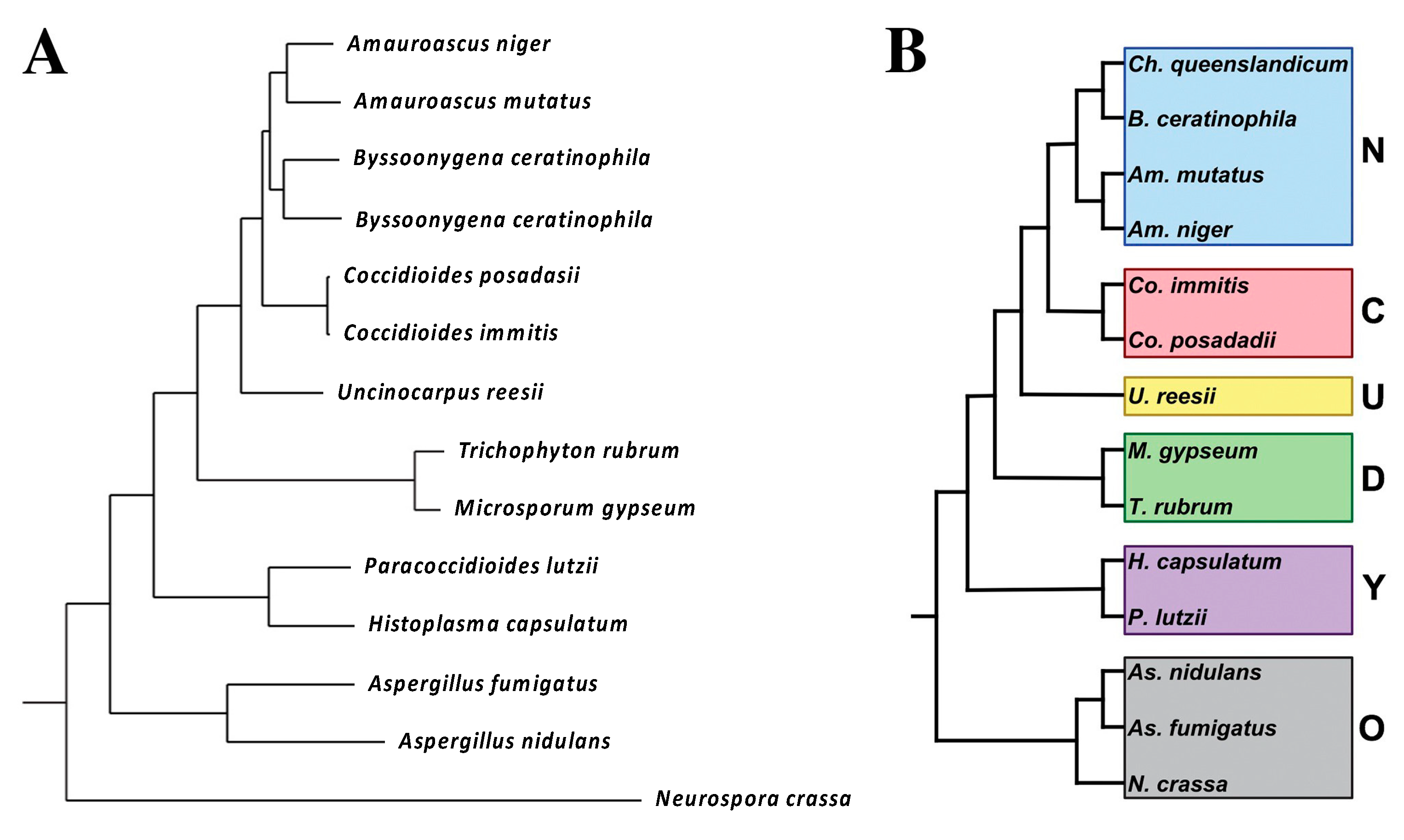
C. Immitis and C. Posadasii
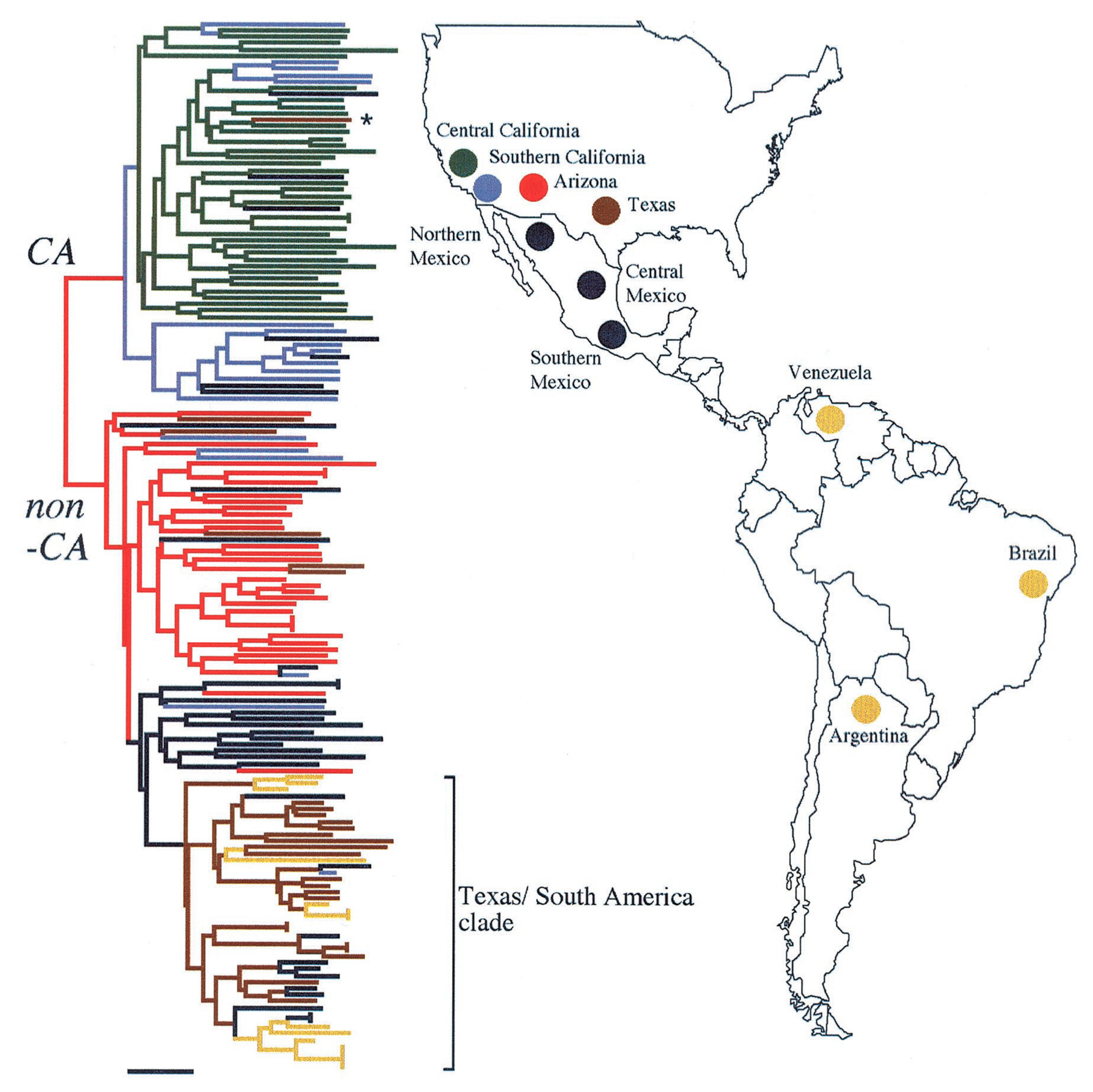
2. Geographic Distribution and Ecology
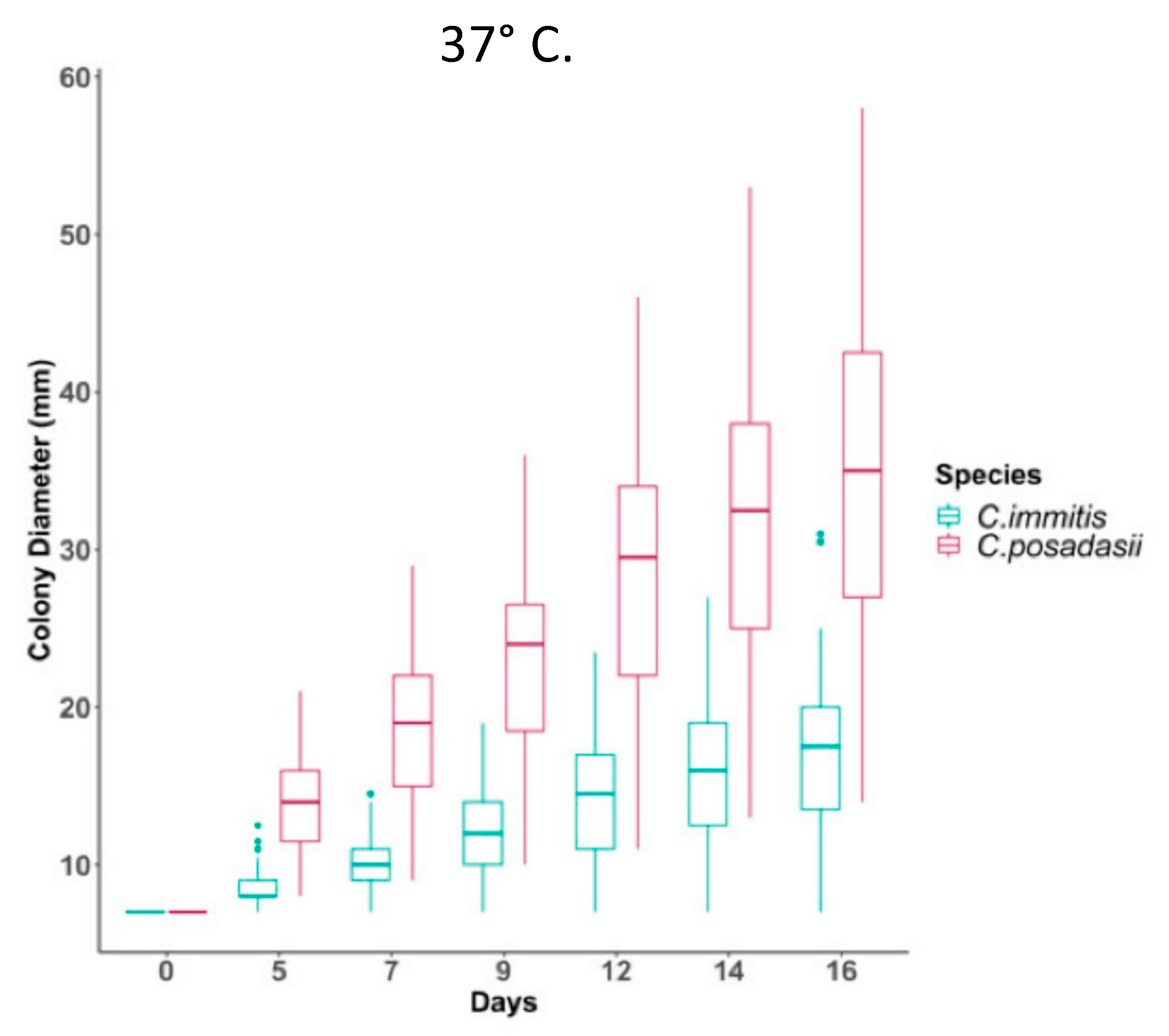
3. Culture, Detection and Morphology of Mycelia and Spherules
3.1. Mycelia and Arthroconidia
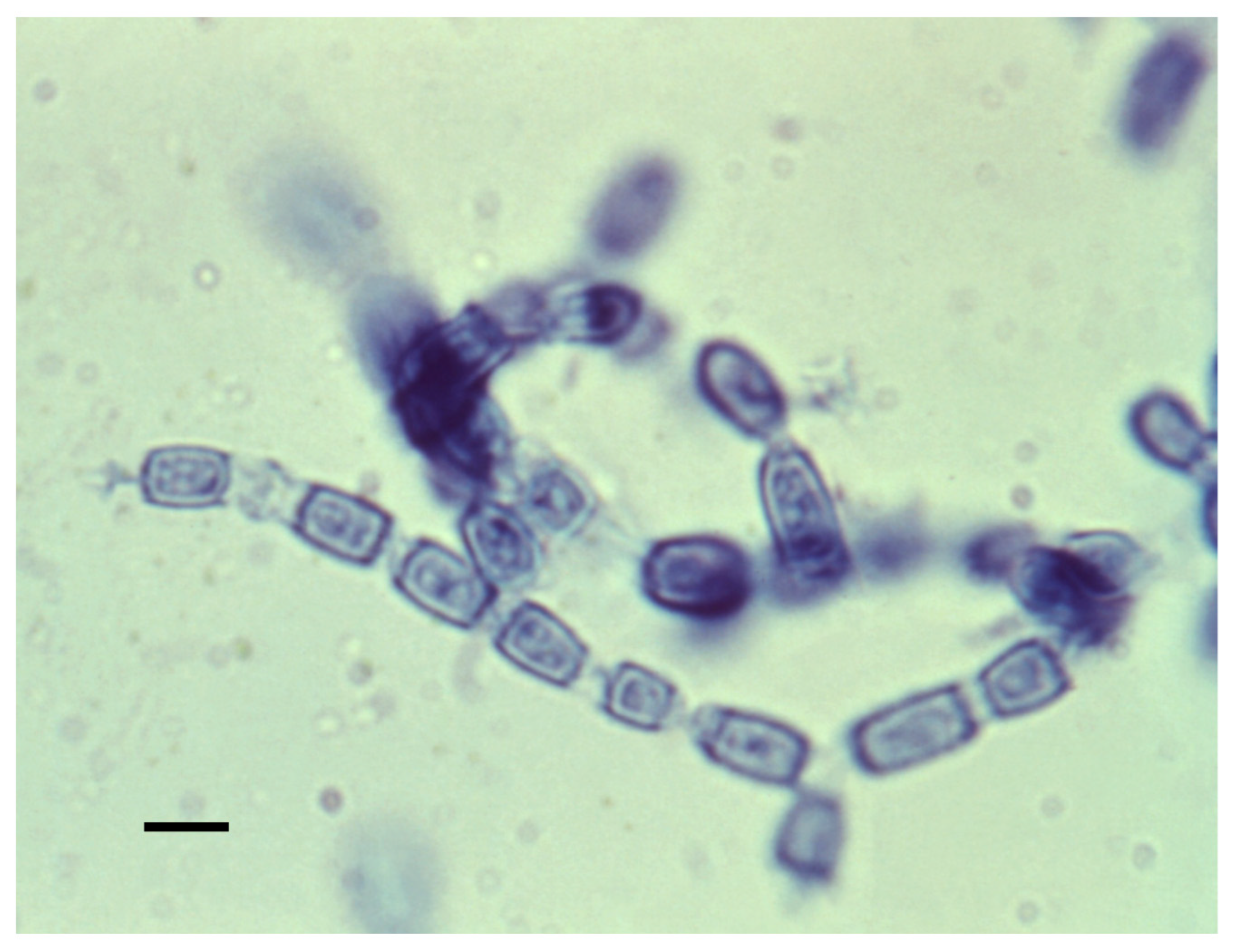
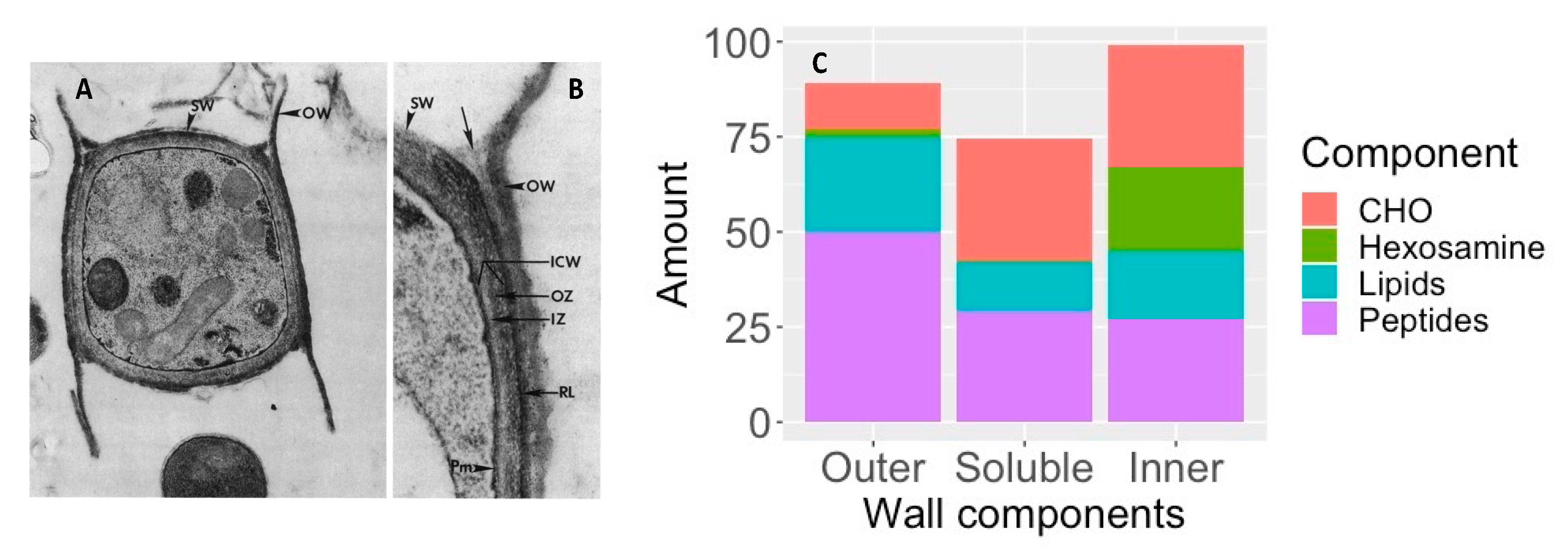
3.2. Spherules
References
- Deresinski, S.; Mirels, L.F. Coccidioidomycosis: What a long strange trip it’s been. Med. Mycol. 2019, 57, S3–S15.
- Deresinski, S.C. History of coccidioidomycosis: “dust to dust”. In Coccidioidomycosis. A Text; Stevens, D.A., Ed.; Plenum Medical Book Company: New York, NY, USA, 1980; pp. 1–20.
- Ophuls, W. Futher observations on a pathogenic mold formerly described as a protozona (Coccidioides immitis, Coccidioides pyogenes). J. Exp. Med. 1905, 6, 443–485.
- Bowman, B.H.; Taylor, J.W.; White, T.J. Molecular evolution of the fungi: Human pathogens. Mol. Biol. Evol. 1992, 9, 893–904.
- Guarro, J.; Gené, J.; Stchigel, A.M. Developments in Fungal Taxonomy. Clin. Microbiol. Rev. 1999, 12, 454–500.
- De Hoog, G.S.; Bowman, B.; Graser, Y.; Haase, G.; El Fari, M.; Gerrits van den Ende, A.H.; Melzer-Krick, B.; Untereiner, W.A. Molecular phylogeny and taxonomy of medically important fungi. Med. Mycol. 1998, 36 (Suppl. 1), 52–56.
- Pan, S.; Sigler, L.; Cole, G.T. Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii (Onygenaceae). Microbiology 1994, 140, 1481–1494.
- Untereiner, W.A.; Scott, J.A.; Naveau, F.A.; Sigler, L.; Bachewich, J.; Angus, A. The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia 2004, 96, 812–821.
- Wang, H.; Xu, Z.; Gao, L.; Hao, B. A fungal phylogeny based on 82 complete genomes using the composition vector method. BMC Evol. Biol. 2009, 9, 195.
- Whiston, E.; Taylor, J.W. Comparative Phylogenomics of Pathogenic and Nonpathogenic Species. G3 2015, 6, 235–244.
- Whiston, E.; Zhang Wise, H.; Sharpton, T.J.; Jui, G.; Cole, G.T.; Taylor, J.W. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS ONE 2012, 7, e41034.
- Carlin, A.F.; Beyhan, S.; Peña, J.F.; Stajich, J.E.; Viriyakosol, S.; Fierer, J.; Kirkland, T.N. Transcriptional Analysis of Coccidioides immitis Mycelia and Spherules by RNA Sequencing. JoF 2021, 7, 366.
- Garcia Garces, H.; Hamae Yamauchi, D.; Theodoro, R.C.; Bagagli, E. PRP8 Intein in Onygenales: Distribution and Phylogenetic Aspects. Mycopathologia 2020, 185, 37–49.
- Fisher, M.C.; Koenig, G.L.; White, T.J.; San-Blas, G.; Negroni, R.; Alvarez, I.G.; Wanke, B.; Taylor, J.W. Biogeographic range expansion into South America by Coccidioides immitis mirrors New world patterns of human migration. Proc. Natl. Acad. Sci. USA 2001, 98, 4558–4562.
- Sil, A.; Andrianopoulos, A. Thermally Dimorphic Human Fungal Pathogens—Polyphyletic Pathogens with a Convergent Pathogenicity Trait. Cold Spring Harb. Perspect. Med. 2014, 5, a019794.
- Koufopanou, V.; Burt, A.; Taylor, J.W. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc. Natl. Acad. Sci. USA 1997, 94, 5478–5482.
- Burt, A.; Carter, D.; Koenig, G.; Whute, T.; Taylor, J.W. Molecular markers reveal cryptic sex in the human pathogen Coccidioides immitis. Proc. Natl. Acad. Sci. USA 1996, 93, 700–773.
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84.
- Mead, H.L.; Hamm, P.S.; Shaffer, I.N.; Teixeira, M.M.; Wendel, C.S.; Wiederhold, N.P.; Thompson, G.R., 3rd; Muniz-Salazar, R.; Castanon-Olivares, L.R.; Keim, P.; et al. Differential Thermotolerance Adaptation between Species of Coccidioides. J. Fungi 2020, 6, 366.
- Barker, B.M.; Tabor, J.A.; Shubitz, L.F.; Perrill, R.; Orbach, M.J. Detection and phylogenetic analysis of Coccidioides posadasii in Arizona soil samples. Fungal Ecol. 2012, 5, 163–176.
- Swatek, F.E. Ecology of Coccidioides immitis. Mycopathol. Mycol. Appl. 1970, 41, 3–12.
- Smith, C.E. Diagnosis of pulmonary coccidioidal infections. Calif. Med. 1951, 75, 385–394.
- Edwards, P.Q.; Palmer, C.E. Prevalence of sensitivity to coccidioidin, with special reference to specific and nonspecific reactions to coccidioidin and to histoplasmin. Dis. Chest 1957, 31, 35–60.
- Huckabone, S.E.; Gulland, F.M.; Johnson, S.M.; Colegrove, K.M.; Dodd, E.M.; Pappagianis, D.; Dunkin, R.C.; Casper, D.; Carlson, E.L.; Sykes, J.E.; et al. Coccidioidomycosis and other systemic mycoses of marine mammals stranding along the central California, USA coast: 1998–2012. J. Wildl. Dis. 2015, 51, 295–308.
- Johnson, S.M.; Carlson, E.L.; Fisher, F.S.; Pappagianis, D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med. Mycol. 2014, 52, 610–617.
- Marsden-Haug, N.; Goldoft, M.; Ralston, C.; Limaye, A.P.; Chua, J.; Hill, H.; Jecha, L.; Thompson, G.R., 3rd; Chiller, T. Coccidioidomycosis acquired in Washington State. Clin. Infect. Dis. 2013, 56, 847–850.
- Marsden-Haug, N.; Hill, H.; Litvintseva, A.P.; Engelthaler, D.M.; Driebe, E.M.; Roe, C.C.; Ralston, C.; Hurst, S.; Goldoft, M.; Gade, L.; et al. Coccidioides immitis identified in soil outside of its known range—Washington, 2013. Morb. Mortal. Wkly. Rep. 2014, 63, 450.
- Litvintseva, A.P.; Marsden-Haug, N.; Hurst, S.; Hill, H.; Gade, L.; Driebe, E.M.; Ralston, C.; Roe, C.; Barker, B.M.; Goldoft, M.; et al. Valley fever: Finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, e1–e3.
- Kollath, D.R.; Teixeira, M.M.; Funke, A.; Miller, K.J.; Barker, B.M. Investigating the Role of Animal Burrows on the Ecology and Distribution of Coccidioides spp. in Arizona Soils. Mycopathologia 2020, 185, 145–159.
- Emmons, C.W.; Ashburn, L.L. The isolation of Haplosporangium parvum n. sp. and Coccidioides immitis from wild rodents. Their relationship to coccidioidomycosis. Public Health Rep. 1942, 57, 1715–1727.
- Kollath, D.R.; Mihalejevic, J.R.; Barker, B.M. PM10 and Other Climatic Variables Are Important Predictors of Seasonal Variability of Coccidioidomycosis in Arizona. Microbiol. Spectr. 2022, 10, e0148321.
- Weaver, E.A.; Kolivras, K.N. Investigating the Relationship Between Climate and Valley Fever (Coccidioidomycosis). Ecohealth 2018, 15, 840–852.
- Coopersmith, E.J.; Bell, J.E.; Benedict, K.; Shriber, J.; McCotter, O.; Cosh, M.H. Relating coccidioidomycosis (valley fever) incidence to soil moisture conditions. Geohealth 2017, 1, 51–63.
- Elconin, A.F.; Egeberg, R.O.; Egeberg, M.C. Significance of soil salinity on the ecology of Coccidioides immitis. J. Bacteriol. 1964, 87, 500–503.
- Maddy, K.T. Observations on Coccidioides immitis found growing naturally in soil. Ariz. Med. 1965, 22, 281–288.
- Lauer, A.; Etyemezian, V.; Nikolich, G.; Kloock, C.; Arzate, A.F.; Sadiq Batcha, F.; Kaur, M.; Garcia, E.; Mander, J.; Kayes Passaglia, A. Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California. Int. J. Environ. Res. Public Health 2020, 17, 5285.
- McCurdy, S.A.; Portillo-Silva, C.; Sipan, C.L.; Bang, H.; Emery, K.W. Risk for Coccidioidomycosis among Hispanic Farm Workers, California, USA, 2018. Emerg. Infect. Dis. 2020, 26, 1430–1437.
- Chow, N.A.; Kangiser, D.; Gade, L.; McCotter, O.Z.; Hurst, S.; Salamone, A.; Wohrle, R.; Clifford, W.; Kim, S.; Salah, Z.; et al. Factors Influencing Distribution of Coccidioides immitis in Soil, Washington State, 2016. Msphere 2021, 6, e0059821.
- Litvintseva, A.P.; Chow, N.A.; Salah, Z. The Association between Coccidioides immitis and Rodent Habitats in Washington State Remains Unresolved. Msphere 2022. Ahead of Print.
- Lauer, A.; Baal, J.D.; Mendes, S.D.; Casimiro, K.N.; Passaglia, A.K.; Valenzuela, A.H.; Guibert, G. Valley Fever on the Rise-Searching for Microbial Antagonists to the Fungal Pathogen Coccidioides immitis. Microorganisms 2019, 7, 31.
- Saubolle, M.A.; Wojack, B.R.; Wertheimer, A.M.; Fuayagem, A.Z.; Young, S.; Koeneman, B.A. Multicenter Clinical Validation of a Cartridge-Based Real-Time PCR System for Detection of Coccidioides spp. in Lower Respiratory Specimens. J. Clin. Microbiol. 2018, 56, e01277-17.
- Mitchell, M.; Dizon, D.; Libke, R.; Peterson, M.; Slater, D.; Dhillon, A. Development of a real-time PCR Assay for identification of Coccidioides immitis by use of the BD Max system. J. Clin. Microbiol. 2015, 53, 926–929.
- Binnicker, M.J.; Buckwalter, S.P.; Eisberner, J.J.; Stewart, R.A.; McCullough, A.E.; Wohlfiel, S.L.; Wengenack, N.L. Detection of Coccidioides species in clinical specimens by real-time PCR. J. Clin. Microbiol. 2007, 45, 173–178.
- Gastelum-Cano, J.M.; Dautt-Castro, M.; Garcia-Galaz, A.; Felix-Murray, K.; Rascon-Careaga, A.; Cano-Rangel, M.A.; Islas-Osuna, M.A. The clinical laboratory evolution in coccidioidomycosis detection: Future perspectives. J. Mycol. Med. 2021, 31, 101159.
- Chaturvedi, S.; Victor, T.R.; Marathe, A.; Sidamonidze, K.; Crucillo, K.L.; Chaturvedi, V. Real-time PCR assay for detection and differentiation of Coccidioides immitis and Coccidioides posadasii from culture and clinical specimens. PLoS Negl. Trop. Dis. 2021, 15, e0009765.
- Bowers, J.R.; Parise, K.L.; Kelley, E.J.; Lemmer, D.; Schupp, J.M.; Driebe, E.M.; Engelthaler, D.M.; Keim, P.; Barker, B.M. Direct detection of Coccidioides from Arizona soils using CocciENV, a highly sensitive and specific real-time PCR assay. Med. Mycol. 2019, 57, 246–255.
- Chow, N.A.; Griffin, D.W.; Barker, B.M.; Loparev, V.N.; Litvintseva, A.P. Molecular detection of airborne Coccidioides in Tucson, Arizona. Med. Mycol. 2016, 54, 584–592.
- Lauer, A.; Talamantes, J.; Castañón Olivares, L.R.; Medina, L.J.; Baal, J.D.H.; Casimiro, K.; Shroff, N.; Emery, K.W. Combining forces—The use of Landsat TM satellite imagery, soil parameter information, and multiplex PCR to detect Coccidioides immitis growth sites in Kern County, California. PLoS ONE 2014, 9, e111921.
- Ocampo-Chavira, P.; Eaton-Gonzalez, R.; Riquelme, M. Of Mice and Fungi: Coccidioides spp. Distribution Models. J. Fungi 2020, 6, 320.
- Dobos, R.R.; Benedict, K.; Jackson, B.R.; McCotter, O.Z. Using soil survey data to model potential Coccidioides soil habitat and inform Valley fever epidemiology. PLoS ONE 2021, 16, e0247263.
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20.
- Del Rocio Reyes-Montes, M.; Perez-Huitron, M.A.; Ocana-Monroy, J.L.; Frias-De-Leon, M.G.; Martinez-Herrera, E.; Arenas, R.; Duarte-Escalante, E. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect. Dis. 2016, 16, 550.
- de Macêdo, R.C.; Rosado, A.S.; da Mota, F.F.; Cavalcante, M.A.; Eulálio, K.D.; Filho, A.D.; Martins, L.M.; Lazéra, M.S.; Wanke, B. Molecular identification of Coccidioides spp. in soil samples from Brazil. BMC Microbiol. 2011, 11, 108.
- Eulálio, K.D.; de Macedo, R.L.; Cavalcanti, M.A.; Martins, L.M.; Lazéra, M.S.; Wanke, B. Coccidioides immitis isolated from armadillos (Dasypus novemcinctus) in the state of Piauí, northeast Brazil. Mycopathologia 2001, 149, 57–61.
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731.
- Chow, E.D.; Liu, O.W.; O’Brien, S.; Madhani, H.D. Exploration of whole-genome responses of the human AIDS-associated yeast pathogen Cryptococcus neoformans var grubii: Nitric oxide stress and body temperature. Curr. Genet. 2007, 52, 137–148.
- Huppert, M.; Sun, S.H. Overview of Mycology, and the Mycology of Coccidioides immitis. In Coccidioidomycosis: A Text; Stevens, D.A., Ed.; Springer: Boston, MA, USA, 1980; pp. 21–46.
- Mead, H.L.; Van Dyke, M.C.C.; Barker, B.M. Proper Care and Feeding of Coccidioides: A Laboratorian’s Guide to Cultivating the Dimorphic Stages of C. immitis and C. posadasii. Curr. Protoc. Microbiol. 2020, 58, e113.
- Cole, G.T. Models of cell differentiation in Conidial fungi. Microbiol. Rev. 1986, 50, 95–132.
- Cole, G.T.; Sun, S.H.; Huppert, M. Isolation and ultrastructural examination of conidial wall components of Coccidioides and Aspergillus. Scan. Electron Microsc. 1982, 1677–1685.
- Lewis, E.R.; Bowers, J.R.; Barker, B.M. Dust devil: The life and times of the fungus that causes valley Fever. PLoS Pathog. 2015, 11, e1004762.
- Yu, J.J.; Holbrook, E.; Liao, Y.R.; Zarnowski, R.; Andes, D.R.; Wheat, L.J.; Malo, J.; Hung, C.Y. Characterization of an Uncinocarpus reesii-expressed recombinant tube precipitin antigen of Coccidioides posadasii for serodiagnosis. PLoS ONE 2019, 14, e0221228.
- Drutz, D.J.; Huppert, M. Coccidioidomycosis: Factors affecting the host-parasite interaction. J. Infect. Dis. 1983, 147, 372–390.
- Klotz, S.A.; Drutz, D.J.; Huppert, M.; Sun, S.H.; DeMarsh, P.L. The critical role of CO2 in the morphogenesis of Coccidioides immitis in cell-free subcutaneous chambers. J. Infect. Dis. 1984, 150, 127–134.
- Converse, J.L.; Besemer, A.R. Nutrition of the Parasitic Phase of Coccidioides immitis in a Chemically Defined Liquid Medium. J. Bacteriol. 1959, 78, 231–239.
- Converse, J.L. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J. Bacteriol. 1957, 74, 106–107.
- Viriyakosol, S.; Singhania, A.; Fierer, J.; Goldberg, J.; Kirkland, T.N.; Woelk, C.H. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol. 2013, 13, 121.
- Mead, H.L.; Teixeira, M.M.; Galgiani, J.N.; Barker, B.M. Characterizing in vitro spherule morphogenesis of multiple strains of both species of Coccidioides. Med. Mycol. 2019, 57, 478–488.
- Brooks, L.D.; Northey, W.T. Studies on Coccidioides immitis. II. Physiological studies on in vitro spherulation. J. Bacteriol. 1963, 85, 12–15.
- Drutz, D.J.; Huppert, M.; Sun, S.H.; McGuire, W.L. Human sex hormones stimulate the growth and maturation of Coccidioides immitis. Infect. Immun. 1981, 32, 897–907.
- Powell, B.L.; Drutz, D.J. Identification of a high-affinity binder for estradiol and a low-affinity binder for testosterone in Coccidioides immitis. Infect. Immun. 1984, 45, 784–786.
- Petkus, A.F.; Baum, L.L.; Ellis, R.B.; Stern, M.; Danley, D.L. Pure spherules of Coccidioides immitis in continuous culture. J. Clin. Microbiol. 1985, 22, 165–167.
- Mitchell, N.M.; Grys, T.E.; Lake, D.F. Carbo-loading in Coccidioides spp.: A quantitative analysis of CAZyme abundance and resulting glycan populations. Glycobiology 2020, 30, 186–197.
- Mitchell, N.M.; Dasari, S.; Grys, T.E.; Lake, D.F. Laser Capture Microdissection-Assisted Protein Biomarker Discovery from Coccidioides-Infected Lung Tissue. J. Fungi 2020, 6, 365.





