
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tomoka Hasegawa | -- | 2228 | 2022-09-27 16:09:52 | | | |
| 2 | Lindsay Dong | Meta information modification | 2228 | 2022-09-28 03:43:48 | | |
Video Upload Options
Bone mineralization entails two mineralization phases: primary and secondary mineralization. Primary mineralization is achieved when matrix vesicles are secreted by osteoblasts, and thereafter, bone mineral density gradually increases during secondary mineralization. Nearby extracellular phosphate ions (PO43−) flow into the vesicles via membrane transporters and enzymes located on the vesicles’ membranes, while calcium ions (Ca2+), abundant in the tissue fluid, are also transported into the vesicles. The accumulation of Ca2+ and PO43− in the matrix vesicles induces crystal nucleation and growth. The calcium phosphate crystals grow radially within the vesicle, penetrate the vesicle’s membrane, and continue to grow outside the vesicle, ultimately forming mineralized nodules. The mineralized nodules then attach to collagen fibrils, mineralizing them from the contact sites (i.e., collagen mineralization). Afterward, the bone mineral density gradually increases during the secondary mineralization process.
1. Introduction
2. Matrix Vesicle-Meditated Mineralization
2.1. Nucleation of Calcium Phosphates in Matrix Vesicles
2.2. Distribution of Ca and P in the Vicinity of Matrix Vesicles in the Osteoid
2.3. Local Synthesis of PO43− by the Activities of TNAP and ENPP1
2.4. Transport of PPi and PO43− via ANK and Pit1/Pit2
2.5. PHOSPHO1 for PO43− Production inside Matrix Vesicles
3. Development of Mineralized Nodules and Collagen Mineralization
3.1. Growth of Mineralized Nodules
The calcium phosphate crystals that are nucleated inside the matrix vesicles grow in all directions and then penetrate the plasma membrane to exit the vesicles, eventually forming mineralized nodules, which are also referred to as calcifying globules [1][3][4]. Under TEM observation, mineralized nodules appear as globular structures composed of radially assembled hydroxyapatite crystals [53][54]. It seems likely that the growth of mineralized nodules is regulated by non-collagenous proteins in the osteoid. Among these materials, osteopontin is especially suited to regulating mineralization because it is a negatively charged and highly phosphorylated molecule that can effectively inhibit hydroxyapatite formation and growth [6][55]. Osteocalcin is another important bone matrix protein subjected to vitamin K-dependent carboxylation at its glutamate residues.
3.2. Collagen Mineralization
4. Osteocyte Network and the Biological Function of Regulating Bone Mineralization
4.1. Distribution of the Osteocyte Network
4.2. Osteocyte-Derived Molecules Involved in Peripheral Mineralization
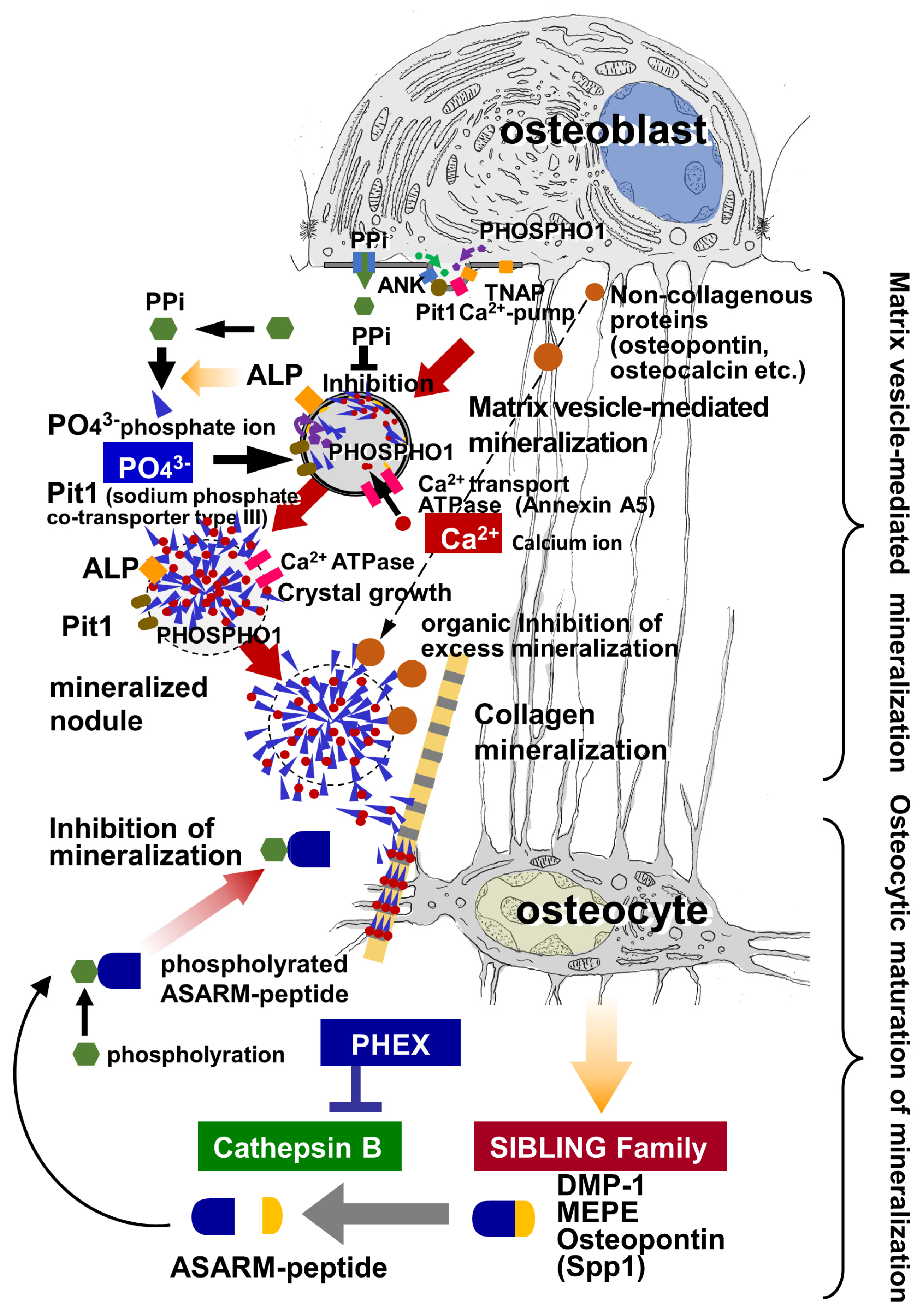
5. Cellular Interplay between Osteoblasts and Osteocytes in Bone Mineralization
6. Conclusions
References
- Ozawa, H.; Yamada, M.; Yamamoto, T. Ultrastructural Observations on the Location of Lead and Calcium in the Mineralizing Dentine of Rat Incisor. In Matrix Vesicles; Ascenzi, A., Bonucci, E., de Bernard, B., Eds.; Wiching Editore Srl: Milano, Italy, 1981; pp. 179–187.
- Hasegawa, T.; Li, M.; Hara, K.; Sasaki, M.; Tabata, C.; de Freitas, P.H.L.; Hongo, H.; Suzuki, R.; Kobayashi, M.; Inoue, K.; et al. Morphological Assessment of Bone Mineralization in Tibial Metaphyses of Ascorbic Acid-Deficient ODS rats. Biomed. Res. 2011, 32, 259–269.
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intra-cellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175.
- Ansari, S.; de Wildt, B.W.; Vis, M.A.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineral-ization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289.
- Matsuzawa, T.; Anderson, H.C. Phosphatases of epiphyseal carti-lage studied by electron microscopic cytochemical methods. J. Histochem. Cytochem. 1971, 19, 801–808.
- De Bruyn, J.R.; Goiko, M.; Mozaffari, M.; Bator, D.; Dauphinee, R.L.; Liao, Y.; Flemming, R.L.; Bramble, M.S.; Hunter, G.K.; Goldberg, H.A. Dynamic light scattering study of inhibition of nucleation and growth of hydroxyapatite crystals by osteopon-tin. PLoS ONE 2013, 8, e56764.
- Kato, K.; Nishimasu, H.; Okudaira, S.; Mihara, E.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 16876–168781.
- Bai, L.; Collins, J.F.; Ghishan, F.K. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am. J. Physiol. Cell. Physiol. 2000, 279, C1135–C1143.
- Bai, L.; Collins, J.F.; Xu, H.; Xu, L.; Ghishan, F.K. Molecular cloning of a murine type III sodium-dependent phosphate co-transporter (Pit-2) gene promoter. Biochim. Biophys. Acta 2001, 1522, 42–45.
- Collins, J.F.; Bai, L.; Ghishan, F.K. The SLC20 family of proteins: Dual functions as sodium-phosphate cotransporters and viral receptors. Pflügers Arch. 2004, 447, 647–652.
- Zoidis, E.; Ghirlanda-Keller, C.; Gosteli-Peter, M.; Zapf, J.; Schmid, C. Regulation of phosphate (Pi) transport and NaPi-III transporter (Pit-1) mRNA in rat osteoblasts. J. Endocrinol. 2004, 181, 531–540.
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 2013, 34, 386–395.
- Roberts, S.J.; Stewart, A.J.; Sadler, P.J.; Farquharson, C. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem. J. 2004, 382, 59–65.
- Roberts, S.; Narisawa, S.; Harmey, D.; Millán, J.L.; Farquharson, C. Functional Involvement of PHOSPHO1 in Matrix Vesicle-Mediated Skeletal Mineralization. J. Bone Miner. Res. 2007, 22, 617–627.
- Ciancaglini, P.; Yadav, M.C.; Simão, A.M.S.; Narisawa, S.; Pizauro, J.M.; Farquharson, C.; Hoylaerts, M.F.; Millán, J.L. Kinetic Analysis of Substrate Utilization by Native and TNAP-, NPP1- or PHOSPHO1-Deficient Matrix Vesicles. J. Bone Miner. Res. 2010, 25, 716–723.
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the mouse ank gene in tissue calcification and arthritis. Science 2000, 15, 265–270.
- Gurley, K.A.; Reimer, R.J.; Kingsley, D.M. Biochemical and Genetic Analysis of ANK in Arthritis and Bone Disease. Am. J. Hum. Genet. 2006, 79, 1017–1029.
- Hasegawa, T.; Yamamoto, T.; Tsuchiya, E.; Hongo, H.; Tsuboi, K.; Kudo, A.; Abe, M.; Yoshida, T.; Nagai, T.; Khadiza, N.; et al. Ultrastructural and biochemical aspects of matrix vesicle-mediated mineralization. Jpn. Dent. Sci. Rev. 2016, 53, 34–45.
- Hasegawa, T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018, 149, 289–304.
- Xu, T.; Bianco, P.; Fisher, L.W.; Longenecker, G.; Smith, E.; Goldstein, S.; Bonadio, J.; Boskey, A.; Heegaard, A.-M.; Sommer, B.; et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat. Genet. 1998, 20, 78–82.
- Corsi, A.; Xu, T.; Chen, X.; Boyde, A.; Liang, J.; Mankani, M.; Sommer, B.; Iozzo, R.V.; Eichstetter, I.; Robey, P.G.; et al. Phenotypic Effects of Biglycan Deficiency Are Linked to Collagen Fibril Abnormalities, Are Synergized by Decorin Deficiency, and Mimic Ehlers-Danlos-Like Changes in Bone and Other Connective Tissues. J. Bone Miner. Res. 2002, 17, 1180–1189.
- Kemp, L.P.; Morris, J.A.; Medina-Gomez, C.; Forgetta, V.; Warrington, N.M.; Youlten, S.E.; Zheng, J.; Gregson, C.L.; Grundberg, E.; Trajanoska, K.; et al. Identification of 153 new loci associated with heel bone mineral density and functional in-volvement of GPC6 in osteoporosis. Nat. Genetics. 2017, 49, 1468–1475.
- Hao, J.-X.; Shen, M.-J.; Wang, C.-Y.; Wei, J.-H.; Wan, Q.-Q.; Zhu, Y.-F.; Ye, T.; Luo, M.-L.; Qin, W.-P.; Li, Y.-T.; et al. Regulation of biomineralization by proteoglycans: From mechanisms to application. Carbohydr. Polym. 2022, 294, 119773.
- Nagai, T.; Hasegawa, T.; Yimin; Yamamoto, T.; Hongo, H.; Abe, M.; Yoshida, T.; Yokoyama, A.; de Freitas, P.H.L.; Li, M.; et al. Immunocytochemical assessment of cell differentiation of podoplanin-positive osteoblasts into os-teocytes in murine bone. Histochem. Cell. Biol. 2021, 155, 369–380.
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605.
- Gould, N.R.; Torre, O.M.; Leser, J.M.; Stains, J.P. The cytoskeleton and connected elements in bone cell mechano-transduction. Bone 2021, 149, 115971.
- Moriishi, T.; Komori, T. Osteocytes: Their Lacunocanalicular Structure and Mechanoresponses. Int. J. Mol. Sci. 2022, 23, 4373.
- Wang, H.; Du, T.; Li, R.; Main, R.P.; Yang, H. Interactive effects of various loading parameters on the fluid dynamics within the lacunar-canalicular system for a single osteocyte. Bone 2022, 158, 116367.
- Sato, T.; Verma, S.; Andrade, C.D.C.; Omeara, M.; Campbell, N.; Wang, J.S.; Cetinbas, M.; Lang, A.; Ausk, B.J.; Brooks, D.J.; et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 2020, 11, 3282.
- Ubaidus, S.; Li, M.; Sultana, S.; de Freitas, P.H.L.; Oda, K.; Maeda, T.; Takagi, R.; Amizuka, N. FGF23 ismainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J. Electron. Microsc. 2009, 58, 381–392.
- Rowe, P.S.; Kumagai, Y.; Gutierrez, G.; Garrett, I.R.; Blacher, R.; Rosen, D.; Cundy, J.; Navvab, S.; Chen, D.; Drezner, M.K.; et al. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 2004, 34, 303–319.
- Rowe, P.S.; Garrett, I.R.; Schwarz, P.M.; Carnes, D.L.; Lafer, E.; Mundy, G.R.; Gutierrez, G.E. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE–ASARM motif: A model for impaired mineralization in X-linked rickets (HYP). Bone 2005, 36, 33–46.
- Sasaki, M.; Hasegawa, T.; Yamada, T.; Hongo, H.; de Freitas, P.H.; Suzuki, R.; Yamamoto, T.; Tabata, C.; Toyosawa, S.; Yamamoto, T.; et al. Altered distribution of bone matrix proteins and defective bone min-eralization in klotho-deficient mice. Bone 2013, 57, 206–219.
- Oya, K.; Ishida, K.; Nishida, T.; Sato, S.; Kishino, M.; Hirose, K.; Ogawa, Y.; Ikebe, K.; Takeshige, F.; Yasuda, H.; et al. Immunohistochemical analysis of dentin matrix protein 1 (Dmp1) phosphorylation by Fam20C in bone: Impli-cations for the induction of biomineralization. Histochem. Cell. Biol. 2017, 147, 341–351.
- Anderson, H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969, 41, 59–72.
- Bonucci, E. Fine Structure and Histochemistry of "Calcifying Globules" in Epiphyseal Cartilage. Cell Tissue Res. 1970, 103, 192–217.
- Amizuka, N.; Hasegawa, T.; Oda, K.; Luiz de Freitas, P.H.; Hoshi, K.; Li, M.; Ozawa, H. Histology of Epiphyseal Cartilage Cal-cification and Endochondral Ossification. Front. Biosci. 2012, 4, 2085–2100.
- Tadross, M.R.; Tsien, R.W.; Yue, D.T. Ca2+ channel nanodomains boost local Ca2+ amplitude. Proc. Natl. Acad. Sci. USA 2013, 110, 15794–15799.
- Melcrova, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035.
- Hoshi, K.; Ejiri, S.; Ozawa, H. Localizational Alterations of Calcium, Phosphorus, and Calcification-Related Organics Such as Proteoglycans and Alkaline Phosphatase During Bone Calcification. J. Bone Miner. Res. 2001, 16, 289–298.
- Genge, B.R.; Wu, L.N.; Wuthier, R.E. Identification of phospholipid-dependent calcium-binding proteins as constituents of ma-trix vesicles. J. Biol. Chem. 1989, 264, 10917–10921.
- Genge, B.R.; Cao, X.; Wu, L.N.; Buzzi, W.R.; Showman, R.W.; Arsenault, A.L.; Ishikawa, Y.; Wuthier, R.E. Establishment of the primary structure of the major lipid dependent Ca2+ binding proteins of chicken growth plate cartilage matrix vesicles: Iden-tity with anchorin CII (annexin V) and annexin II. J. Bone. Miner. Res. 1992, 7, 807–819.
- Balcerzak, M.; Malinowska, A.; Thouverey, C.; Sekrecka, A.; Dadlez, M.; Buchet, R.; Pikula, S. Proteome analysis of matrix vesicles isolated from femurs of chicken embryo. Proteomics 2007, 8, 192–205.
- Terkeltaub, R.; Rosenbach, M.; Fong, F.; Goding, J. Causal link between nucleotide pyrophosphohydrolase overactivity and increased intracellular inorganic pyrophosphate generation demonstrated by transfection of cultured fibroblasts and osteo-blasts with plasma cell membrane glycoprotein-1. Arthritis. Rheum. 1994, 37, 934–941.
- Johnson, K.; Vaingankar, S.; Chen, Y.; Moffa, A.; Goldring, M.; Sano, K.; Jin-Hua, P.; Sali, A.; Goding, J.; Terkeltaub, R. Dif-ferential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondro-cytes. Arthritis. Rheum. 1999, 42, 1986–1997.
- Johnson, K.; Moffa, A.; Chen, Y.; Pritzker, K.; Goding, J.; Terkeltaub, R. Matrix vesicle plasma membrane glycoprotein-1 reg-ulates mineralization by murine osteoblastic MC3T3 cells. J. Bone. Miner. Res. 1999, 14, 883–892.
- Nakano, Y.; Beertsen, W.; van den Bos, T.; Kawamoto, T.; Oda, K.; Takano, Y. Site-specific localization of two distinct phos-phatasesalong the osteoblast plasma membrane: Tissue non-specificalkaline phosphatase and plasma membrane calcium ATPase. Bone 2004, 35, 1077–1085.
- Yamamoto, T.; Hasegawa, T.; Mae, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Nasoori, A.; Morimoto, Y.; Maruoka, H.; Kubota, K.; et al. Comparative immunolocalization of tissue nonspecific alkaline phosphatase and ectonucleotide pyrophosphatase/phosphodiesterase 1 in murine bone. J. Oral Biosci. 2021, 63, 259–264.
- Beck, L.; Leroy, C.; Salaün, C.; Margall-Ducos, G.; Desdouets, C.; Friedlander, G. Identification of a Novel Function of PiT1 Critical for Cell Proliferation and Independent of Its Phosphate Transport Activity. J. Biol. Chem. 2009, 284, 31363–31374.
- Salaün, C.; Leroy, C.; Rousseau, A.; Boitez, V.; Beck, L.; Friedlander, G. Identification of a novel transport-independent function of PiT1/SLC20A1 in the regulation of TNF-induced apoptosis. J. Biol. Chem. 2010, 285, 34408–34418.
- Forand, A.; Koumakis, E.; Rousseau, A.; Sassier, Y.; Journe, C.; Merlin, J.F.; Leroy, C.; Boitez, V.; Codogno, P.; Friedlander, G.; et al. Disruption of the Phosphate Transporter Pit1 in Hepatocytes Improves Glucose Metabolism and Insulin Signaling by Modulating the USP7/IRS1 Interaction. Cell. Rep. 2016, 16, 2736–2748.
- Houston, B.; Stewart, A.J.; Farquharson, C. PHOSPHO1—A novel phosphatase specifically expressed at sites of mineralisation in bone and cartilage. Bone 2004, 34, 629–637.
- Ozawa, H. Ultrastructural concepts on biological calcification; Focused on matrix vesicles. Jpn. J. Oral Biol. 1985, 27, 751–774.
- Weiner, S. Organization of extracellularly mineralized tissues: A comparative study of biological crystal growth. CRC Crit. Rev. Biochem. 1986, 20, 365–408.
- Boskey, A.L.; Christensen, B.; Taleb, H.; Sørensen, E.S. Post-translational modification of osteopontin: Effects on in vitro hy-droxyapatite formation and growth. Biochem. Biophys. Res. Commun. 2012, 419, 333–338.
- Boskey, A.L.; Spevak, L.; Doty, S.B.; Rosenberg, L. Effects of bone CS-proteoglycans, DS-decorin, and DS-biglycan on hydrox-yapatite formation in a gelatin gel. Calcif. Tissue. Int. 1997, 61, 298–305.
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462.
- Wang, K.; Ren, Y.; Lin, S.; Jing, Y.; Ma, C.; Wang, J.; Yuan, X.B.; Han, X.; Zhao, H.; Wang, Z.; et al. Osteocytes but not osteoblasts directly build mineralized bone structures. Int. J. Biol. Sci. 2021, 17, 2430–2448.
- Hoshi, K.; Kemmotsu, S.; Takeuchi, Y.; Amizuka, N.; Ozawa, H. The Primary Calcification in Bones Follows Removal of Decorin and Fusion of Collagen Fibrils. J. Bone Miner. Res. 1999, 14, 273–280.
- Liu, S.; Rowe, P.S.N.; Vierthaler, L.; Zhou, J.; Quarles, L.D. Phosphorylated acidic serine–aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J. Endocrinol. 2007, 192, 261–267.
- Staines, K.A.; MacRae, V.E.; Farquharson, C. The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J. Endocrinol. 2012, 214, 241–255.
- Yamada, T.; Matsukawa, N.; Matsumoto, M.; Morimoto, S.; Ogihara, T.; Ochi, T.; Yoshikawa, H.; Nampei, A.; Hashimoto, J.; Hayashida, K.; et al. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J. Bone Miner. Metab. 2004, 22, 176–184.
- Hoac, B.; Østergaard, M.; Wittig, N.K.; Boukpessi, T.; Buss, D.J.; Chaussain, C.; Birkedal, H.; Murshed, M.; McKee, M.D. Genetic Ablation of Osteopontin in Osteomalacic Hyp Mice Partially Rescues the Deficient Mineralization Without Correcting Hypo-phosphatemia. J. Bone. Miner. Res. 2020, 35, 2032–2048.
- Feng, J.Q.; Ward, L.M.; Liu, S.; Lu, Y.; Xie, Y.; Yuan, B.; Yu, X.; Rauch, F.; Davis, S.I.; Zhang, S.; et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 2006, 38, 1310–1315.
- Mäkitie, O.; Pereira, R.C.; Kaitila, I.; Turan, S.; Bastepe, M.; Laine, T.; Kröger, H.; Cole, W.G.; Jüppner, H. Long-term clinical outcome and carrier phenotype in autosomal recessive hypophosphatemia caused by a novel DMP1 mutation. J. Bone Miner. Res. 2010, 25, 2165–2174.




