Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gilbert Noun | -- | 5899 | 2022-09-26 08:31:54 | | | |
| 2 | Conner Chen | -105 word(s) | 5794 | 2022-09-27 05:00:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Noun, G.; Cascio, M.L.; Spano, D.; Marras, S.; Sirca, C. Methods, Technologies, and Approaches to Monitoring PWS. Encyclopedia. Available online: https://encyclopedia.pub/entry/27575 (accessed on 08 February 2026).
Noun G, Cascio ML, Spano D, Marras S, Sirca C. Methods, Technologies, and Approaches to Monitoring PWS. Encyclopedia. Available at: https://encyclopedia.pub/entry/27575. Accessed February 08, 2026.
Noun, Gilbert, Mauro Lo Cascio, Donatella Spano, Serena Marras, Costantino Sirca. "Methods, Technologies, and Approaches to Monitoring PWS" Encyclopedia, https://encyclopedia.pub/entry/27575 (accessed February 08, 2026).
Noun, G., Cascio, M.L., Spano, D., Marras, S., & Sirca, C. (2022, September 26). Methods, Technologies, and Approaches to Monitoring PWS. In Encyclopedia. https://encyclopedia.pub/entry/27575
Noun, Gilbert, et al. "Methods, Technologies, and Approaches to Monitoring PWS." Encyclopedia. Web. 26 September, 2022.
Copy Citation
Global climate change presents a threat for the environment, and it is aggravated by the mismanagement of water use in the agricultural sector. Since plants are the intermediate component of the soil–plant–atmosphere continuum, and their physiology is directly affected by water availability, plant-based approaches proved to be sensitive and effective in estimating plant water status and can be used as a possible water-saving strategy in crop irrigation scheduling. The plant water status (PWS) assessment is an approach that aims to help farmers elaborate an irrigation schedule as a possible water-saving strategy.
climate change
irrigation scheduling
water stress
water saving
precision Agriculture 4.0
smart irrigation
1. Introduction
Global climate change is a devastating threat for the environment due to the constant increase in average air and surface temperatures, as well as the erratic alterations of rainfall patterns [1]. The Mediterranean Climate Region (the Mediterranean basin, California, Central Chile, the Cape Region of South Africa, and the southernmost regions of Australia) in particular is affected by these drastic climatic oscillations that increase water deficits [2][3][4]. This directly alters agricultural productivity by causing a reduction in crop yields, particularly orchards and vineyards, damaging fruit’s quality, and subsequently negatively impacting the economic sector [2][5][6][7]. In parallel, agriculture is the main consumer of water resources worldwide, and irrigated lands increase yearly to maintain the population’s food demands [8]. Consequently, mismanagement of water use in the agricultural sector will aggravate the impact of climate change by increasing water losses [8][9].
Therefore, adaptation measures in irrigation measurement need to be implemented to guarantee efficient use of the available water, reduce water losses, and ensure both quality and quantity of crop yield [10]. Irrigation scheduling is, then, a priority, and improved and standardized methods are required to help farmers knowing when and how much water to apply while maintaining promising yields. Regulated deficit irrigation (RDI) strategies have been implemented as a way to balance drought periods and plant’s irrigation needs during specific phenological periods [11][12][13]. RDI is a practice where crops are irrigated with an amount of water slightly below the crop coefficient during the least water stress-sensitive growing period with predefined water stress thresholds during each period [14][15], in order to improve crop production while saving water [16]. This approach was particularly successful in grapevines [17], olives [18], pomegranate [19], citrus trees [20], and peach [21], among others. On the other hand, it can negatively impact the yield in some crops, such as sweet cherry [22]. Therefore, for a remunerative and sustainable production process [23], an efficient plant-based irrigation program is needed, which strictly depends on the most sensitive indicator used to assess water stress per each crop.
In this regard, the plant water status (PWS) assessment is an approach that aims to help farmers elaborate an irrigation schedule as a possible water-saving strategy. Formerly, PWS was estimated based on soil water content, the readily available water, and the assessment of evapotranspiration [24][25], but such soil-based methods are highly influenced by soil texture [26][27], and soil water status indirectly affects plant growth rather than directly [28][29]. Additionally, plant physiological response to water deficit is affected essentially by changes in leaf and stem water content, rather than by soil water dynamics which are highly variable [27][30]. For these reasons, more recently, plant-based approaches proved to be more accurate and sensitive in estimating PWS [10][31][32], particularly in woody crops since the deep nature of their root systems presents difficulties in estimating soil water contents [33].
Plants are the intermediate components of the soil–plant–atmosphere continuum, and their physiology is directly affected by water availability [28][34]. Several studies analyzed and monitored PWS through various correlated physiological variables [33][35][36], whereas others focused on developing approaches, methods, and sensors that can operate continuously and remotely [37][38][39]. Stomatal conductance (gs) [40], leaf turgor [41], stem diameter variation [42][43][44], leaf thickness (LT) [45], water potential [27][46][47], relative water content (RWC) [48][49], and sap flow (SF) [50][51][52] can be indirect indicators or proxies of water stress deficit. Each of these physiological variables has a certain response to water availability. In the case of water stress, the partial closure of stomata reduces water loss but simultaneously reduces photosynthetic activity and, thus, reduces growth and productivity [53]. Loss of turgidity affects cell enlargement by reducing plant growth and leaf area while increasing LT [54]. Moreover, trunk diameter decreases evidently, and shrinking is clear as water losses and evapotranspiration increase [55]. Lower water potentials may lead to complete desiccation or plant death [56]. On the other hand, the higher the RWC of a plant is, the greater it tolerates and survives under drought stress conditions. Sap flow decreases and shoot growth decreases when water is withheld [57].
2. Methods, Technologies, and Approaches to Monitoring Plant Water Status
The technical characteristics, strengths, and limitations of the methodologies and technologies are summarized in Table 1, while a graphic representation is portrayed in Figure 1.
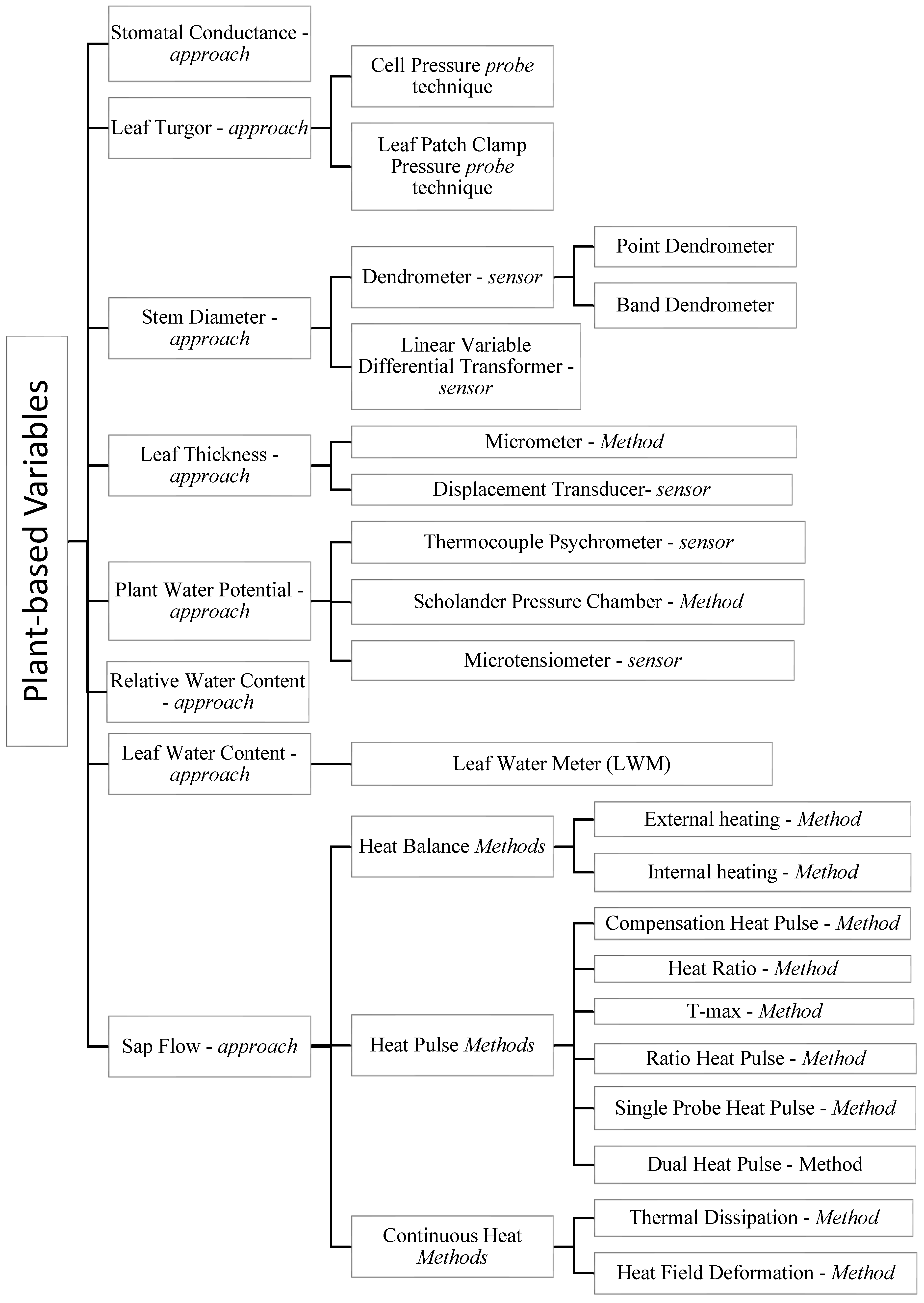
Figure 1. Graphical scheme describing the different plant-based variables and approaches, and the respective technology or sensors used to estimate plant water status. The approaches measure physiological variables by means of different methods and instruments.
Table 1. A summary of the main plant-based water stress indicators, measured variables, respective sensors and methods with their technical functions, and their main strengths and limitations for better irrigation scheduling.
| Indicators, Measured Variables, Sensors, and Methods. | ||||
|---|---|---|---|---|
| Technical Function | Strengths | Limitations | Main References | |
| (1) Stomatal conductance gs (maximum daily stomatal aperture) approach | ||||
| (a) Porometer | Computes gs to Water Vapor (WV) | - Effective - Sensitive |
- Handheld - Not automated - Leaf-to-leaf variation - Affected by nature of crop |
|
| (b) Infrared gas analyzer (IRGA) | Computes gs to WV and CO2 | [58] | ||
| (2) Leaf turgor (cell turgor pressure) approach | ||||
| (a) Cell pressure probe technique | Measures the turgor pressure equilibrium sap/oil | - Continuous and accurate measurement | - Invasive- Not suitable for long-term outdoor applications | [59][60] |
| (b) Leaf patch clamp pressure probe | Measures attenuated output pressure, in response to magnetic clamp pressure | - Noninvasive - Sensitive - Accurate - Continuous |
- Possible leaf-to-leaf variation - Level of accuracy depends on crop |
[61] |
| (3) Stem diameter variation (maximum daily shrinkage) approach | ||||
| (a) Dendrometer | Measures potential difference of either swelling or shrinking of the stem and translates it into an electrical signal | - Continuously and automatically recorded | - Affected by environmental changes and plant age - Variable and inaccurate |
[62][63] |
| (b) Linear variable differential transformer | Converts linear displacements of the stem to an electrical signal | - Robust - High precision - Automated |
- Needs individual calibration | [49] |
| (4) Leaf thickness approach | ||||
| (a) Micrometer | Pressure–volume curve. | - Automated | - Invasive method (requires leaf cut) | [64][65] |
| (b) Linear variable displacement transducers | Distance separating the sensor head of the metal target and leaf probe | - Noninvasive method | - Sensitivity limited by lateral shrinkage - Expensive instrumentation |
[66] |
| (5) Leaf water content | ||||
| (a) Leaf Water Meter (LWM) | Measures leaf water content through the measurement of the absorption of radiation | - Noninvasive - Sensitive - Non-destructive |
- Novel instrumentation | [67] |
| (6) Plant water potential (free energy of water) approach | ||||
| (a) Thermocouple psychrometer | Measure temperature and voltage variations due to vapor pressure | - Noninvasive | - Not automated | [68] |
| (b) Scholander pressure chamber | Balancing pressure measured with a pressure chamber and the osmotic potential of the xylem sap | - Simple - Effective |
- Uses highly compressed gases - Time-consuming - Not continuous - Misrepresentation |
[69] |
| (c) Pump-up pressure | Pressure applied by means of pump | - Avoids use of compressed gases - Mainly designed for irrigation scheduling and monitoring |
- Novel instrumentation | [70] |
| (d) Microtensiometer | Sensor embedded in trunk to directly measure Stem Water Potential (SWP) | - Continuous - Accurate - Automated |
- Underestimate SWP values below -1.5 MPa - Inaccurate measurement under high Vapor Pressure Deficit (VPD) condition |
[71][72] |
| (7) Relative water content (relative amount of water present in the plant tissues) approach | ||||
| (a) Mass weighing | Weighing fresh, dry, and turgid masses of the leaf | - Easy to measure - Directly related to physiological function |
- Difficult to obtain uniform replication | [48] |
| (8) Sap flow (movement of fluid) approach | ||||
| (a) Heat balance method | ||||
| (i) Stem heat balance | Heat input from the heater to the entire circumference is balanced by the heat fluxes out of the stem | - Used for woody and herbaceous stems | - Invasive - Sensors are rigid and fixed - Cannot be used for thick stems |
[51] |
| (ii) Trunk sector heat balance method | Heat applied to a segment of the stem | - Used for large stem diameters | - Invasive - Sensors are rigid and fixed |
[73] |
| (b) Heat pulse method | ||||
| (i) Compensation heat pulse method | Heat pulse velocity is calculated by measuring temperature differences | - Consistent results | - Need to be corrected - Unable to measure low sap flow rates and reverse flow |
[50] |
| (ii) Heat ratio method | Measures the ratio of the increase in temperature | - Measures reverse flow | - More accurate than Compensation Heat Pulse Method (CHPM) - Less reliable at high flux densities |
[74] |
| (iii) T-max method | Calculates time delay for a maximum temperature rise to occur at the downstream temperature sensor | - Single temperature sensor - Measures simultaneously the heat wave at several depths in the trunk |
- Noisy measurements at night - Unable to measure low flow rates |
[75] |
| (iv) TmRatio heat pulse method | Calculates heat pulse velocity using the ratio of the maximum temperature increase between the downstream and side probe | - Low-cost - Easily replicated - Able to measure low flow and at night |
- Novel instrumentation | [76] |
| (v) Sapflow+ method | Calculates conduction and convection of a short-duration heat pulse | - Nondestructive measurement of high, low, and reverse sap flows | - Requires temperature correction | [77] |
| (vi) Single probe heat pulse | Measures sap velocity using a probe | - Simple and small size, less physical damage, less errors | - Unreliable in determining low sap velocity | [78] |
| (vii) Dual heat pulse method | Measures diverse flow ranges such as low and high flow rates, as well as reverse flows, using two heat pulse techniques | - Effective in tracking water demands associated with changing microclimatic conditions | - Limited efficacy (research purposes) | [79] |
| (c) Continuous heat method | ||||
| (i) Thermal dissipation probe | Calculate temperature difference between two probes | - Simple - Accurate - Low-cost |
- Needs calibration - Errors in estimating sap flow for whole tree - High electrical consumption |
[80] |
| (ii) Heat field deformation method | Continuous linear heating system | - Shows plants’ responses to sudden environmental changes and water stress - Measures at different depths in the sapwood, high, low, and reverse flows |
- Can cause errors in estimations | [81] |
2.1. Stomatal Conductance-Based Approach
In the leaf, the role of stomata is to regulate carbon dioxide (CO2) assimilation with respect to water vapor (WV) loss. Although water loss through transpiration during high-temperature conditions cools down plants, stomatal closure during drought periods is crucial to limit transpiration and prevent possible xylem dysfunction [58][82]. Therefore, stomatal regulation of leaf gas exchange is vital for plant survival under arid and semi-arid conditions when potential evapotranspiration is above precipitation [10][83].
The measurement of stomatal aperture or gs is the inverse of the stomata resistance to the rate of passage of CO2 entering and WV exiting the leaf. Many variables, such as alternations in soil water status and atmospheric demand, cause the stomatal aperture to change regularly [84][85]. The constant variation of gs measurements reflects the plant response to water stress and is considered one of the most effective and sensitive water stress indicators [32][86]. The maximum daily gs (gsmax) is the gs value measured at the broadest possible stomatal aperture when optimal gas exchange is achieved and is widely considered a water stress indicator [10].
The gs is measured using porometers and infrared gas analyzers (IRGAs). The porometer computes gs to WV, whereas the IRGAs compute gs to both WV and CO2. Both devices have a chamber in which the whole leaf, or part of it, is clamped. If the leaf cuticle is permeable to WV and CO2, the apparatus measures the leaf conductance (gl). If the leaf has an impermeable cuticle, the device computes the gs. Toro [87] showed that the measured gs strongly differed between the IRGA and the porometer depending on the plant species, water availability, and environmental conditions. Under maximum water stress, gs measured with the leaf porometer was higher than those measured with the IRGA.
2.2. Leaf Turgor-Based Approach
Leaf turgor is the pressure exerted on the cell walls to maintain its rigidity and form. The leaf loses rigidity and wilts because of water stress and deficit. The osmotic flow of water regulates this pressure. Stomatal closure and aperture control transpiration, which in turn affects leaf water status and subsequently leaf turgor pressure [88]. The decrease in turgor pressure was shown to be directly proportional to the transpiration rate and stomatal closure [41][89]. After studying diurnal oscillations of turgor pressure, Zimmermann [90] also found that leaf water status can be evaluated according to the size of turgor pressure loss around noon and the time needed for its recovery in the afternoon.
2.2.1. Cell Pressure Probe Technique
The cell pressure probe technique was introduced as a method intended to continuously measure cell turgor [59][60][91][92]. The pressure probe comprises a microcapillary, a pressure chamber containing a pressure transducer, and a metal rod, with the whole device filled with silicone oil. The probe is then attached to the leaf by inserting the microcapillary into the cell, and pressure is exerted by releasing the oil. Consequently, turgor pressure pushes the sap to exit the cell into the microcapillary, decreasing the cell pressure. Again, oil is released, causing an increase of pressure until the boundary sap–oil reaches an equilibrium, and the pressure on the oil read by the pressure transducer becomes equal to cell sap. This technique is accurate, robust, and straightforward in determining leaf turgor [93].
2.2.2. Leaf Patch Clamp Pressure Probe
More recently, researchers studied a noninvasive leaf patch clamp pressure probe (LPCPP) designed to measure leaf turgor [61][94][95]. The probes are made up of pressure sensors clamped to the leaves using two magnets to monitor relative water status changes. To consider the measurements accurate, the patches should be in osmotic contact with the whole leaf, and the stomata should be closed to avoid water loss. For these conditions to be achieved, the upper magnet can be moved and clamped according to LT and rigidity, while keeping constant the pressure exerted by the magnets. The probe measures the pressure transfer function of the leaf patch, i.e., the attenuated output pressure (Pp), in response to the magnetic clamp pressure (Pclamp), with cell turgor pressure (Pc) measured on the leaf patch being opposed to this output pressure (Pp) [61][96].
A non-invasive magnetic LPCPP (the ZIM-Probe) was developed by Zimmermann [97] to measure changes in leaf turgor continuously and in real-time.
2.3. Leaf Thickness
The first studies dedicated to the relationship between LT and PWS showed a decrease in LT during plant dehydration, followed by a rapid compensation upon irrigation, causing changes in leaf and stem thickness indicators of water deficit [45]. Studies showed a correlation between LT and plant water potential [64][66], providing an early stress detection measurement. More recent studies showed that LT can be used to measure leaf RWC and overall plant water content [98].
LT can be measured using micrometers [64][65] or using the linear variable displacement transducers, also known as linear variable differential transformers (LVDTs), that similarly measure stem diameter [66].
2.3.1. Micrometers
The sample leaf is cut and submerged in water after being inserted in a polyethylene bag to prevent evaporative water loss, and then stored in darkness. The leaf is allowed to regain full hydration before measuring its thickness at full turgor. The gear-wheel type micrometer is used to measure LT through an internal spring that exerts pressure when released. A pressure–volume curve is then constructed to calculate thickness and RWC [64][65]. Micrometers are considered bulky and hard to automate [98].
2.3.2. Displacement Transducers
A displacement transducer is a device consisting of a leaf clamp holding a probe and a metal target or rod [66]. When the instrument is clamped around a sample leaf, an alternating current passes through the probe, generating an alternating magnetic field that induces eddy currents within the target. The circuit is then transformed into a voltage and linearized as a function of the distance separating the sensor head of the metal target and the leaf probe, this distance being the LT. These transducers were introduced in an attempt to allow automated LT measurement [99].
2.4. Leaf Water Content
The authors of ref. [67] introduced a novel sensor, the leaf water meter (LWM), that measures leaf water content through the measurement of the absorption of radiation when this propagates through the leaf tissues. The non-invasive tool is based on the photon attenuation of the passage of radiation through the leaf. Three plastic clamp cables are connected to a readout system, which is equipped with climatic sensors. LWM was shown to be a sensitive, non-destructive, and reliable device to monitor plant water status continuously and in real-time during water stress progression.
2.5. Stem Diameter-Based Approach
Stem diameter variation (SDV) is a PWS indicator that permits the early detection of water stress. A strong relation exists between daily variations in PWS and daily variations in stem diameter [42][43][44][100][101][102]. As transpiration (Ep) occurs in the plant leaves, a tension arises in the evaporative surface and extends to all water-storing organs. This rapid response to atmospheric changes causes systematically diurnal diameter changes in all plant parts, including the stem, branches, roots, leaves, and fruits [102][103][104][105][106]. As a result, as Ep increases, water loss increases, leading to a decrease in trunk diameter.
Nevertheless, these changes in water content represented by shrinkage and swelling of the tissues are reversible, leading to diurnal SDV. Daily, the fluctuations record SDV-derived variables: a maximum daily stem diameter and a minimum daily stem diameter, with the difference between them being the maximum daily shrinkage (MDS). Another recorded measurement is stem growth rate, which corresponds to the difference between the maximum stem diameter of two consecutive days [107][108]. Significant differences in stem diameter variation under different irrigation levels exist as water shortage results in larger maximum daily shrinkage and smaller daily increase [109].
2.5.1. Dendrometers
Dendrometers are instruments used to measure stem and trunk diameter variation and growth. They give high-resolution data of diurnal stem size variations and seasonal tree growth and water storage fluctuations over the year [62][101][110].
Point Dendrometers
Point dendrometers measure stem growth along the radius or diameter of a tree using a linear potentiometer or sensor consisting of a rod nailed or screwed outside the trunk. The sensor measures a potential difference of either swelling or shrinking of the stem, and translates it into an electrical signal [63]. An output voltage will then be obtained, indicating the stem’s growth.
Band Dendrometers
Band dendrometers measure the circumference and linear displacement of a band wrapped around the trunk, stem, or branch using a linear potentiometer. Similar to the point dendrometer, as the stem swells or shrinks, the band expands and contracts, transmitting a signal to the potentiometer [62].
2.5.2. Linear Variable Differential Transformers
LVDTs are sensors fixed on the main trunk by a metal frame of Invar, a metal alloy with minimal thermal expansion. They function by converting stem linear displacements they are coupled to into an electrical signal through a displacement transducer. The sensors should be individually calibrated using a precision micrometer. The LVDT sensors are robust and of high precision [107]. They are sensitive to small changes in stem growth.
2.6. Plant Water Potential-Based Approach
Water potential or free water energy measures the potential energy of water that allows water to move up the plant [111].
Leaf water potential (LWP) is measured on a single leaf and can represent local leaf water demand, soil water availability, internal plant hydraulic conductivity, and stomatal regulation [46][112]. Xylem water potential (XWP) is measured on a non-transpiring leaf since, when leaves do not transpire, their potential is considered to correspond to stem water potential (SWP) [7][113]. XWP is the result of whole-plant transpiration and soil and root/soil hydraulic conductivity. Subsequently, it indicates the ability of plants to conduct water from the soil to the atmosphere [113]. Studies showed SWP to be a water deficit indicator [35][114][115], and since SWP is equal to XWP, it can replace LWP as a more accurate water stress indicator [27][116]. According to Van Leeuwen [7], under conditions established by dry soil cultivation, plants tend to maintain LWP, especially at midday, through increased stomatal closure to avoid severe water losses. Furthermore, Choné [46] established a relationship between leaf transpiration and ΔΨ for grapevines. PWP is measured using three main methodologies: thermocouple psychrometers, pressure chambers and Scholander pressure chambers [65][69][117][118],and more recently, the microtensiometer [71][72][119].
2.6.1. Thermocouple Psychrometers
Thermocouple psychrometers are noninvasive instruments that measure leaf water status on site. Isopiestic psychrometers work by enclosing the sample leaf and a thermocouple in a small container or chamber while maintaining constant temperature [68]. The thermocouple is made up of two junctions: the reference junction, which measures the chamber temperature, and the measurement junction, which measures the air temperature. As water evaporates from the leaf, air humidity is measured, and water vapor pressure is determined. When evaporation takes place, vapor pressure increases, and subsequently, temperature and voltage detected by the thermocouples decrease. Contrarily, when condensation occurs, vapor pressure drops, and temperature and voltage increase. Nevertheless, when the temperature is kept stable, and neither condensation nor evaporation occurs, vapor pressure is considered equal to air humidity, thus equivalent to the plant water potential [68].
The water activity meter is considered a subgroup of the psychrometer technique that measures plant water potential based on the chilled-mirror dewpoint technique [120][121]. The instrument is made up of a sealed chamber that contains a mirror and a means of detecting condensation. At equilibrium, the water potential of the air in the chamber is equivalent to the water potential of the sample.
2.6.2. The Scholander Pressure Chamber
The Scholander pressure chamber is a simple and effective instrument widely used to measure LWP [69]. The method consists of increasing the pressure using a high-pressure compressed gas around a leaf until sap from the xylem appears at the end of the shoot, extends outside the chamber, and is exposed to atmospheric pressure [68]. The pressure needed to keep this condition is equal to the negative pressure existing in the intact stem. The quantity of pressure necessary to force water out of the leaf cells into the xylem is a function of the water potential of the leaf cells [68]. LWPs are then estimated from the sum of the balancing pressure measured with a pressure chamber, and the osmotic potential of the xylem sap in leafy shoots or leaves.
The pump-up pressure chamber is a newly designed pressure chamber that avoids the use of compressed gases in the Scholander design, achieving the required pressure through a pump [70]. This novel pressure chamber is mainly designed for irrigation scheduling and monitoring, particularly for managing deficit irrigation.
The authors of ref. [122] proposed modifications in the sampling technique to obtain more accurate and consistent results, emphasizing the knowledge and proper training of the operator.
2.6.3. Microtensiometer
Since the previous methods do not measure water potential continuously and are labor consuming, microtensiometers were studied as an option for continuous monitoring of water status [71][119]. These sensors measure water potential based on a microelectromechanical pressure sensor that is embedded in the trunk and directly measures stem water potential. This method can be automated, providing continuous data in easy-to-interpret pressure units similar to the traditional pressure chamber stem water potential methods [119]. Blanco and Kalcsits [71] found that microtensiometers gave accurate continuous measurements of SWP in trees during the growing season across a large range of environmental conditions and soil water content. On the other hand, the author of ref. [72] found that microtensiometers are sensitive in representing diurnal and seasonal changes in water potential, except under high VPD conditions.
2.7. Relative Water Content-Based Method
RWC represents the relative amount of water present in the plant tissues, i.e., the correlation of the actual water content of a tissue to the highest attainable water content at full turgor [123]. It is used as a water deficit indicator [37][124][125]. Diurnal RWC is closely related to stem diameter changes and varies inversely with the change in solar radiation, increasing when the radiation decreases and decreasing as radiation increases [49]. Mathematically, the RWC of plant tissue is calculated according to Equation (1). [48]:
RWC (%) = [(FW − DW)/(TW − DW)] × 100
FW, DW, and TW are the fresh, dry, and turgid masses, respectively, of the tissue.
FW is the mass weighed immediately after leaf collection, TW is obtained after floating the leaf in distilled water, and DW is the weight taken after placing the leaf in a heated oven.
2.8. Sap Flow-Based Approach
SF is the movement of fluid in the roots, stems, and branches of plants, and is typically measured in the xylem of plants [126]. The measurement of the rate at which the sap ascends a plant, whether the whole plant, individual branches, or tillers, can determine the transpiration rate. Since transpiration depends on PWS, and given that the effect is controlled by stomatal opening and gs, SF can be used as an indicator of PWS and water stress [126][127][128]. According to Alarcón [127], SF is greatest on warm, sunny days of high vapor pressure deficit, and the least on cooler, cloudy days of low VPD. Additionally, SF would decrease progressively once irrigation water is suspended, and vice versa, increase when irrigation is resumed [127].
Two main approaches exist to measure SF: one calculating the sap-flow rate through the heat balance methods, the other calculating sap-flux density through either the heat pulse methods or the continuous thermal dissipation methods [128].
SAPFLUXNET is a global database of SF measurements [129]. The metadata surveys SF datasets from field studies on species around the globe in order to serve as a benchmark for research.
2.8.1. Heat Balance Methods
Heat balance methods calculate the mass of flow rate of sap, determining, by difference, the amount of heat transported in the moving sap after being subjected to a known amount of heat.
Heat Balance with External Heating, or Stem Heat Balance Method
The stem heat balance (SHB) method, introduced by Sakuratani [51], is used to measure SF in both woody [130] and herbaceous [131] stems. A SHB gauge is made up of a flexible heater (thermopile) and thermocouples to sense temperature differences wrapped around the conductive organ. A small quantity of heat is then applied continuously through the heater, and the connected thermocouple junctions sense the increase of temperature of the enclosed stem.
Energy conservation between the energy put into the stem and the energy losses is calculated, i.e., the heat input from the heater is balanced by the heat fluxes out of the stem, thus obtaining SF [51][131].
Heat Balance with Internal Heating, or the Trunk Sector Heat Balance Method
The trunk sector heat balance method of SF measurement used on tree trunks with diameters greater than 120 mm. Similarly to the stem heat balance method, SF rates are derived from the heat balance of a heated stem tissue. However, in the trunk sector heat balance method, heat is applied internally to only a segment of the trunk, instead of externally to the entire circumference of the enclosed stem. Stainless steel electrode plates, as well as thermocouples, are inserted into the trunk to transfer heat. Temperature increase ΔT between the inside and the outside of the trunk is calculated to measure the SF rate at the center of the heated trunk sector [73].
2.8.2. Heat-Pulse Methods
Heat-pulse techniques are noninvasive methods used to measure SF in plant stems without disrupting the sap stream of the conductive organ [52][75][132]. The obtained measurements are consistent, use low-priced technology, and provide a good time resolution of SF, as well as automated data collection and storage [133]. The sequential or simultaneous measurements on numerous trees can estimate transpiration from whole stands of trees [132].
Compensation Heat Pulse method
The compensation heat pulse method (CHPM), introduced by Marshall [50], is a technique intended to study SF [127][134][135][136]. Since its introduction, simple instrumentation, robust probes, and reliable measurements have been developed [133].
This technique uses two temperature probes asymmetrically placed on either side of a central line heater inserted radially into the tree xylem through drilling holes into the sapwood. The heater probe then releases a heat pulse that is then carried via convection and conduction as a tracer in the conducting organ. The heat pulse velocity is then calculated by measuring temperature differences, with the application of a set of theoretically derived corrections to correct errors that might occur due to a stem wound following the drilling [52][132][135].
The comparison between the values of SF and transpiration rates measured underlined the robustness and high sensitivity of the compensation heat-pulse technique for estimating transpiration [133][137].
The Heat Ratio Method
The heat ratio method (HRM), an improved heat-pulse-based technique, was developed by Burgess [74] to modify the CHPM.
The HRM measures the ratio of the increase in temperature, following the release of a heat pulse through a central heater, at points equidistant downstream and upstream. With the HRM, placement errors of the equidistant probes can be tested in situ and mathematically corrected, making it more accurate than CHPM asymmetrical probes [74].
The velocity of the heat pulse can be calculated from the temperature ratio between the two sensor probes, the thermal diffusivity of the sapwood, and the distance between the heater and the sensor probes [50][74], and then converted into sap flux density [74][138].
A recently developed external heat ratio (EHR) method aims to obtain a noninvasive and accurate bidirectional SF, and is further adapted to thin stems [139][140]. The EHR consists of a small heater and two thermocouples installed on the stem equidistantly, a few millimeters from the center of the heater.
T-Max Method, The Cohen’s Heat-Pulse Method
Marshall’s [50] analytical theory was used by Cohen [75] to develop an alternative improved heat pulse method, the T-max method, which, as opposed to other heat-pulse methods that rely on two temperature sensors or thermocouples, uses a single temperature sensor inserted downstream of the line heater. This method simultaneously measures the heatwave at several depths in the trunk by recording the time delay for a maximum temperature rise at the sensor location. A second probe located upstream of the heater serves as a reference probe to compensate for any background changes in stem temperature during the T-max measurement. SF is then determined from the time delay for a maximum temperature rise to occur at the downstream temperature sensor.
Green [135] described the procedure used to convert raw heat-pulse data into values of volumetric SF by presenting a set of theoretical correction factors for this purpose.
The Ratio Heat Pulse Method
Miner [76] developed the TmRatio method using a gauge consisting of three needle probes: the central probe applies a heat pulse, one temperature probe located above the heater probe and the other placed on the side of the heater. The aim is to calculate heat pulse velocity using the ratio of the maximum temperature increase between the downstream and side probe.
The Sapflow+ Method
Vandegehuchte and Steppe [77] developed a sap flow method that simultaneously measures sap flow density and stem water content, without disrupting the sap flow. The combination of determining heat velocity and water content results in the sap flux density values. These parameters are determined based on the conduction and convection of a short-duration heat pulse, a finite length away from an infinite line source in the anisotropic sapwood. The sensor is formed of a four-needle probe consisting of a linear heater and three measurement needles located at specific distances axially upstream, downstream, and tangentially from the heater [77].
Measurements can be conducted at different depths to obtain a radial sap flux density profile. Therefore, heat velocity, axial, and tangential thermal conductivity, as well as volumetric heat capacity, are thus derived after fitting the correct heat conduction–convection equation to the measured temperature profiles.
Single Probe Heat Pulse Method
The authors of ref. [78] presented and tested a new heat pulse method using a single probe, called the single probe heat pulse (SPHP) to monitor sap velocity. This method uses a single needle as opposed to the two to four needles needed in the other traditional methods. The advantages of a single-probe sensor, apart from its simplicity and smaller size, is the decreased physical damage from the insertion of the needle, the lesser thermal trauma and power requirements, in addition to the prevention of measurement errors. Nevertheless, this method was shown to be unreliable in determining low sap velocity [78]. The authors of ref. [141] developed an improved single probe method with finite heating duration (F-SPHP) to enlarge the SF density measurement range even at low flow rates. Compared with Sapflow+, F-SPHP needed calibration to enable water content determination. These single probe methods, combined with their simplicity and low cost, present many advantages compared to multi-probe methods [78][141].
Dual Heat Pulse Method
The authors of ref. [79] validated the combination of two heat pulse techniques, HRM and CHPM, in a single set of sensor probes, the dual SF sensor. The integration of the methods allows the measurement of diverse flow ranges such as low and high flow rates, as well as reverse flows. This novel sensor proved to be effective in tracking water demands associated with changing microclimatic conditions. Nevertheless, its efficacy was limited to research purposes due to the variability of the sensors, needing to be evaluated against other techniques [79][133].
2.8.3. Continuous Heat
Thermal Dissipation Probe
Granier [80] developed a simple yet accurate and low-cost constant heating method to relate the dissipation of heat to sap flux density empirically. The thermal dissipation SF meter is composed of two probes inserted radially into the xylem. One of the two probes, the thermal dissipation probe, is heated with constant energy input. In contrast, the other, the reference probe, remains unheated, i.e., keeps the same ambient temperature of the wood. Sap flux density is then calculated as a function of the temperature difference between the two probes, assuming that under thermal equilibrium conditions of the system and constant sap flux density, input of heat is equal to heat dissipated by convection and conduction [80][142]. This technique requires species-specific calibration to allow accurate SF measurements [138][143]. Alizadeh [144] developed a new sensor that aims to overcome the limitations of the thermal dissipation probe. The novel trunk RWC sensor (TRWC) includes, in addition to the two probes, a microprocessor and data acquisition, data processing, and heater control system. The microprocessor turns the heater on and off according to temperature changes in the trunk, and then correlates the elapsed time with the water content. The TRWC is designed to reduce the effect of outside temperature.
The Heat Field Deformation Method
The heat field deformation (HFD) method [145] enables sap flux density measurements to be made through a continuous linear heating system. This constant heating technique shows plant responses to sudden environmental changes and water stress. The sensor used comprises a needle-like heater radially inserted in the sapwood and two pairs of differential thermocouples. The lower reference thermometer of the asymmetrical pair of thermocouples is then positioned in one common needle and placed below the heater. The upper thermometer is placed next to the heater, whereas the thermometers are positioned equidistantly from the heater in the symmetrical pair of thermocouples. This placement allows simultaneous recording of the dissipation and deformation of heat in axial and tangential directions around the linear heater [145]. The HFD method measures both asymmetrical and symmetrical temperature gradients, dTsym and dTas, respectively. It, therefore, eliminates any limitations in the measurements due to the separate application of thermometers as in other methods.
The dTsym/dTas ratio thus calculated is proportional to SF rates [145][146], and dTsym is also known as the SF index, which can be used as a stress indicator [145]. The method is designed to measure SF measurements in tree organs with a diameter greater than 3 cm, as well as those with a diameter less than 2 cm using baby sensors [147][148].
References
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. IPCC, 2019: Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019; Unpublished work.
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769.
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104.
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Chang. 2018, 18, 1481–1493.
- Gosling, S.N.; Arnell, N.W. A Global Assessment of the Impact of Climate Change on Water Scarcity. Clim. Change 2016, 134, 371–385.
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014; ISBN 978-92-9169-143-2.
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514.
- Velasco-Muñoz, J.; Aznar-Sánchez, J.; Belmonte-Ureña, L.; Román-Sánchez, I. Sustainable Water Use in Agriculture: A Review of Worldwide Research. Sustainability 2018, 10, 1084.
- Xiloyannis, C.; Montanaro, G.; Dichio, B. Irrigation in Mediterranean Fruit Tree Orchards. In Irrigation Systems and Practices in Challenging Environments; InTech: London, UK, 2012.
- Fernández, J. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 35.
- El Jaouhari, N.; Abouabdillah, A.; Bouabid, R.; Bourioug, M.; Aleya, L.; Chaoui, M. Assessment of Sustainable Deficit Irrigation in a Moroccan Apple Orchard as a Climate Change Adaptation Strategy. Sci. Total Environ. 2018, 642, 574–581.
- Khapte, P.S.; Kumar, P.; Burman, U.; Kumar, P. Deficit Irrigation in Tomato: Agronomical and Physio-Biochemical Implications. Sci. Hortic. (Amsterdam). 2019, 248, 256–264.
- Yu, L.; Zhao, X.; Gao, X.; Siddique, K.H.M. Improving/Maintaining Water-Use Efficiency and Yield of Wheat by Deficit Irrigation: A Global Meta-Analysis. Agric. Water Manag. 2020, 228, 105906.
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Review. Deficit Irrigation in Fruit Trees and Vines in Spain. Spanish J. Agric. Res. 2010, 8, 5.
- Marsal, J.; Casadesus, J.; Lopez, G.; Mata, M.; Bellvert, J.; Girona, J. Sustainability of Regulated Deficit Irrigation in a Mid-Maturing Peach Cultivar. Irrig. Sci. 2016, 34, 201–208.
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated Deficit Irrigation for Crop Production under Drought Stress. A Review. Agron. Sustain. Dev. 2016, 36, 3.
- Munitz, S.; Netzer, Y.; Schwartz, A. Sustained and Regulated Deficit Irrigation of Field-Grown Merlot Grapevines. Aust. J. Grape Wine Res. 2017, 23, 87–94.
- Cano-Lamadrid, M.; Girón, I.F.; Pleite, R.; Burló, F.; Corell, M.; Moriana, A.; Carbonell-Barrachina, A.A. Quality Attributes of Table Olives as Affected by Regulated Deficit Irrigation. LWT - Food Sci. Technol. 2015, 62, 19–26.
- Galindo, A.; Calín-Sánchez, Á.; Griñán, I.; Rodríguez, P.; Cruz, Z.N.; Girón, I.F.; Corell, M.; Martínez-Font, R.; Moriana, A.; Carbonell-Barrachina, A.A.; et al. Water Stress at the End of the Pomegranate Fruit Ripening Stage Produces Earlier Harvest and Improves Fruit Quality. Sci. Hortic. (Amsterdam). 2017, 226, 68–74.
- Ballester, C.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Response of Navel Lane Late Citrus Trees to Regulated Deficit Irrigation: Yield Components and Fruit Composition. Irrig. Sci. 2013, 31, 333–341.
- Mirás-Avalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Maximum Daily Trunk Shrinkage for Estimating Water Needs and Scheduling Regulated Deficit Irrigation in Peach Trees. Irrig. Sci. 2017, 35, 69–82.
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of Regulated Deficit Irrigation and Environmental Conditions on Reproductive Response of Sweet Cherry Trees. Plants 2020, 9, 94.
- Egea, G.; Fernández, J.E.; Alcon, F. Financial Assessment of Adopting Irrigation Technology for Plant-Based Regulated Deficit Irrigation Scheduling in Super High-Density Olive Orchards. Agric. Water Manag. 2017, 187, 47–56.
- Stričevič, R.; Čaki, E. Relationships between Available Soil Water and Indicators of Plant Water Status of Sweet Sorghum to Be Applied in Irrigation Scheduling. Irrig. Sci. 1997, 18, 17–21.
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating Water Stress in Irrigated Olives: Correlation of Soil Water Status, Tree Water Status, and Thermal Imagery. Irrig. Sci. 2009, 27, 367–376.
- Schmitz, M.; Sourell, H. Variability in Soil Moisture Measurements. Irrig. Sci. 2000, 19, 147–151.
- McCutchan, H.; Shackel, K.A. Stem-Water Potential as a Sensitive Indicator of Water Stress in Prune Trees (Prunus domestica L. Cv. French). J. Am. Soc. Hortic. Sci. 1992, 117, 607–611.
- Kramer, P.J. Water Stress and Plant Growth. Ecology 1963, 51, 164–165.
- Jones, H.G. Irrigation Scheduling – Comparison of Soil, Plant and Atmosphere Monitoring Approaches. Acta Hortic. 2008, 792, 391–403.
- Intrigliolo, D.S.; Castel, J.R. Continuous Measurement of Plant and Soil Water Status for Irrigation Scheduling in Plum. Irrig. Sci. 2004, 23, 93–102.
- Jones, H.G. Monitoring Plant and Soil Water Status: Established and Novel Methods Revisited and Their Relevance to Studies of Drought Tolerance. J. Exp. Bot. 2006, 58, 119–130.
- Jones, H.G. Irrigation Scheduling: Advantages and Pitfalls of Plant-Based Methods. J. Exp. Bot. 2004, 55, 2427–2436.
- Blanco-Cipollone, F.; Lourenço, S.; Silvestre, J.; Conceição, N.; Moñino, M.; Vivas, A.; Ferreira, M. Plant Water Status Indicators for Irrigation Scheduling Associated with Iso- and Anisohydric Behavior: Vine and Plum Trees. Horticulturae 2017, 3, 47.
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86.
- Blanco, V.; Domingo, R.; Pérez-Pastor, A.; Blaya-Ros, P.J.; Torres-Sánchez, R. Soil and Plant Water Indicators for Deficit Irrigation Management of Field-Grown Sweet Cherry Trees. Agric. Water Manag. 2018, 208, 83–94.
- Tuccio, L.; Lo Piccolo, E.; Battelli, R.; Matteoli, S.; Massai, R.; Scalabrelli, G.; Remorini, D. Physiological Indicators to Assess Water Status in Potted Grapevine (Vitis vinifera L.). Sci. Hortic. (Amsterdam). 2019, 255, 8–13.
- Scalisi, A.; O’Connell, M.G.; Stefanelli, D.; Lo Bianco, R. Fruit and Leaf Sensing for Continuous Detection of Nectarine Water Status. Front. Plant Sci. 2019, 10.
- Martínez-Gimeno, M.A.; Castiella, M.; Rüger, S.; Intrigliolo, D.S.; Ballester, C. Evaluating the Usefulness of Continuous Leaf Turgor Pressure Measurements for the Assessment of Persimmon Tree Water Status. Irrig. Sci. 2017, 35, 159–167.
- Marino, G.; Pernice, F.; Marra, F.P.; Caruso, T. Validation of an Online System for the Continuous Monitoring of Tree Water Status for Sustainable Irrigation Managements in Olive (Olea europaea L.). Agric. Water Manag. 2016, 177, 298–307.
- Turner, N.C. Measurement and Influence of Environmental and Plant Factors on Stomatal Conductance in the Field. Agric. For. Meteorol. 1991, 54, 137–154.
- Palta, J.A.; Wyn-Jones, R.G.; Tomos, A.D. Leaf Diffusive Conductance and Top Root Cell Turgor Pressure of Sugarbeet. Plant, Cell Environ. 1987, 10, 735–740.
- Klepper, B.; Browning, V.D.; Taylor, H.M. Stem Diameter in Relation to Plant Water Status. Plant Physiol. 1971, 48, 683–685.
- Huck, M.G.; Klepper, B. Water Relations of Cotton. II. Continuous Estimates of Plant Water Potential from Stem Diameter Measurements. Agron. J. 1977, 69, 593–597.
- Kozlowski, T.T. Cambial Growth, Root Growth, and Reproductive Growth; Academic Press: Cambridge, MA, USA, 1971; ISBN 9780323163149.
- Meidner, H. An Instrument for the Continuous Determination of Leaf Thickness Changes in the Field. J. Exp. Bot. 1952, 3, 319–325.
- Choné, X. Stem Water Potential Is a Sensitive Indicator of Grapevine Water Status. Ann. Bot. 2001, 87, 477–483.
- Garnier, E.; Berger, A. Testing Water Potential in Peach Trees as an Indicator of Water Stress. J. Hortic. Sci. 1985, 60, 47–56.
- Weatherley, P.E. Studies in the Water Relations of the Cotton Plant. I. the Field Measurement of Water Deficits in Leaves. New Phytol. 1950, 49, 81–97.
- Simonneau, T.; Habib, R.; Goutouly, J.-P.; Huguet, J.-G. Diurnal Changes in Stem Diameter Depend Upon Variations in Water Content: Direct Evidence in Peach Trees. J. Exp. Bot. 1993, 44, 615–621.
- Marshall, D.C. Measurement of Sap Flow in Conifers by Heat Transport. Plant Physiol. 1958, 33, 385–396.
- Sakuratani, T. A Heat Balance Method for Measuring Water Flux in the Stem of Intact Plants. J. Agric. Meteorol. 1981, 37, 9–17.
- Swanson, R.H.; Whitfield, D.W.A. A Numerical Analysis of Heat Pulse Velocity Theory and Practice. J. Exp. Bot. 1981, 32, 221–239.
- Escalona, J.M.; Flexas, J.; Medrano, H. Drought Effects on Water Flow, Photosynthesis and Growth of Potted Grapevines. Vitis 2002, 41, 57–62.
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water Stress Affects Leaf Anatomy, Gas Exchange, Water Relations and Growth of Two Avocado Cultivars. Sci. Hortic. (Amsterdam) 2002, 95, 39–50.
- Cohen, M.; Goldhamer, D.A.; Fereres, E.; Girona, J.; Mata, M. Assessment of Peach Tree Responses to Irrigation Water Ficits by Continuous Monitoring of Trunk Diameter Changes. J. Hortic. Sci. Biotechnol. 2001, 76, 55–60.
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global Convergence in the Vulnerability of Forests to Drought. Nature 2012, 491, 752–755.
- Gavloski, J.E.; Ellis, C.R.; Whitfield, G.H. Effect of Restricted Watering on Sap Flow and Growth in Corn (Zea mays L.). Can. J. Plant Sci. 1992, 72, 361–368.
- Tyree, M.T.; Sperry, J.S. Vulnerability of Xylem to Cavitation and Embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 19–36.
- Zimmermann, U.; Steudle, E. Physical Aspects of Water Relations of Plant Cells. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1979; Volume 6, pp. 45–117.
- Hüsken, D.; Steudle, E.; Zimmermann, U. Pressure Probe Technique for Measuring Water Relations of Cells in Higher Plants. Plant Physiol. 1978, 61, 158–163.
- Zimmermann, D.; Reuss, R.; Westhoff, M.; Geßner, P.; Bauer, W.; Bamberg, E.; Bentrup, F.-W.; Zimmermann, U. A Novel, Non-Invasive, Online-Monitoring, Versatile and Easy Plant-Based Probe for Measuring Leaf Water Status. J. Exp. Bot. 2008, 59, 3157–3167.
- Deslauriers, A.; Rossi, S.; Anfodillo, T. Dendrometer and Intra-Annual Tree Growth: What Kind of Information Can Be Inferred? Dendrochronologia 2007, 25, 113–124.
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Morin, H.; Saracino, A.; Motta, R.; Borghetti, M. Conifers in Cold Environments Synchronize Maximum Growth Rate of Tree-ring Formation with Day Length. New Phytol. 2006, 170, 301–310.
- Bùrquez, A. Leaf Thickness and Water Deficit in Plants: A Tool for Field Studies. J. Exp. Bot. 1987, 38, 109–114.
- Turner, N.C.; Spurway, R.A.; Schulze, E.-D. Comparison of Water Potentials Measured by In Situ Psychrometry and Pressure Chamber in Morphologically Different Species. Plant Physiol. 1984, 74, 316–319.
- McBurney, T. The Relation Between Leaf Thickness and Plant Water Potential. J. Exp. Bot. 1992, 43, 327–335.
- Cecilia, B.; Francesca, A.; Dalila, P.; Carlo, S.; Antonella, G.; Francesco, F.; Marco, R.; Mauro, C. On-Line Monitoring of Plant Water Status: Validation of a Novel Sensor Based on Photon Attenuation of Radiation through the Leaf. Sci. Total Environ. 2022, 817, 152881.
- Boyer, J.S.; Knipling, E.B. Isopiestic Technique for Measuring Leaf Water Potentials with a Thermocouple Psychrometer. Natl. Acad. Sci. 1965, 54, 1044–1051.
- Scholander, P.F.; Hammel, H.T.; Hemmingsen, E.A.; Bradstreet, E.D. Hydrostatic Pressure and Osmotic Potential in Leaves of Mangroves and Some Other Plants. Proc. Natl. Acad. Sci. 1964, 52, 119–125.
- Goldhamer, D.; Fereres, E. Irrigation Scheduling Protocols Using Continuously Recorded Trunk Diameter Measurements. Irrig. Sci. 2001, 20, 115–125.
- Blanco, V.; Kalcsits, L. Microtensiometers Accurately Measure Stem Water Potential in Woody Perennials. Plants 2021, 10, 2780.
- Pagay, V. Evaluating a Novel Microtensiometer for Continuous Trunk Water Potential Measurements in Field-Grown Irrigated Grapevines. Irrig. Sci. 2022, 40, 45–54.
- Čermák, J.; Kučera, J. The Compensation of Natural Temperature Gradient at the Measuring Point during the Sap Flow Rate Determination in Trees. Biol. Plant. 1981, 23, 469–471.
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Beverly, C.R.; Ong, C.K.; Khan, A.A.H.; Bleby, T.M. Erratum: An Improved Heat Pulse Method to Measure Low and Reverse Rates of Sap Flow in Woody Plants. Tree Physiol. 2001, 21, 589–598.
- Cohen, Y.; Fuchs, M.; Green, G.C. Improvement of the Heat Pulse Method for Determining Sap Flow in Trees. Plant, Cell Environ. 1981, 4, 391–397.
- Miner, G.L.; Ham, J.M.; Kluitenberg, G.J. A Heat-Pulse Method for Measuring Sap Flow in Corn and Sunflower Using 3D-Printed Sensor Bodies and Low-Cost Electronics. Agric. For. Meteorol. 2017, 246, 86–97.
- Vandegehuchte, M.W.; Steppe, K. Sapflow+: A Four-needle Heat-pulse Sap Flow Sensor Enabling Nonempirical Sap Flux Density and Water Content Measurements. New Phytol. 2012, 196, 306–317.
- López-Bernal, Á.; Testi, L.; Villalobos, F.J. A Single-Probe Heat Pulse Method for Estimating Sap Velocity in Trees. New Phytol. 2017, 216, 321–329.
- Pearsall, K.R.; Williams, L.E.; Castorani, S.; Bleby, T.M.; Mcelrone, A.J. Evaluating the Potential of a Novel Dual Heat-Pulse Sensor to Measure Volumetric Water Use in Grapevines under a Range of Flow Conditions. Funct. Plant Biol. 2014, 41, 874–883.
- Granier, A. Une Nouvelle Méthode Pour La Mesure Du Flux de Sève Brute Dans Le Tronc Des Arbres. Ann. des Sci. For. 1985, 42, 193–200.
- Nadezhdina, N. Revisiting the Heat Field Deformation (HFD) Method for Measuring Sap Flow. iForest - Biogeosciences For. 2018, 11, 118–130.
- Lavoie-Lamoureux, A.; Sacco, D.; Risse, P.A.; Lovisolo, C. Factors Influencing Stomatal Conductance in Response to Water Availability in Grapevine: A Meta-Analysis. Physiol. Plant. 2017, 159, 468–482.
- Wagner, Y.; Pozner, E.; Bar-On, P.; Ramon, U.; Raveh, E.; Neuhaus, E.; Cohen, S.; Grünzweig, J.; Klein, T. Rapid Stomatal Response in Lemon Saves Trees and Their Fruit Yields under Summer Desiccation, but Fails under Recurring Droughts. Agric. For. Meteorol. 2021, 307, 108487.
- Miranda, M.T.; Da Silva, S.F.; Silveira, N.M.; Pereira, L.; Machado, E.C.; Ribeiro, R.V. Root Osmotic Adjustment and Stomatal Control of Leaf Gas Exchange Are Dependent on Citrus Rootstocks Under Water Deficit. J. Plant Growth Regul. 2021, 40, 11–19.
- Lepaja, L.; Kullaj, E.; Lepaja, K.; Avdiu, V.; Zajmi, A. Effect of Water Stress on Some Physiological Indices in Young Pear Trees. Acta Hortic. 2019, 1253, 71–76.
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological Tools for Irrigation Scheduling in Grapevine (Vitis vinifera L.). Agric. Ecosyst. Environ. 2005, 106, 159–170.
- Toro, G.; Flexas, J.; Escalona, J.M. Contrasting Leaf Porometer and Infra-Red Gas Analyser Methodologies: An Old Paradigm about the Stomatal Conductance Measurement. Theor. Exp. Plant Physiol. 2019, 31, 483–492.
- Ehrenberger, W.; Rüger, S.; Fitzke, R.; Vollenweider, P.; Günthardt-Goerg, M.; Kuster, T.; Zimmermann, U.; Arend, M. Concomitant Dendrometer and Leaf Patch Pressure Probe Measurements Reveal the Effect of Microclimate and Soil Moisture on Diurnal Stem Water and Leaf Turgor Variations in Young Oak Trees. Funct. Plant Biol. 2012, 39, 297.
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most Stomatal Closure in Woody Species under Moderate Drought Can Be Explained by Stomatal Responses to Leaf Turgor. Plant Cell Environ. 2016, 39, 2014–2026.
- Zimmermann, U.; Rüger, S.; Shapira, O.; Westhoff, M.; Wegner, L.H.; Reuss, R.; Gessner, P.; Zimmermann, G.; Israeli, Y.; Zhou, A.; et al. Effects of Environmental Parameters and Irrigation on the Turgor Pressure of Banana Plants Measured Using the Non-Invasive, Online Monitoring Leaf Patch Clamp Pressure Probe. Plant Biol. 2010, 12, 424–436.
- Tomos, A.D.; Leigh, R.A. THE PRESSURE PROBE: A Versatile Tool in Plant Cell Physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 447–472.
- Kim, Y.X.; Stumpf, B.; Sung, J.; Lee, S.J. The Relationship between Turgor Pressure Change and Cell Hydraulics of Midrib Parenchyma Cells in the Leaves of Zea Mays. Cells 2018, 7, 180.
- Knoche, M.; Grimm, E.; Schlegel, H.J. Mature Sweet Cherries Have Low Turgor. J. Am. Soc. Hortic. Sci. 2014, 139, 3–12.
- Bramley, H.; Ehrenberger, W.; Zimmermann, U.; Palta, J.A.; Rüger, S.; Siddique, K.H.M. Non-Invasive Pressure Probes Magnetically Clamped to Leaves to Monitor the Water Status of Wheat. Plant Soil 2013, 369, 257–268.
- Rüger, S.; Ehrenberger, W.; Arend, M.; Geßner, P.; Zimmermann, G.; Zimmermann, D.; Bentrup, F.-W.; Nadler, A.; Raveh, E.; Sukhorukov, V.L.; et al. Comparative Monitoring of Temporal and Spatial Changes in Tree Water Status Using the Non-Invasive Leaf Patch Clamp Pressure Probe and the Pressure Bomb. Agric. Water Manag. 2010, 98, 283–290.
- Westhoff, M.; Reuss, R.; Zimmermann, D.; Netzer, Y.; Gessner, A.; Geßner, P.; Zimmermann, G.; Wegner, L.H.; Bamberg, E.; Schwartz, A.; et al. A Non-Invasive Probe for Online-Monitoring of Turgor Pressure Changes under Field Conditions. Plant Biol. 2009, 11, 701–712.
- Zimmermann, U.; Bitter, R.; Marchiori, P.E.R.; Rüger, S.; Ehrenberger, W.; Sukhorukov, V.L.; Schüttler, A.; Ribeiro, R.V. A Non-Invasive Plant-Based Probe for Continuous Monitoring of Water Stress in Real Time: A New Tool for Irrigation Scheduling and Deeper Insight into Drought and Salinity Stress Physiology. Theor. Exp. Plant Physiol. 2013, 25, 2–11.
- Afzal, A.; Duiker, S.W.; Watson, J.E. Leaf Thickness to Predict Plant Water Status. Biosyst. Eng. 2017, 156, 148–156.
- Seelig, H.-D.; Stoner, R.J.; Linden, J.C. Irrigation Control of Cowpea Plants Using the Measurement of Leaf Thickness under Greenhouse Conditions. Irrig. Sci. 2012, 30, 247–257.
- De Swaef, T.; Steppe, K.; Lemeur, R. Determining Reference Values for Stem Water Potential and Maximum Daily Trunk Shrinkage in Young Apple Trees Based on Plant Responses to Water Deficit. Agric. Water Manag. 2009, 96, 541–550.
- Downes, G.M.; Drew, D.; Battaglia, M.; Schulze, D. Measuring and Modelling Stem Growth and Wood Formation: An Overview. Dendrochronologia 2009, 27, 147–157.
- Silber, A.; Naor, A.; Israeli, Y.; Assouline, S. Combined Effect of Irrigation Regime and Fruit Load on the Patterns of Trunk-Diameter Variation of “Hass” Avocado at Different Phenological Periods. Agric. Water Manag. 2013, 129, 87–94.
- Ueda, M.; Shibata, E. Diurnal Changes in Branch Diameter as Indicator of Water Status of Hinoki Cypress Chamaecyparis Obtusa. Trees 2001, 15, 315–318.
- Sevanto, S.; Vesala, T.; Perämäki, M.; Nikinmaa, E. Time Lags for Xylem and Stem Diameter Variations in a Scots Pine Tree. Plant. Cell Environ. 2002, 25, 1071–1077.
- Cermak, J.; Kucera, J.; Bauerle, W.L.; Phillips, N.; Hinckley, T.M. Tree Water Storage and Its Diurnal Dynamics Related to Sap Flow and Changes in Stem Volume in Old-Growth Douglas-Fir Trees. Tree Physiol. 2007, 27, 181–198.
- Abdelfatah, A.; Aranda, X.; Savé, R.; de Herralde, F.; Biel, C. Evaluation of the Response of Maximum Daily Shrinkage in Young Cherry Trees Submitted to Water Stress Cycles in a Greenhouse. Agric. Water Manag. 2013, 118, 150–158.
- Fernández, J.E.; Cuevas, M.V. Irrigation Scheduling from Stem Diameter Variations: A Review. Agric. For. Meteorol. 2010, 150, 135–151.
- Martín-Palomo, M.J.; Corell, M.; Girón, I.; Andreu, L.; Trigo, E.; López-Moreno, Y.E.; Torrecillas, A.; Centeno, A.; Pérez-López, D.; Moriana, A. Pattern of Trunk Diameter Fluctuations of Almond Trees in Deficit Irrigation Scheduling during the First Seasons. Agric. Water Manag. 2019, 218, 115–123.
- Ru, C.; Hu, X.; Wang, W.; Ran, H.; Song, T.; Guo, Y. Signal Intensity of Stem Diameter Variation for the Diagnosis of Drip Irrigation Water Deficit in Grapevine. Horticulturae 2021, 7, 154.
- Hernandez-Santana, V.; Fernández, J.E.; Cuevas, M.V.; Perez-Martin, A.; Diaz-Espejo, A. Photosynthetic Limitations by Water Deficit: Effect on Fruit and Olive Oil Yield, Leaf Area and Trunk Diameter and Its Potential Use to Control Vegetative Growth of Super-High Density Olive Orchards. Agric. Water Manag. 2017, 184, 9–18.
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995.
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of Drought Stress on Photosynthetic Response, Leaf Water Potential, and Stem Sap Flow in Two Cultivars of Bi-Leader Apple Trees (Malus × Domestica Borkh.). Sci. Hortic. (Amsterdam) 2019, 246, 535–543.
- Begg, J.E.; Turner, N.C. Water Potential Gradients in Field Tobacco. Plant Physiol. 1970, 46, 343–346.
- Memmi, H.; Gijón, M.C.; Couceiro, J.F.; Pérez-López, D. Water Stress Thresholds for Regulated Deficit Irrigation in Pistachio Trees: Rootstock Influence and Effects on Yield Quality. Agric. Water Manag. 2016, 164, 58–72.
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Morales-Sillero, A.; Martín-Palomo, M.J.; Mayr, S.; Beikircher, B.; Fernández, J.E. Shoot Hydraulic Characteristics, Plant Water Status and Stomatal Response in Olive Trees under Different Soil Water Conditions. Plant Soil 2013, 373, 77–87.
- Abrisqueta, I.; Conejero, W.; Valdés-Vela, M.; Vera, J.; Ortuño, M.F.; Ruiz-Sánchez, M.C. Stem Water Potential Estimation of Drip-Irrigated Early-Maturing Peach Trees under Mediterranean Conditions. Comput. Electron. Agric. 2015, 114, 7–13.
- Schaefer, N.L.; Trickett, E.S.; Ceresa, A.; Barrs, H.D. Continuous Monitoring of Plant Water Potential. Plant Physiol. 1986, 81, 45–49.
- Turner, N.C. Techniques and Experimental Approaches for the Measurement of Plant Water Status. Plant Soil 1981, 58, 339–366.
- Pagay, V.; Santiago, M.; Sessoms, D.A.; Huber, E.J.; Vincent, O.; Pharkya, A.; Corso, T.N.; Lakso, A.N.; Stroock, A.D. A Microtensiometer Capable of Measuring Water Potentials below -10 MPa. Lab Chip 2014, 14, 2806–2817.
- Martínez, E.M.; Cancela, J.J.; Cuesta, T.S.; Neira, X.X. Review. Use of Psychrometers in Field Measurements of Plant Material: Accuracy and Handling Difficulties. Spanish J. Agric. Res. 2011, 9, 313.
- Martínez, E.M.; Rey, B.J.; Fandiño, M.; Cancela, J.J. Comparison of Two Techniques for Measuring Leaf Water Potential in Vitis Vinifera Var. Albariño. Cienc. e Tec. Vitivinic. 2013, 28, 29–41.
- Levin, A.D. Re-Evaluating Pressure Chamber Methods of Water Status Determination in Field-Grown Grapevine (Vitis Spp.). Agric. Water Manag. 2019, 221, 422–429.
- Ihuoma, S.O.; Madramootoo, C.A. Recent Advances in Crop Water Stress Detection. Comput. Electron. Agric. 2017, 141, 267–275.
- Ammar, A.; Ben Aissa, I.; Mars, M.; Gouiaa, M. Comparative Physiological Behavior of Fig (Ficus Carica L.) Cultivars in Response to Water Stress and Recovery. Sci. Hortic. (Amsterdam). 2020, 260, 108881.
- Rodríguez, P.; Mellisho, C.D.; Conejero, W.; Cruz, Z.N.; Ortuño, M.F.; Galindo, A.; Torrecillas, A. Plant Water Relations of Leaves of Pomegranate Trees under Different Irrigation Conditions. Environ. Exp. Bot. 2012, 77, 19–24.
- Giménez, C.; Gallardo, M.; Thompson, R.B. Plant–Water Relations. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–8. ISBN 9780124095489.
- Alarcón, J.J.; Domingo, R.; Green, S.R.; Sánchez-Blanco, M.J.; Rodríguez, P.; Torrecillas, A. Sap Flow as an Indicator of Transpiration and the Water Status of Young Apricot Trees. Plant Soil 2000, 227, 77–85.
- Flo, V.; Martinez-Vilalta, J.; Steppe, K.; Schuldt, B.; Poyatos, R. A Synthesis of Bias and Uncertainty in Sap Flow Methods. Agric. For. Meteorol. 2019, 271, 362–374.
- Poyatos, R.; Granda, V.; Flo, V.; Molowny-Horas, R.; Steppe, K.; Mencuccini, M.; Martínez-Vilalta, J. SAPFLUXNET: A Global Database of Sap Flow Measurements. Zenodo 2020.
- Steinberg, S.; van Bavel, C.H.M.; McFarland, M.J. A Gauge to Measure Mass Flow Rate of Sap in Stems of Woody Plants.Pdf. J. Am. Soc. Hortic. Sci. 1989, 114, 466–472.
- Baker, J.M.; van Bavel, C.H.M. Measurement of Mass Flow of Water in the Stems of Herbaceous Plants. Plant, Cell Environ. 1987, 10, 777–782.
- Green, S.R.; Clothier, B.E. Water Use of Kiwifruit Vines and Apple Trees by the Heat-Pulse Technique. J. Exp. Bot. 1988, 39, 115–123.
- Forster, M. How Reliable Are Heat Pulse Velocity Methods for Estimating Tree Transpiration? Forests 2017, 8, 350.
- Fernández, J.; Palomo, M.; Díaz-Espejo, A.; Clothier, B.; Green, S.; Girón, I.; Moreno, F. Heat-Pulse Measurements of Sap Flow in Olives for Automating Irrigation: Tests, Root Flow and Diagnostics of Water Stress. Agric. Water Manag. 2001, 51, 99–123.
- Green, S.; Clothier, B.; Jardine, B. Theory and Practical Application of Heat Pulse to Measure Sap Flow. Agron. J. 2003, 95, 1371–1379.
- Green, S.R. Radiation Balance, Transpiration and Photosynthesis of an Isolated Tree. Agric. For. Meteorol. 1993, 64, 201–221.
- Alarcón, J.J.; Ortuno, M.F.; Nicolas, E.; Torres, R.; Torrecillas, A. Compensation Heat-Pulse Measurements of Sap Flow for Estimating Transpiration in Young Lemon Trees. Biol. Plant. 2005, 49, 527–532.
- Fuchs, S.; Leuschner, C.; Link, R.; Coners, H.; Schuldt, B. Calibration and Comparison of Thermal Dissipation, Heat Ratio and Heat Field Deformation Sap Flow Probes for Diffuse-Porous Trees. Agric. For. Meteorol. 2017, 244–245, 151–161.
- Clearwater, M.J.; Luo, Z.; Mazzeo, M.; Dichio, B. An External Heat Pulse Method for Measurement of Sap Flow through Fruit Pedicels, Leaf Petioles and Other Small-Diameter Stems. Plant. Cell Environ. 2009, 32, 1652–1663.
- Wang, S.; Fan, J.; Ge, J.; Wang, Q.; Yong, C.; You, W. New Design of External Heat-Ratio Method for Measuring Low and Reverse Rates of Sap Flow in Thin Stems. For. Ecol. Manag. 2018, 419–420, 10–16.
- Ren, R.; von der Crone, J.; Horton, R.; Liu, G.; Steppe, K. An Improved Single Probe Method for Sap Flow Measurements Using Finite Heating Duration. Agric. For. Meteorol. 2020, 280, 107788.
- Cabibel, B. Mesures Thermiques Des Flux de Sève et Comportement Hydrique Des Arbres. III. Influence Sur Les Flux de Sève Des Modalités d’apport d’eau En Irrigation Localisée Sur Sol Fissuré. Agronomie 1991, 11, 877–888.
- Rabbel, I.; Diekkrüger, B.; Voigt, H.; Neuwirth, B. Comparing ΔTmax Determination Approaches for Granier-Based Sapflow Estimations. Sensors 2016, 16, 2042.
- Alizadeh, A.; Toudeshki, A.; Ehsani, R.; Migliaccio, K.; Wang, D. Detecting Tree Water Stress Using a Trunk Relative Water Content Measurement Sensor. Smart Agric. Technol. 2021, 1, 100003.
- Nadezhdina, N. Sap Flow Index as an Indicator of Plant Water Status. Tree Physiol. 1999, 19, 885–891.
- Nadezhdina, N. A Simplified Equation for Sap Flow Calculation Based on the Heat- Field Deformation (HFD) Measurements. Acta Hortic. 2012, 951, 117–120.
- Nadezhdina, N. Heat Field Deformation Sensors for Sap Flow Measurements in Small Stems. Acta Hortic. 2013, 991, 53–60.
- Hanssens, J.; De Swaef, T.; Nadezhdina, N.; Steppe, K. Measurement of Sap Flow Dynamics through the Tomato Peduncle Using a Non-Invasive Sensor Based on the Heat Field Deformation Method. Acta Hortic. 2013, 991, 409–416.
More
Information
Subjects:
Agricultural Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
998
Revisions:
2 times
(View History)
Update Date:
27 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




