
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bryan Mathis | -- | 3540 | 2022-09-21 22:16:47 | | | |
| 2 | Lindsay Dong | -2 word(s) | 3538 | 2022-09-22 08:34:08 | | |
Video Upload Options
Obesity is a chronic, progressive disease of caloric energy storage that manifests as excess visceral or subcutaneous lipid deposition. A systemic condition, surplus adipose tissue generates inflammation through secretion of cytokines while the neuroendocrine and metabolic energy balance systems resist loss of stored fat as a conserved survival mechanism. Thus, obesity is not disease of storage, but a metabolic condition that resets energy homeostasis and inflicts gradual damage to the cardiopulmonary and glucose management systems. Obesity without this concomitantly increased cardiopulmonary risk is termed metabolically healthy obesity (MHO) while metabolically unhealthy obesity (MUHO) represents the endpoint of obesogenesis, but increases in cardiovascular, cancer, and all-cause mortality.
1. Introduction
From the economic standpoint of the rapidly aging Asian population alone, an obesity pandemic would be unsustainable, as care for type 2 diabetes and cardiovascular diseases are expensive and long-term commitments. Additionally, obesogenesis in East Asian children is also increasing, which will affect population-level quality of life and further increase demand on limited resources for obesity-related care [2]. Therefore, if East Asians currently suffering from obesity can maintain MHO status for as long as possible before intervention, it would buffer the burden on the socialized medical systems found in East Asia as well as improving quality of life with regard to metabolic syndrome. However, since excessive fat has been found to increase peak loading of the musculoskeletal movement chain, the effect of weight stress could increase the failure rate of hip implants in the aging and cause balance issues even in younger populations [3][4] Thus, even if it not a state of total health, MHO is preferable to MUHO since a healthy metabolism may facilitate weight loss through exercise by reducing weight stress on the legs and hips.
In general, the first-line treatment strategy of “eat less, move more” is ineffective for MUHO in the long-term (partly due to obesity effects on the movement chain’s peak loading stress) but, as MHO is characterized by cardiopulmonary fitness and normal glucose regulation, maintenance of MHO and weight loss through diet and exercise may be possible in these individuals if targeted interventions remove known causes of MUHO pathogenesis [3]. While bariatric surgery (expensive and permanent) has shown some promise, obesity is a nearly incurable disease and, thus, prevention of obesogenesis and maintenance of MHO are of utmost importance to relieve the socialized medical systems of East Asia. However, in-depth analyses of the causes of obesity and potential factors for shifting from MHO to MUHO in East Asia are scarce.
2. Factors in Metabolically Healthy and Unhealthy Obesity
2.1. Age
2.2. CICO vs. CIM
2.3. Damage from Reactive Oxygen Species
2.4. Environmental Pollution
2.5. Seed Oils and Allergies
2.6. Hormonal Changes, Age, and Menopause
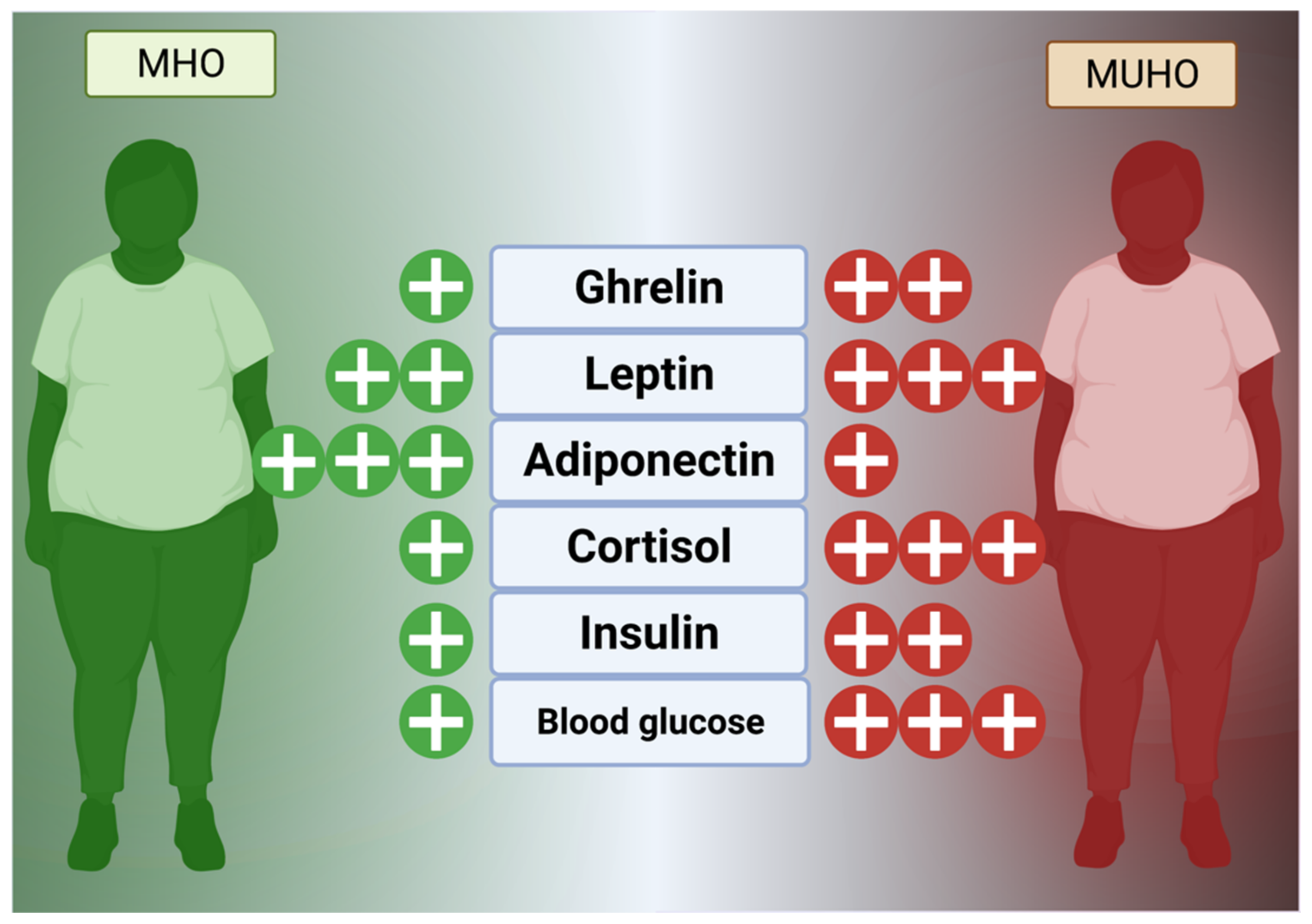
Hormones, as a comparatively slower but wide-reaching chemical messenger system, play a key role in obesogenesis and maintenance of excessive fat stores. In general, the hunger and fat management mechanism is currently known to consist of hormones, such as glucagon-like peptide 1 (GLP-1), visfatin, ghrelin, cholecystokinin (CCK), leptin, and enterostatin, that reside in the digestive tract and fat to control satiety and peristalsis in concert with the hypothalamus via vagus nerve signaling (Figure 1) [52][53].
2.7. Immune Factors
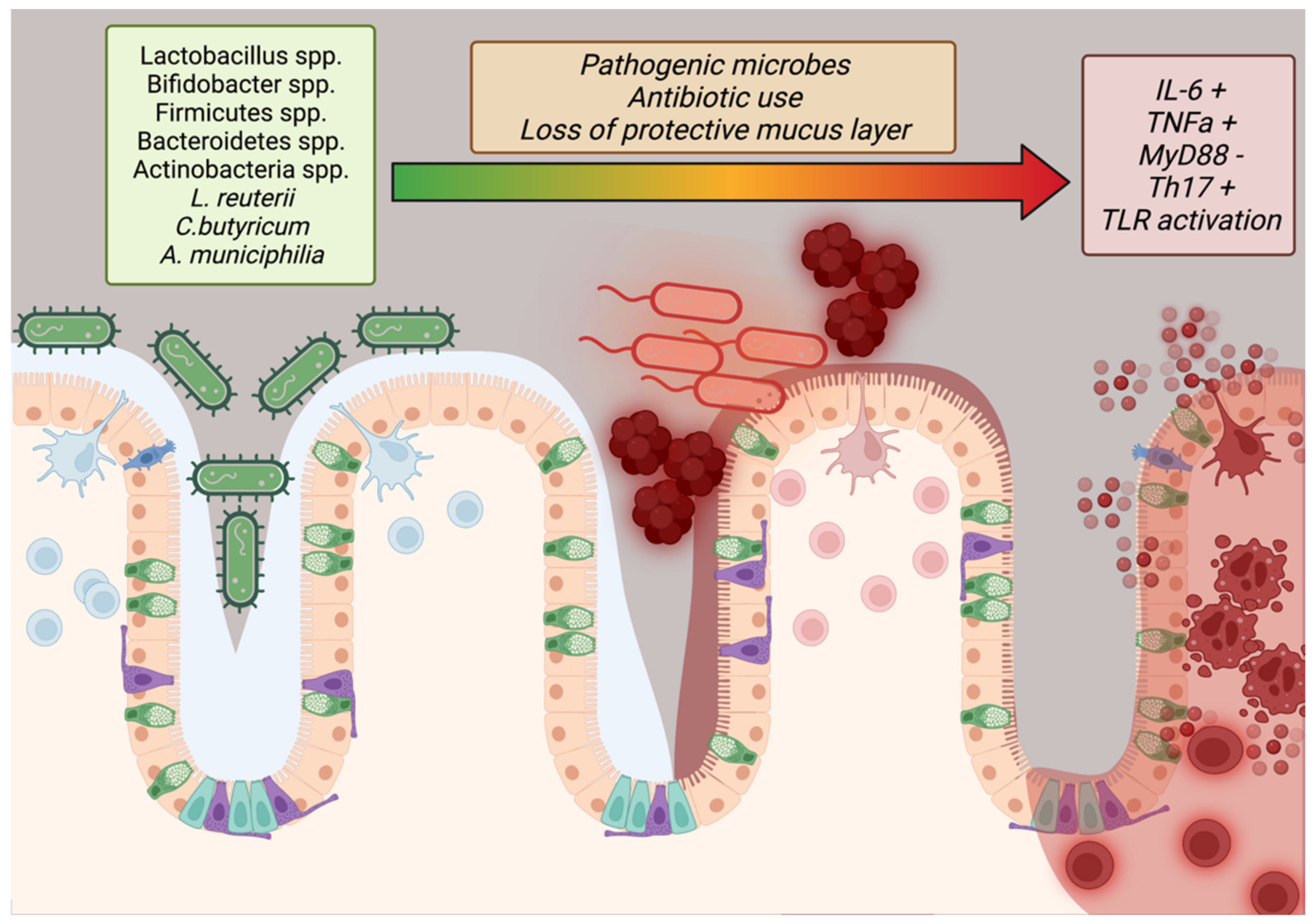
2.8. The Microbiome
2.9. Social Aspect
2.10. Vitamin and Micronutrient Deficiencies
A lack of vitamins or other micronutrients has been implicated in obesogenesis and animal studies have shown that obesity features a lack of Vitamins A and D, with Vitamin A regulating metabolism through RBP3 and ALDH1A1 and Vitamin D playing key roles in adipokine synthesis, calcium balance, and glucose metabolism [76][77][78]. Vitamin D, shown to be sequestered in fat tissue and, therefore, of low bioavailability in the obese, additionally regulates the immune system through the Vitamin D receptor on T cells, reducing the effect of chronic inflammation on obesogenesis [78][79].
2.11. Weight Fluctuation and Rebound Effect
In East Asia: Although studies on rebound are scarce, several small studies in Japan and China implicated a return to previous overfeeding patterns due to social contact or post-surgical pain that limited activity [81][82]. A large Korean study of 3678 adults, among whom half experienced high weight variability, found increased risk of death and rebound was attributed to homeostatic feedback after weight loss that creates a biochemical milieu favorable to weight gain [83].
2.12. Genetic Factors
In East Asia: A study of 1213 Chinese children found that KCNQ1-rs2237897 is associated with cardiovascular and KCNQ1-rs2237892 is associated with insulin resistance risk in MHO while another Chinese study of 1790 MHO children found FTO-rs9939609 or CYP17A1-rs11191548 predictive of cardiovascular risk in addition to GNPDA2-rs10938397 or KCTD15-rs29941 being predictive for insulin resistance [84][85]. A separate Chinese study did find that adiponectin-related gene polymorphisms, especially rs6773957, were related to diet and MUHO, in line with a similar Russian study of 503 obese patients that found similar connections to polymorphisms such as G45G [86][87]. A genome-wide association study of 49,915 Koreans found that LPL, APOA5, CETP, GCKR, CDKAL1, and CDKN2B (all lipid metabolism genes) were associated with MHO, thus lending weight to the concept that the MHO condition is a discrete genetic phenotype and that shifts to MUHO may involve epigenetic regulation from synergistic internal (reactive oxygen species, stress, hormones, etc.) and/or external (pollution, diet, etc.) sources [88].
References
- Helble, M.; Francisco, K. The Upcoming Obesity Crisis in Asia and the Pacific: First Cost Estimates; ADBI Working Paper 743; ADB Institute: Tokyo, Japan, 2017.
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542.
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribol. 2022, 33, 31–38.
- Teasdale, N.; Simoneau, M.; Corbeil, P.; Handrigan, G.; Tremblay, A.; Hue, O. Obesity Alters Balance and Movement Control. Curr. Obes. Rep. 2013, 2, 235–240.
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220.
- Lührmann, P.M.; Edelmann-Schafer, B.; Neuhäuser-Berthold, M. Changes in resting metabolic rate in an elderly German population: Cross-sectional and longitudinal data. J. Nutr. Health Aging 2010, 14, 232–236.
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904.
- Lu, J.; Liu, Q. Four decades of studies on population aging in China. China Popul. Dev. Stud. 2019, 3, 24–36.
- Tahara, Y. Cardiopulmonary Resuscitation in a Super-Aging Society—Is There an Age Limit for Cardiopulmonary Resuscitation? Circ. J. 2016, 80, 1102–1103.
- Jang, I.-Y.; Lee, H.Y.; Lee, E. The 50th Anniversary Committee of Korean Geriatrics Society Geriatrics Fact Sheet in Korea 2018 From National Statistics. Ann. Geriatr. Med. Res. 2019, 23, 50–53.
- Howell, S.; Kones, R. “Calories in, calories out” and macronutrient intake: The hope, hype, and science of calories. Am. J. Physiol. Metab. 2017, 313, E608–E612.
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254.
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103.
- Russell-Jones, D.; Khan, R. Insulin-associated weight gain in diabetes—Causes, effects and coping strategies. Diabetes Obes. Metab. 2006, 9, 799–812.
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of Metformin on Weight Loss in Non-Diabetic Individuals with Obesity. Exp. Clin. Endocrinol. Diabetes 2012, 121, 27–31.
- Brunani, A.; Caumo, A.; Graci, S.; Castagna, G.; Viberti, G.; Liuzzi, A. Rosiglitazone is more effective than metformin in improving fasting indexes of glucose metabolism in severely obese, non-diabetic patients. Diabetes Obes. Metab. 2008, 10, 460–467.
- Berne, C.; The Orlistat Swedish Type 2 Diabetes Study Group. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabet. Med. 2005, 22, 612–618.
- Odawara, M.; Aoi, S.; Takeshima, T.; Iwasaki, K. Comparative Effects of Metformin and Dipeptidyl Peptidase-4 Inhibitors in Japanese Obese Patients with Type 2 Diabetes: A Claims Database Study. Diabetes Ther. 2021, 12, 2165–2177.
- Rebello, A.S.; Koh, H.; Chen, C.; Naidoo, N.; Odegaard, O.A.; Koh, W.-P.; Butler, L.M.; Yuan, J.-M.; van Dam, R. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: A prospective cohort study. Am. J. Clin. Nutr. 2014, 100, 53–64.
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444.
- Lefranc, C.; Friederich-Persson, M.; Palacios, R.; Cat, A.N.D. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159.
- McMurray, F.; Patten, D.A.; Harper, M.-E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310.
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973.
- Al-Lahham, R.; Deford, J.H.; Papaconstantinou, J. Mitochondrial-generated ROS down regulates insulin signaling via activation of the p38MAPK stress response pathway. Mol. Cell. Endocrinol. 2016, 419, 1–11.
- Kogawa, T.; Kashiwakura, I. Relationship between obesity and serum reactive oxygen metabolites in adolescents. Environ. Health Prev. Med. 2013, 18, 451–457.
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Health Sci. 2016, 6, 225–230.
- Na, I.J.; Park, J.S.; Park, S.B. Association between Abdominal Obesity and Oxidative Stress in Korean Adults. Korean J. Fam. Med. 2019, 40, 395–398.
- Wei, C.; Feng, R.; Hou, X.; Peng, T.; Shi, T.; Hu, X. Nanocolloids in drinking water increase the risk of obesity in mice by modulating gut microbes. Environ. Int. 2020, 146, 106302.
- Kusumoto, M.; Mathis, B. Biologic Treatments for Asthma and Chronic Obstructive Pulmonary Disorder. Allergies 2021, 10, 7.
- Wang, Y.; Hollis-Hansen, K.; Ren, X.; Qiu, Y.; Qu, W. Do environmental pollutants increase obesity risk in humans? Obes. Rev. 2016, 17, 1179–1197.
- Dirinck, E.; Jorens, P.G.; Covaci, A.; Geens, T.; Roosens, L.; Neels, H.; Mertens, I.; Van Gaal, L. Obesity and Persistent Organic Pollutants: Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity 2011, 19, 709–714.
- Moon, S.; Seo, M.Y.; Choi, K.; Chang, Y.-S.; Kim, S.-H.; Park, M.J. Urinary bisphenol A concentrations and the risk of obesity in Korean adults. Sci. Rep. 2021, 11, 1603.
- Lee, I.; Park, Y.J.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Park, H.; et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2020, 146, 106227.
- McLachlan, M.S.; Czub, G.; MacLeod, M.; Arnot, J.A. Bioaccumulation of Organic Contaminants in Humans: A Multimedia Perspective and the Importance of Biotransformation. Environ. Sci. Technol. 2010, 45, 197–202.
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-Induced Oxidative Stress and Mitochondrial Damage in the Nasal Mucosa of Rats. Int. J. Environ. Res. Public Health 2017, 14, 134.
- Jiang, J.; Li, Y.; Liang, S.; Sun, B.; Shi, Y.; Xu, Q.; Zhang, J.; Shen, H.; Duan, J.; Sun, Z. Combined exposure of fine particulate matter and high-fat diet aggravate the cardiac fibrosis in C57BL/6J mice. J. Hazard. Mater. 2020, 391, 122203.
- Deschenes, O.; Wang, H.; Wang, S.; Zhang, P. The effect of air pollution on body weight and obesity: Evidence from China. J. Dev. Econ. 2020, 145, 102461.
- Cao, S.; Guo, Q.; Xue, T.; Wang, B.; Wang, L.; Duan, X.; Zhang, J. Long-term exposure to ambient PM2.5 increase obesity risk in Chinese adults: A cross-sectional study based on a nationwide survey in China. Sci. Total Environ. 2021, 778, 145812.
- Paoin, K.; Ueda, K.; Ingviya, T.; Buya, S.; Phosri, A.; Seposo, X.T.; Seubsman, S.-A.; Kelly, M.; Sleigh, A.; Honda, A.; et al. Long-term air pollution exposure and self-reported morbidity: A longitudinal analysis from the Thai cohort study (TCS). Environ. Res. 2020, 192, 110330.
- Deol, S.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci. Rep. 2017, 7, 12488.
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver. PLoS ONE 2015, 10, e0132672.
- El-Magd, N.F.A.; El-Mesery, M.; El-Karef, A.; El-Shishtawy, M.M. Amelioration effect of black seed oil against high-fat diet-induced obesity in rats through Nrf2/HO-1 pathway. J. Food Biochem. 2021, 45, e13693.
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651.
- Wang, L.; Zhang, L.; Ristovski, Z.D.; Zheng, X.; Wang, H.-L.; Li, L.; Gao, J.; Salimi, F.; Gao, Y.; Jing, S.; et al. Assessing the Effect of Reactive Oxygen Species and Volatile Organic Compound Profiles Coming from Certain Types of Chinese Cooking on the Toxicity of Human Bronchial Epithelial Cells. Environ. Sci. Technol. 2020, 54, 8868–8877.
- Zhuang, P.; Mao, L.; Wu, F.; Wang, J.; Jiao, J.; Zhang, Y. Cooking Oil Consumption Is Positively Associated with Risk of Type 2 Diabetes in a Chinese Nationwide Cohort Study. J. Nutr. 2020, 150, 1799–1807.
- Kim, S.; Moon, S.; Popkin, B.M. The nutrition transition in South Korea. Am. J. Clin. Nutr. 2000, 71, 44–53.
- Gu, Y.; Guo, X.; Sun, S.; Che, H. High-Fat Diet-Induced Obesity Aggravates Food Allergy by Intestinal Barrier Destruction and Inflammation. Int. Arch. Allergy Immunol. 2021, 183, 80–92.
- Visness, C.M.; London, S.J.; Daniels, J.L.; Kaufman, J.S.; Yeatts, K.B.; Siega-Riz, A.-M.; Liu, A.H.; Calatroni, A.; Zeldin, D.C. Association of obesity with IgE levels and allergy symptoms in children and adolescents: Results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2009, 123, 1163–1169.e4.
- Hayashi, K.; Tsujiguchi, H.; Hori, D.; Yamada, Y.; Shimizu, Y.; Nguyen, T.T.T.; Hibino, Y.; Kambayashi, Y.; Hara, A.; Nakamura, H. The association between overweight and prevalence of food allergy in Japanese children: A cross-sectional study. Environ. Health Prev. Med. 2021, 26, 44.
- Lei, Y.; Yang, H.; Zhen, L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China). Asia Pac. Allergy 2016, 6, 101–104.
- Kim, S.Y.; Choi, S.H.; Kim, J.D.; Sol, I.S.; Kim, M.J.; Kim, Y.H.; Jung, Y.-C.; Sohn, M.H.; Kim, K.W. Korean Youth with Comorbid Allergic Disease and Obesity Show Heightened Psychological Distress. J. Pediatr. 2019, 206, 99–104.e4.
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2015, 40, 622–632.
- Duque, A.P.; Junior, L.F.R.; Mediano, M.F.F.; Tibiriça, E.; De Lorenzo, A. Emerging concepts in metabolically healthy obesity. Am. J. Cardiovasc. Dis. 2020, 10, 48–61.
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; De La Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664.
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971.
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48.
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose Tissue Macrophage Polarization in Healthy and Unhealthy Obesity. Front. Nutr. 2021, 8, 625331.
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359.
- Kern, L.; Mittenbühler, M.; Vesting, A.; Ostermann, A.; Wunderlich, C.; Wunderlich, F. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24.
- Moschen, A.; Molnar, C.; Geiger, S.; Graziadei, I.; Ebenbichler, C.; Weiss, H.; Kaser, S.; Kaser, A.; Tilg, H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor expression. Gut 2010, 59, 1259–1264.
- Tang, H.; Liu, N.; Feng, X.; Yang, Y.; Fang, Y.; Zhuang, S.; Dai, Y.; Liu, M.; Tang, L. Circulating levels of IL-33 are elevated by obesity and positively correlated with metabolic disorders in Chinese adults. J. Transl. Med. 2021, 19, 52.
- Ji, Y.-F.; Jiang, X.; Li, W.; Ge, X. Impact of interleukin-6 gene polymorphisms and its interaction with obesity on osteoporosis risk in Chinese postmenopausal women. Environ. Health Prev. Med. 2019, 24, 48.
- Dai, W.; Liu, X.; Su, H.; Li, X.; Xu, Y.; Yu, Y. Influence of adipose tissue immune dysfunction on childhood obesity. Cytokine Growth Factor Rev. 2022, 65, 27–38.
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123.
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219.
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211.
- Iacob, S.; Iacob, D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front. Microbiol. 2019, 10, 1676.
- Naito, E.; Yoshida, Y.; Kunihiro, S.; Makino, K.; Kasahara, K.; Kounoshi, Y.; Aida, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: A randomised, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 9–18.
- Rosenbaum, D.L.; White, K.S. The role of anxiety in binge eating behavior: A critical examination of theory and empirical literature. Health Psychol. Res. 2013, 1, e19.
- Giletta, M.; Slavich, G.M.; Rudolph, K.D.; Hastings, P.D.; Nock, M.K.; Prinstein, M. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J. Child Psychol. Psychiatry 2017, 59, 129–139.
- Brixval, C.S.; Rayce, S.L.B.; Rasmussen, M.; Holstein, B.; Due, P. Overweight, body image and bullying--an epidemiological study of 11- to 15-years olds. Eur. J. Public Health 2011, 22, 126–130.
- Kubo, T. Common approach to childhood obesity in Japan. J. Pediatr. Endocrinol. Metab. 2014, 27, 581–592.
- Cai, Y.; Zhu, X.; Wu, X. Overweight, obesity, and screen-time viewing among Chinese school-aged children: National prevalence estimates from the 2016 Physical Activity and Fitness in China—The Youth Study. J. Sport Health Sci. 2017, 6, 404–409.
- Koyanagi, A.; Veronese, N.; Vancampfort, D.; Stickley, A.; Jackson, S.E.; Oh, H.; Shin, J.I.; Haro, J.M.; Stubbs, B.; Smith, L. Association of bullying victimization with overweight and obesity among adolescents from 41 low- and middle-income countries. Pediatr. Obes. 2019, 15, e12571.
- Terunuma, Y.; Mathis, B.J. Cultural sensitivity in brain death determination: A necessity in end-of-life decisions in Japan. BMC Med. Ethic. 2021, 22, 58.
- Trasino, S.E.; Tang, X.-H.; Jessurun, J.; Gudas, L.J. Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci. Rep. 2015, 5, 15893.
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178.
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103.
- McKay, J.; Ho, S.; Jane, M.; Pal, S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020, 6, 12.
- Hong, H.C.; Lee, J.-S.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Seo, A.J.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metabolism 2013, 62, 1305–1312.
- Yamaguchi, S. Cause of Weight Rebound with Obese Women Who Had Lost Weight and Effect of Intervention by Telephone and Newsletters. Jpn. J. Nutr. Diet. 2007, 65, 21–28.
- Zuo, D.; Xiao, X.; Yang, S.; Gao, Y.; Wang, G.; Ning, G. Effects of bariatric surgery in Chinese with obesity and type 2 diabetes mellitus. Medicine 2020, 99, e21673.
- Oh, T.J.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, K.S.; Cho, N.H.; Jang, H.C. Body-Weight Fluctuation and Incident Diabetes Mellitus, Cardiovascular Disease, and Mortality: A 16-Year Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2018, 104, 639–646.
- Li, L.; Yin, J.; Cheng, H.; Wang, Y.; Gao, S.; Li, M.; Grant, S.F.; Li, C.; Mi, J.; Li, M. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: Data from the BCAMS study. J. Clin. Endocrinol. Metab. 2016, 101, 1816–1825.
- Gao, L.; Zhang, M.; Mi, J. Lbps 01-06 genetic and environmental factors of metabolically healthy obesity in Chinese children. J. Hypertens. 2016, 34, e175.
- Li, G.; Zhong, L.; Han, L.; Wang, Y.; Li, B.; Wang, D.; Zhao, Y.; Li, Y.; Zhang, Q.; Qi, L.; et al. Genetic variations in adiponectin levels and dietary patterns on metabolic health among children with normal weight versus obesity: The BCAMS study. Int. J. Obes. 2021, 46, 325–332.
- Berezina, A.; Belyaeva, O.; Berkovich, O.; Baranova, E.; Karonova, T.; Bazhenova, E.; Brovin, D.; Grineva, E.; Shlyakhto, E. Prevalence, Risk Factors, and Genetic Traits in Metabolically Healthy and Unhealthy Obese Individuals. BioMed Res. Int. 2015, 2015, 548734.
- Park, J.-M.; Park, D.-H.; Song, Y.; Kim, J.O.; Choi, J.-E.; Kwon, Y.-J.; Kim, S.-J.; Lee, J.-W.; Hong, K.-W. Understanding the genetic architecture of the metabolically unhealthy normal weight and metabolically healthy obese phenotypes in a Korean population. Sci. Rep. 2021, 11, 2279.




