Obesity is a chronic, progressive disease of caloric energy storage that manifests as excess visceral or subcutaneous lipid deposition. A systemic condition, surplus adipose tissue generates inflammation through secretion of cytokines while the neuroendocrine and metabolic energy balance systems resist loss of stored fat as a conserved survival mechanism. Thus, obesity is not disease of storage, but a metabolic condition that resets energy homeostasis and inflicts gradual damage to the cardiopulmonary and glucose management systems. Obesity without this concomitantly increased cardiopulmonary risk is termed metabolically healthy obesity (MHO) while metabolically unhealthy obesity (MUHO) represents the endpoint of obesogenesis, but increases in cardiovascular, cancer, and all-cause mortality.
- body mass index
- diabetes
- metabolically healthy obesity
- obesity
1. Introduction
From the economic standpoint of the rapidly aging Asian population alone, an obesity pandemic would be unsustainable, as care for type 2 diabetes and cardiovascular diseases are expensive and long-term commitments. Additionally, obesogenesis in East Asian children is also increasing, which will affect population-level quality of life and further increase demand on limited resources for obesity-related care [3][2]. Therefore, if East Asians currently suffering from obesity can maintain MHO status for as long as possible before intervention, it would buffer the burden on the socialized medical systems found in East Asia as well as improving quality of life with regard to metabolic syndrome. However, since excessive fat has been found to increase peak loading of the musculoskeletal movement chain, the effect of weight stress could increase the failure rate of hip implants in the aging and cause balance issues even in younger populations [4,5][3][4] Thus, even if it not a state of total health, MHO is preferable to MUHO since a healthy metabolism may facilitate weight loss through exercise by reducing weight stress on the legs and hips.
In general, the first-line treatment strategy of “eat less, move more” is ineffective for MUHO in the long-term (partly due to obesity effects on the movement chain’s peak loading stress) but, as MHO is characterized by cardiopulmonary fitness and normal glucose regulation, maintenance of MHO and weight loss through diet and exercise may be possible in these individuals if targeted interventions remove known causes of MUHO pathogenesis [4][3]. While bariatric surgery (expensive and permanent) has shown some promise, obesity is a nearly incurable disease and, thus, prevention of obesogenesis and maintenance of MHO are of utmost importance to relieve the socialized medical systems of East Asia. However, in-depth analyses of the causes of obesity and potential factors for shifting from MHO to MUHO in East Asia are scarce.
2. Factors in Metabolically Healthy and Unhealthy Obesity
2.1. Age
2.2. CICO vs. CIM
The Calories In Calories Out (CICO) model, or energy balance model (EBM), treats every calorie as equal without accounting for the variability of resting energy expenditure based on time of day or other factors. While over 60–70% of daily caloric expenditure stems from basal metabolism, around 10% comes from the thermogenic effect of food intake (the energy used for digestion and nutrient assimilation) while the remaining 20% or so is the energy expended to move or exercise [12][11]. In this model, all calories are equal from an obesogenic perspective and substitutions (e.g., replacing fat calories with carbohydrate calories) are expected to have an effect dictated by simple mathematics (if calories in < calories out, then weight loss will occur). However, this model does assert that the brain tightly controls energy balance within the body through a network of nervous/endocrine messaging and that modern, processed food is confusing the neurological system with regard to food reward and dopamine release [13][12]. In this regard, the EBM is considered as a holistic system that measures caloric demand by internal body sensors but does not control for the degradation of assimilative and communicative machinery within the body (e.g., the inability of brains affected by leptin resistance to respond properly to leptin signaling [13][12]. The Carbohydrate–Insulin Model (CIM), on the other hand, focuses on the differential effects of metabolic glucose dysregulation and hormonal action on shifting calories from even healthy foods directly into fat via sustained increases in insulin response after meals [14][13]. In this model, processed carbohydrates (especially fructose and other insulinogenic starches) and excess protein spike insulin as a defensive response against uncontrolled blood sugar, driving the liver to convert sugars directly into heart- and artery-damaging lipids (blood triglycerides, cholesterol, and visceral fat) [14][13]. This effect is mirrored in type 2 diabetics, who receive insulin shots and experience a concomitant and tightly associated weight gain [15][14]. This theory is also bolstered by studies in non-diabetic obese patients who take metformin (gluconeogenesis inhibitor) or rosiglitazone (insulin response enhancer) and lose significantly more body weight than controls [16,17,18][15][16][17]. This model, however, does not take into account non-carbohydrate obesogenesis and insulin control, which could be substantial in individuals who consume excessive fat on low-carbohydrate diets and continue to gain non-muscle body weight [13][12]. In East Asia: The metabolic model may be relevant to prevent the sequelae of MUHO in East Asia as a health insurance study in Japan that summarevieweized 2548 total patients of normal or obese statuses taking either metformin or dipeptidyl peptidase-4 (glucose metabolism) inhibitors found that metformin resulted in a significant HbA1c reduction, increasing insulin sensitivity independent of BMI [19][18]. However, a study of 53,469 Singaporean adults of Han Chinese heritage found that, while the carbohydrate load of the typical Asian diet was not related to heart disease pathogenesis, transitioning from simple carbohydrates to produce and slow-digesting whole grains did reduce risk significantly [20][19]. Taken together, study results indicate that the CICO model could be outdated and MUHO is most likely driven, at least in part, by insulin resistance. This could explain why a majority of MUHO cases proceed to type 2 diabetes while pathogenesis in MHO is delayed significantly because these individuals still retain a normal glucose response and insulin sensitivity. Thus, with regard to MHO, shifting Asians from white rice to whole grains and produce could be protective against MHO to MUHO progression.2.3. Damage from Reactive Oxygen Species
Metabolic damage results from excessive reactive oxygen species (ROS) generated by chronic inflammation, NADPH oxidases during hyperglycemia, polyol and hexosamine sugar shunting, excess blood lipids that generate superoxide anion radicals, and increased hydroxyl radicals generated by sustained, high leptin levels that also generate nitrogen radicals [21,22][20][21]. Over time, this cumulative damage could induce a shift from MHO to MUHO due to excess NADH that imbalances the membrane proton gradient and reduces insulin sensitivity through a vicious cycle mediated by JNK, p38MAPK, and NF-κB that phosphorylate IRS-1 and IRS-2 insulin response proteins [21,23,24,25][20][22][23][24]. While Nrf2, a master antioxidant transcription factor, would normally be activated by enhanced ROS production, excess activation of NF-κB may downregulate or blunt the endogenous Nrf2 response by upregulating Nrf2 constitutive repressor Keap1 via p65 under non-autophagic conditions (excessive glucose) [24][23]. Excessive processed carbohydrate consumption in the East, typically from white rice and white flour or instant noodles, may thus be causative in driving the pathogenesis of ROS-induced damage through chronic upregulation of Nrf2-repressing factors under hyperglycemic conditions. In East Asia: While browning of WAT into a calorie-metabolizing, “beige,” BAT-like phenotype can occur through the mineralocorticoid receptor, ERK1/2, MAPK, and AKT, the long-term implications of this shift are not clear and the effect of beige fat in East Asian phenotypes with regard to MHO has not been detailed in sufficient studies [22][21]. However, studies in China, Japan, and South Korea have found solid links between ROS and obesogenesis, even in children, that may result in eventual MUHO pathogenesis [39,40,41][25][26][27].2.4. Environmental Pollution
Associations of air and water pollution with obesity may stem from immune-mediated reactions to particles in the air (asthma, COPD) or interactions between chemicals and gut flora as has been observed in animal studies [42,43][28][29]. A 2016 systematic review of 35 human cohort studies found that 46% reported positive links between environmental pollution and obesity, similar to a study in 98 people with obesity and 47 normal weight volunteers which found at least some associations between liquid organic pollutants (i.e., pesticides/herbicides) and obesity-relevant biomarkers, such as insulin resistance [44,45][30][31]. In East Asia: The BPA-obesity link was mirrored in a Korean study of 10,021 volunteers in which obese adults had significantly higher BPA levels in their urine and another Korean study of 3782 adults that found additional correlations between paraben levels and type 2 diabetes/obesity risks [48,49][32][33]. Clearly, ground/water-borne pollutants, preservatives, and plasticizers may bioaccumulate in lipid tissue, but parabens have been found to concentrate in fingernails, especially those of women, and the sequestration of such products may have an endocrine or immune-disrupting effect that has yet to be fully determined in Asians, especially with regard to the MHO to MUHO shift [50][34]. Air particulate matter (pm), as defined by size in microns (e.g., pm2.5, pm10), may have a more solid connection to obesity through multiple factors. First, air pollution prevents outdoor exercise, especially in asthmatics or those with allergies (who are already at increased risk of obesity). Second, ROS generated by infiltration of fine particulate matter into the body could promote cumulative damage to the mitochondria and, by extension, the overall energy metabolism [51][35]. Finally, damage to the cardiopulmonary system by pm2.5 could reduce exercise capacity crucial in maintaining MHO status by increased fibrotic damage [52][36]. In East Asia: A study using Chinese data from 13,741 adults over a 26-year-period determined that every microgram of pm2.5 increase resulted in a 0.27% rise in BMI via a lack of outdoor exercise [53][37]. Another Chinese study of 91,121 adults similarly found that each 10-microgram increment of pm2.5 pollution resulted in an 8% increase to the obesity risk [54][38]. This was mirrored in an Asian study that found, over 9 years, significant associations between airborne sulfur dioxide levels, high blood pressure, and type 2 diabetes [55][39].2.5. Seed Oils and Allergies
Traditional use of unrefined animal fats (e.g., butter or lard) has given way over the past 70 years to the use of highly refined, easily oxidized seed oils (rapeseed, safflower, sunflower, and soybean) chosen for their high smoke point and neutral taste. However, animal studies have reported that linoleic acid in refined soy oil causes both fatty liver and obesity while black seed oil (cold-pressed and unrefined) has been shown to reduce this effect via upregulation of antioxidant master transcription factor Nrf2 [57,58,59][40][41][42]. Unrefined oils (especially fish oil) high in polyunsaturated omega 3 long-chain fatty acids (e.g., EPA/DHA), inversely to seed-based cooking oils, have been repeatedly shown to reduce cardiovascular disease (CVD) risk, chronic inflammation, and obesity risk in animals and humans alike [60][43]. Cumulative intake of oxidized oils may thus increase metabolic damage and spur progression of MHO to MUHO in populations that rely on such high-heat methods of cooking. In East Asia: High-heat seed oils may not be causative for all Asian obesity trends since only the Chinese cooking styles features foods fried in excessive oil at high temperatures, which could introduce ROS or other oxidized oil byproducts into the body [61][44]. A study of 15,022 Chinese adults over 14 years found positive associations between refined lard, peanut oil, canola oil, and sesame oils and type 2 diabetes, indicative of metabolic damage while, conversely, Korean traditional cooking features addition of oil after preparation and would not be a source of ROS from oxidized oils [62,63][45][46]. Japanese traditional cooking similarly relies on low oil use but recent increases in animal product consumption and the popularity of Western/Chinese cuisine featuring heavy seed oil use in both Japan and South Korea may be of concern in preventing MUHO pathogenesis. Obesity, often associated with allergies (even in children), mediates allergic pathogenesis due to activation of chronic inflammatory mechanisms and generation of ROS via sustained hyperglycemia. Another putative mechanism elucidated in animal studies is the breakdown in the intestinal barrier system via the PPARγ/NF-κB pathway and also a higher circulating IgE level [64,65][47][48]. The sustained inflammation caused by repeated allergen challenge and immune dysregulation may, thus, synergistically increase the risks associated with obesity under conditions of hyperglycemia and subsequent ROS generation. In East Asia: A study of 1772 Japanese children found that girls with overweight status were more likely to self-report food allergies and a Chinese study of 3327 children in Wuhan, China found a link between obesity and allergic rhinitis [66,67][49][50]. A Korean mental health study of 703,869 children found that, of 440,411 enrolled participants with some form of allergic disease (atopic dermatitis, rhinitis, or asthma), 21,836 (~5.0%) had comorbid obesity [68][51]. Specific links between MHO, the East Asian phenotype, and allergies are scarce in the literature.2.6. Hormonal Changes, Age, and Menopause
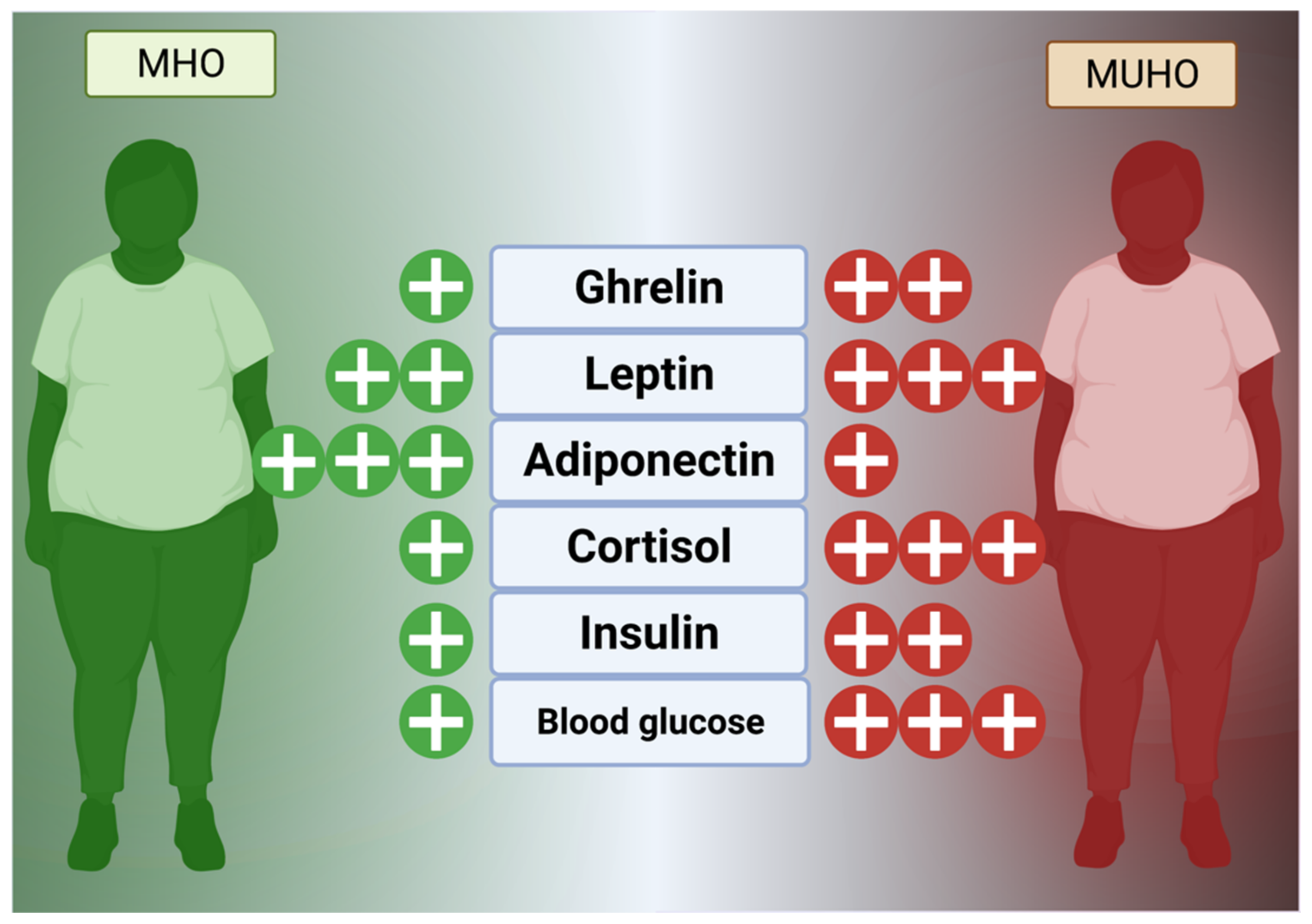
Hormones, as a comparatively slower but wide-reaching chemical messenger system, play a key role in obesogenesis and maintenance of excessive fat stores. In general, the hunger and fat management mechanism is currently known to consist of extensively reviewed hormones, such as glucagon-like peptide 1 (GLP-1), visfatin, ghrelin, cholecystokinin (CCK), leptin, and enterostatin, that reside in the digestive tract and fat to control satiety and peristalsis in concert with the hypothalamus via vagus nerve signaling (Figure 21) [69,70][52][53].
2.7. Immune Factors
2.8. The Microbiome
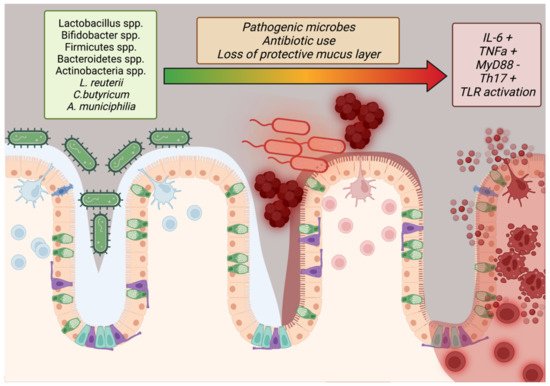
Only within the last 40 years has the importance of the microbiome to human health conditions (such as obesity) been intensely studied. Studies have shown that imbalances in the gut flora, especially with regard to high Firmicutes to Bacteroidetes ratios, are indicative of obesity but the complete effect of stable microbes in the human digestive system have not been fully elucidated [114,115][64][65]. Additional studies have reported that microbial maintenance of the mucous barrier within the intestines, bolstered by mucinogens such as Akkermansia muciniphilia, reduces systemic inflammation by reducing allergen ingress from digesting food while Lactobacillus reuterii, Clostridium butyricum, and other potentially transient species may contribute to barrier function and lipid/glucose homeostasis by regulation of IL-10, GLP1, GLUT2, and TLR2/AMPK pathways (Figure 32) [115,116][65][66]. In addition, the epithelium of the small intestine has tight junctions to prevent ingress of particles into the bloodstream, a function mediated by MyD88 secreted by Paneth cells (Figure 32) [117][67].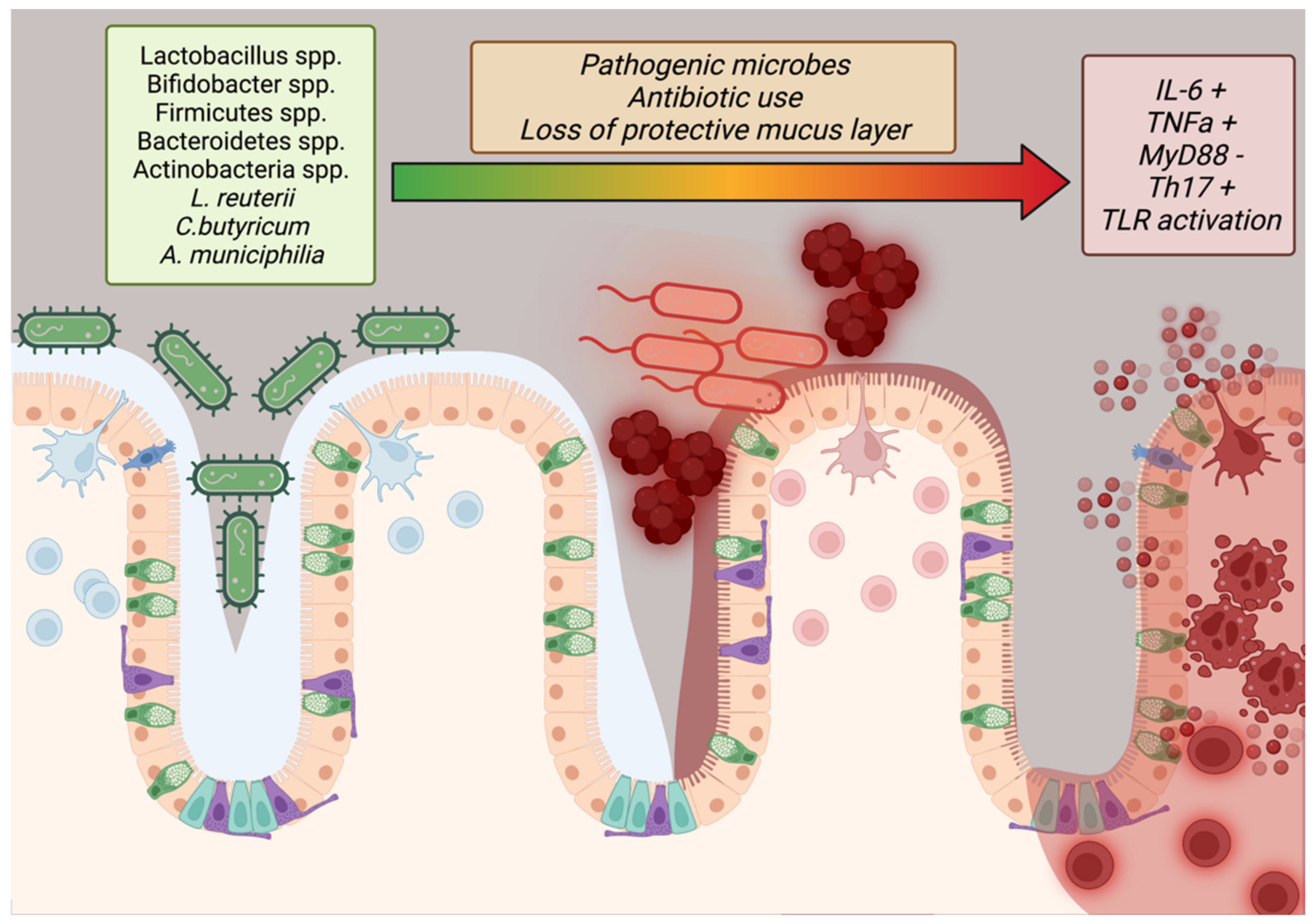
2.9. Social Aspect
2.10. Vitamin and Micronutrient Deficiencies
A lack of vitamins or other micronutrients has been implicated in obesogenesis and animal studies have shown that obesity features a lack of Vitamins A and D, with Vitamin A regulating metabolism through RBP3 and ALDH1A1 and Vitamin D playing key roles in adipokine synthesis, calcium balance, and glucose metabolism [129,130,131][76][77][78]. Vitamin D, shown to be sequestered in fat tissue and, therefore, of low bioavailability in the obese, additionally regulates the immune system through the Vitamin D receptor on T cells, reducing the effect of chronic inflammation on obesogenesis [131,132][78][79].
2.11. Weight Fluctuation and Rebound Effect
In East Asia: Although studies on rebound are scarce, several small studies in Japan and China implicated a return to previous overfeeding patterns due to social contact or post-surgical pain that limited activity [141,142][81][82]. A large Korean study of 3678 adults, among whom half experienced high weight variability, found increased risk of death and rebound was attributed to homeostatic feedback after weight loss that creates a biochemical milieu favorable to weight gain [140][83].
2.12. Genetic Factors
In East Asia: A study of 1213 Chinese children found that KCNQ1-rs2237897 is associated with cardiovascular and KCNQ1-rs2237892 is associated with insulin resistance risk in MHO while another Chinese study of 1790 MHO children found FTO-rs9939609 or CYP17A1-rs11191548 predictive of cardiovascular risk in addition to GNPDA2-rs10938397 or KCTD15-rs29941 being predictive for insulin resistance [145,146][84][85]. A separate Chinese study did find that adiponectin-related gene polymorphisms, especially rs6773957, were related to diet and MUHO, in line with a similar Russian study of 503 obese patients that found similar connections to polymorphisms such as G45G [147,148][86][87]. A genome-wide association study of 49,915 Koreans found that LPL, APOA5, CETP, GCKR, CDKAL1, and CDKN2B (all lipid metabolism genes) were associated with MHO, thus lending weight to the concept that the MHO condition is a discrete genetic phenotype and that shifts to MUHO may involve epigenetic regulation from synergistic internal (reactive oxygen species, stress, hormones, etc.) and/or external (pollution, diet, etc.) sources [149][88].
References
- Helble, M.; Francisco, K. The Upcoming Obesity Crisis in Asia and the Pacific: First Cost Estimates; ADBI Working Paper 743; ADB Institute: Tokyo, Japan, 2017.
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542.
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribol. 2022, 33, 31–38.
- Teasdale, N.; Simoneau, M.; Corbeil, P.; Handrigan, G.; Tremblay, A.; Hue, O. Obesity Alters Balance and Movement Control. Curr. Obes. Rep. 2013, 2, 235–240.
- Hildrum, B.; Mykletun, A.; Hole, T.; Midthjell, K.; Dahl, A.A. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: The Norwegian HUNT 2 study. BMC Public Health 2007, 7, 220.
- Lührmann, P.M.; Edelmann-Schafer, B.; Neuhäuser-Berthold, M. Changes in resting metabolic rate in an elderly German population: Cross-sectional and longitudinal data. J. Nutr. Health Aging 2010, 14, 232–236.
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904.
- Lu, J.; Liu, Q. Four decades of studies on population aging in China. China Popul. Dev. Stud. 2019, 3, 24–36.
- Tahara, Y. Cardiopulmonary Resuscitation in a Super-Aging Society—Is There an Age Limit for Cardiopulmonary Resuscitation? Circ. J. 2016, 80, 1102–1103.
- Jang, I.-Y.; Lee, H.Y.; Lee, E. The 50th Anniversary Committee of Korean Geriatrics Society Geriatrics Fact Sheet in Korea 2018 From National Statistics. Ann. Geriatr. Med. Res. 2019, 23, 50–53.
- Howell, S.; Kones, R. “Calories in, calories out” and macronutrient intake: The hope, hype, and science of calories. Am. J. Physiol. Metab. 2017, 313, E608–E612.
- Hall, K.D.; Farooqi, I.S.; Friedman, J.M.; Klein, S.; Loos, R.J.F.; Mangelsdorf, D.J.; O’Rahilly, S.; Ravussin, E.; Redman, L.M.; Ryan, D.H.; et al. The energy balance model of obesity: Beyond calories in, calories out. Am. J. Clin. Nutr. 2022, 115, 1243–1254.
- Ludwig, D.S.; Ebbeling, C.B. The Carbohydrate-Insulin Model of Obesity: Beyond “Calories In, Calories Out”. JAMA Intern. Med. 2018, 178, 1098–1103.
- Russell-Jones, D.; Khan, R. Insulin-associated weight gain in diabetes—Causes, effects and coping strategies. Diabetes Obes. Metab. 2006, 9, 799–812.
- Seifarth, C.; Schehler, B.; Schneider, H.J. Effectiveness of Metformin on Weight Loss in Non-Diabetic Individuals with Obesity. Exp. Clin. Endocrinol. Diabetes 2012, 121, 27–31.
- Brunani, A.; Caumo, A.; Graci, S.; Castagna, G.; Viberti, G.; Liuzzi, A. Rosiglitazone is more effective than metformin in improving fasting indexes of glucose metabolism in severely obese, non-diabetic patients. Diabetes Obes. Metab. 2008, 10, 460–467.
- Berne, C.; The Orlistat Swedish Type 2 Diabetes Study Group. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabet. Med. 2005, 22, 612–618.
- Odawara, M.; Aoi, S.; Takeshima, T.; Iwasaki, K. Comparative Effects of Metformin and Dipeptidyl Peptidase-4 Inhibitors in Japanese Obese Patients with Type 2 Diabetes: A Claims Database Study. Diabetes Ther. 2021, 12, 2165–2177.
- Rebello, A.S.; Koh, H.; Chen, C.; Naidoo, N.; Odegaard, O.A.; Koh, W.-P.; Butler, L.M.; Yuan, J.-M.; van Dam, R. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: A prospective cohort study. Am. J. Clin. Nutr. 2014, 100, 53–64.
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444.
- Lefranc, C.; Friederich-Persson, M.; Palacios, R.; Cat, A.N.D. Mitochondrial oxidative stress in obesity: Role of the mineralocorticoid receptor. J. Endocrinol. 2018, 238, R143–R159.
- McMurray, F.; Patten, D.A.; Harper, M.-E. Reactive Oxygen Species and Oxidative Stress in Obesity-Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310.
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973.
- Al-Lahham, R.; Deford, J.H.; Papaconstantinou, J. Mitochondrial-generated ROS down regulates insulin signaling via activation of the p38MAPK stress response pathway. Mol. Cell. Endocrinol. 2016, 419, 1–11.
- Kogawa, T.; Kashiwakura, I. Relationship between obesity and serum reactive oxygen metabolites in adolescents. Environ. Health Prev. Med. 2013, 18, 451–457.
- Roh, H.-T.; Cho, S.-Y.; So, W.-Y. Obesity promotes oxidative stress and exacerbates blood-brain barrier disruption after high-intensity exercise. J. Sport Health Sci. 2016, 6, 225–230.
- Na, I.J.; Park, J.S.; Park, S.B. Association between Abdominal Obesity and Oxidative Stress in Korean Adults. Korean J. Fam. Med. 2019, 40, 395–398.
- Wei, C.; Feng, R.; Hou, X.; Peng, T.; Shi, T.; Hu, X. Nanocolloids in drinking water increase the risk of obesity in mice by modulating gut microbes. Environ. Int. 2020, 146, 106302.
- Kusumoto, M.; Mathis, B. Biologic Treatments for Asthma and Chronic Obstructive Pulmonary Disorder. Allergies 2021, 10, 7.
- Wang, Y.; Hollis-Hansen, K.; Ren, X.; Qiu, Y.; Qu, W. Do environmental pollutants increase obesity risk in humans? Obes. Rev. 2016, 17, 1179–1197.
- Dirinck, E.; Jorens, P.G.; Covaci, A.; Geens, T.; Roosens, L.; Neels, H.; Mertens, I.; Van Gaal, L. Obesity and Persistent Organic Pollutants: Possible Obesogenic Effect of Organochlorine Pesticides and Polychlorinated Biphenyls. Obesity 2011, 19, 709–714.
- Moon, S.; Seo, M.Y.; Choi, K.; Chang, Y.-S.; Kim, S.-H.; Park, M.J. Urinary bisphenol A concentrations and the risk of obesity in Korean adults. Sci. Rep. 2021, 11, 1603.
- Lee, I.; Park, Y.J.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Park, H.; et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2020, 146, 106227.
- McLachlan, M.S.; Czub, G.; MacLeod, M.; Arnot, J.A. Bioaccumulation of Organic Contaminants in Humans: A Multimedia Perspective and the Importance of Biotransformation. Environ. Sci. Technol. 2010, 45, 197–202.
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM2.5-Induced Oxidative Stress and Mitochondrial Damage in the Nasal Mucosa of Rats. Int. J. Environ. Res. Public Health 2017, 14, 134.
- Jiang, J.; Li, Y.; Liang, S.; Sun, B.; Shi, Y.; Xu, Q.; Zhang, J.; Shen, H.; Duan, J.; Sun, Z. Combined exposure of fine particulate matter and high-fat diet aggravate the cardiac fibrosis in C57BL/6J mice. J. Hazard. Mater. 2020, 391, 122203.
- Deschenes, O.; Wang, H.; Wang, S.; Zhang, P. The effect of air pollution on body weight and obesity: Evidence from China. J. Dev. Econ. 2020, 145, 102461.
- Cao, S.; Guo, Q.; Xue, T.; Wang, B.; Wang, L.; Duan, X.; Zhang, J. Long-term exposure to ambient PM2.5 increase obesity risk in Chinese adults: A cross-sectional study based on a nationwide survey in China. Sci. Total Environ. 2021, 778, 145812.
- Paoin, K.; Ueda, K.; Ingviya, T.; Buya, S.; Phosri, A.; Seposo, X.T.; Seubsman, S.-A.; Kelly, M.; Sleigh, A.; Honda, A.; et al. Long-term air pollution exposure and self-reported morbidity: A longitudinal analysis from the Thai cohort study (TCS). Environ. Res. 2020, 192, 110330.
- Deol, S.; Fahrmann, J.; Yang, J.; Evans, J.R.; Rizo, A.; Grapov, D.; Salemi, M.; Wanichthanarak, K.; Fiehn, O.; Phinney, B.; et al. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci. Rep. 2017, 7, 12488.
- Deol, P.; Evans, J.R.; Dhahbi, J.; Chellappa, K.; Han, D.S.; Spindler, S.; Sladek, F.M. Soybean Oil Is More Obesogenic and Diabetogenic than Coconut Oil and Fructose in Mouse: Potential Role for the Liver. PLoS ONE 2015, 10, e0132672.
- El-Magd, N.F.A.; El-Mesery, M.; El-Karef, A.; El-Shishtawy, M.M. Amelioration effect of black seed oil against high-fat diet-induced obesity in rats through Nrf2/HO-1 pathway. J. Food Biochem. 2021, 45, e13693.
- Lorente-Cebrián, S.; Costa, A.G.V.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651.
- Wang, L.; Zhang, L.; Ristovski, Z.D.; Zheng, X.; Wang, H.-L.; Li, L.; Gao, J.; Salimi, F.; Gao, Y.; Jing, S.; et al. Assessing the Effect of Reactive Oxygen Species and Volatile Organic Compound Profiles Coming from Certain Types of Chinese Cooking on the Toxicity of Human Bronchial Epithelial Cells. Environ. Sci. Technol. 2020, 54, 8868–8877.
- Zhuang, P.; Mao, L.; Wu, F.; Wang, J.; Jiao, J.; Zhang, Y. Cooking Oil Consumption Is Positively Associated with Risk of Type 2 Diabetes in a Chinese Nationwide Cohort Study. J. Nutr. 2020, 150, 1799–1807.
- Kim, S.; Moon, S.; Popkin, B.M. The nutrition transition in South Korea. Am. J. Clin. Nutr. 2000, 71, 44–53.
- Gu, Y.; Guo, X.; Sun, S.; Che, H. High-Fat Diet-Induced Obesity Aggravates Food Allergy by Intestinal Barrier Destruction and Inflammation. Int. Arch. Allergy Immunol. 2021, 183, 80–92.
- Visness, C.M.; London, S.J.; Daniels, J.L.; Kaufman, J.S.; Yeatts, K.B.; Siega-Riz, A.-M.; Liu, A.H.; Calatroni, A.; Zeldin, D.C. Association of obesity with IgE levels and allergy symptoms in children and adolescents: Results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2009, 123, 1163–1169.e4.
- Hayashi, K.; Tsujiguchi, H.; Hori, D.; Yamada, Y.; Shimizu, Y.; Nguyen, T.T.T.; Hibino, Y.; Kambayashi, Y.; Hara, A.; Nakamura, H. The association between overweight and prevalence of food allergy in Japanese children: A cross-sectional study. Environ. Health Prev. Med. 2021, 26, 44.
- Lei, Y.; Yang, H.; Zhen, L. Obesity is a risk factor for allergic rhinitis in children of Wuhan (China). Asia Pac. Allergy 2016, 6, 101–104.
- Kim, S.Y.; Choi, S.H.; Kim, J.D.; Sol, I.S.; Kim, M.J.; Kim, Y.H.; Jung, Y.-C.; Sohn, M.H.; Kim, K.W. Korean Youth with Comorbid Allergic Disease and Obesity Show Heightened Psychological Distress. J. Pediatr. 2019, 206, 99–104.e4.
- Lean, M.E.J.; Malkova, D. Altered gut and adipose tissue hormones in overweight and obese individuals: Cause or consequence? Int. J. Obes. 2015, 40, 622–632.
- Duque, A.P.; Junior, L.F.R.; Mediano, M.F.F.; Tibiriça, E.; De Lorenzo, A. Emerging concepts in metabolically healthy obesity. Am. J. Cardiovasc. Dis. 2020, 10, 48–61.
- Landecho, M.F.; Tuero, C.; Valentí, V.; Bilbao, I.; De La Higuera, M.; Frühbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664.
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971.
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48.
- Ruggiero, A.D.; Key, C.-C.C.; Kavanagh, K. Adipose Tissue Macrophage Polarization in Healthy and Unhealthy Obesity. Front. Nutr. 2021, 8, 625331.
- Maurya, R.; Sebastian, P.; Namdeo, M.; Devender, M.; Gertler, A. COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality. Front. Immunol. 2021, 12, 649359.
- Kern, L.; Mittenbühler, M.; Vesting, A.; Ostermann, A.; Wunderlich, C.; Wunderlich, F. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation—Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24.
- Moschen, A.; Molnar, C.; Geiger, S.; Graziadei, I.; Ebenbichler, C.; Weiss, H.; Kaser, S.; Kaser, A.; Tilg, H. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor expression. Gut 2010, 59, 1259–1264.
- Tang, H.; Liu, N.; Feng, X.; Yang, Y.; Fang, Y.; Zhuang, S.; Dai, Y.; Liu, M.; Tang, L. Circulating levels of IL-33 are elevated by obesity and positively correlated with metabolic disorders in Chinese adults. J. Transl. Med. 2021, 19, 52.
- Ji, Y.-F.; Jiang, X.; Li, W.; Ge, X. Impact of interleukin-6 gene polymorphisms and its interaction with obesity on osteoporosis risk in Chinese postmenopausal women. Environ. Health Prev. Med. 2019, 24, 48.
- Dai, W.; Liu, X.; Su, H.; Li, X.; Xu, Y.; Yu, Y. Influence of adipose tissue immune dysfunction on childhood obesity. Cytokine Growth Factor Rev. 2022, 65, 27–38.
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123.
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219.
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211.
- Iacob, S.; Iacob, D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front. Microbiol. 2019, 10, 1676.
- Naito, E.; Yoshida, Y.; Kunihiro, S.; Makino, K.; Kasahara, K.; Kounoshi, Y.; Aida, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: A randomised, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 9–18.
- Rosenbaum, D.L.; White, K.S. The role of anxiety in binge eating behavior: A critical examination of theory and empirical literature. Health Psychol. Res. 2013, 1, e19.
- Giletta, M.; Slavich, G.M.; Rudolph, K.D.; Hastings, P.D.; Nock, M.K.; Prinstein, M. Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J. Child Psychol. Psychiatry 2017, 59, 129–139.
- Brixval, C.S.; Rayce, S.L.B.; Rasmussen, M.; Holstein, B.; Due, P. Overweight, body image and bullying--an epidemiological study of 11- to 15-years olds. Eur. J. Public Health 2011, 22, 126–130.
- Kubo, T. Common approach to childhood obesity in Japan. J. Pediatr. Endocrinol. Metab. 2014, 27, 581–592.
- Cai, Y.; Zhu, X.; Wu, X. Overweight, obesity, and screen-time viewing among Chinese school-aged children: National prevalence estimates from the 2016 Physical Activity and Fitness in China—The Youth Study. J. Sport Health Sci. 2017, 6, 404–409.
- Koyanagi, A.; Veronese, N.; Vancampfort, D.; Stickley, A.; Jackson, S.E.; Oh, H.; Shin, J.I.; Haro, J.M.; Stubbs, B.; Smith, L. Association of bullying victimization with overweight and obesity among adolescents from 41 low- and middle-income countries. Pediatr. Obes. 2019, 15, e12571.
- Terunuma, Y.; Mathis, B.J. Cultural sensitivity in brain death determination: A necessity in end-of-life decisions in Japan. BMC Med. Ethic. 2021, 22, 58.
- Trasino, S.E.; Tang, X.-H.; Jessurun, J.; Gudas, L.J. Obesity Leads to Tissue, but not Serum Vitamin A Deficiency. Sci. Rep. 2015, 5, 15893.
- Blaner, W.S. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol. Ther. 2019, 197, 153–178.
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103.
- McKay, J.; Ho, S.; Jane, M.; Pal, S. Overweight & obese Australian adults and micronutrient deficiency. BMC Nutr. 2020, 6, 12.
- Hong, H.C.; Lee, J.-S.; Choi, H.Y.; Yang, S.J.; Yoo, H.J.; Seo, A.J.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; et al. Liver enzymes and vitamin D levels in metabolically healthy but obese individuals: Korean National Health and Nutrition Examination Survey. Metabolism 2013, 62, 1305–1312.
- Yamaguchi, S. Cause of Weight Rebound with Obese Women Who Had Lost Weight and Effect of Intervention by Telephone and Newsletters. Jpn. J. Nutr. Diet. 2007, 65, 21–28.
- Zuo, D.; Xiao, X.; Yang, S.; Gao, Y.; Wang, G.; Ning, G. Effects of bariatric surgery in Chinese with obesity and type 2 diabetes mellitus. Medicine 2020, 99, e21673.
- Oh, T.J.; Moon, J.H.; Choi, S.H.; Lim, S.; Park, K.S.; Cho, N.H.; Jang, H.C. Body-Weight Fluctuation and Incident Diabetes Mellitus, Cardiovascular Disease, and Mortality: A 16-Year Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2018, 104, 639–646.
- Li, L.; Yin, J.; Cheng, H.; Wang, Y.; Gao, S.; Li, M.; Grant, S.F.; Li, C.; Mi, J.; Li, M. Identification of genetic and environmental factors predicting metabolically healthy obesity in children: Data from the BCAMS study. J. Clin. Endocrinol. Metab. 2016, 101, 1816–1825.
- Gao, L.; Zhang, M.; Mi, J. Lbps 01-06 genetic and environmental factors of metabolically healthy obesity in Chinese children. J. Hypertens. 2016, 34, e175.
- Li, G.; Zhong, L.; Han, L.; Wang, Y.; Li, B.; Wang, D.; Zhao, Y.; Li, Y.; Zhang, Q.; Qi, L.; et al. Genetic variations in adiponectin levels and dietary patterns on metabolic health among children with normal weight versus obesity: The BCAMS study. Int. J. Obes. 2021, 46, 325–332.
- Berezina, A.; Belyaeva, O.; Berkovich, O.; Baranova, E.; Karonova, T.; Bazhenova, E.; Brovin, D.; Grineva, E.; Shlyakhto, E. Prevalence, Risk Factors, and Genetic Traits in Metabolically Healthy and Unhealthy Obese Individuals. BioMed Res. Int. 2015, 2015, 548734.
- Park, J.-M.; Park, D.-H.; Song, Y.; Kim, J.O.; Choi, J.-E.; Kwon, Y.-J.; Kim, S.-J.; Lee, J.-W.; Hong, K.-W. Understanding the genetic architecture of the metabolically unhealthy normal weight and metabolically healthy obese phenotypes in a Korean population. Sci. Rep. 2021, 11, 2279.
