Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lavinia Cosmina Ardelean | -- | 1526 | 2022-09-21 11:36:00 | | | |
| 2 | Camila Xu | Meta information modification | 1526 | 2022-09-22 02:21:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Beschiu, L.M.; Ardelean, L.C.; Tigmeanu, C.V.; Rusu, L. Cranial and Odontological Methods for Sex Estimation. Encyclopedia. Available online: https://encyclopedia.pub/entry/27435 (accessed on 11 January 2026).
Beschiu LM, Ardelean LC, Tigmeanu CV, Rusu L. Cranial and Odontological Methods for Sex Estimation. Encyclopedia. Available at: https://encyclopedia.pub/entry/27435. Accessed January 11, 2026.
Beschiu, Laura Maria, Lavinia Cosmina Ardelean, Codruta Victoria Tigmeanu, Laura-Cristina Rusu. "Cranial and Odontological Methods for Sex Estimation" Encyclopedia, https://encyclopedia.pub/entry/27435 (accessed January 11, 2026).
Beschiu, L.M., Ardelean, L.C., Tigmeanu, C.V., & Rusu, L. (2022, September 21). Cranial and Odontological Methods for Sex Estimation. In Encyclopedia. https://encyclopedia.pub/entry/27435
Beschiu, Laura Maria, et al. "Cranial and Odontological Methods for Sex Estimation." Encyclopedia. Web. 21 September, 2022.
Copy Citation
The estimation of sex from osteological remains can be achieved using three major types of methods: morphological assessment (non-metric) of teeth and bone traits that exhibit dimorphic features, morphometric assessment (by measuring specific quantifiable features of bones and teeth) and biochemical analysis.
sex estimation
cranial methods
odontological methods
1. Introduction
The estimation of sex from osteological and dental records has long been an interdisciplinary field of dentistry, forensic medicine and anthropology alike, as it concerns all the above mentioned specialties.
In both forensic and archaeological cases, a reliable method to establish the sex of the deceased is paramount, as it is the first step towards a more detailed analysis of the human remains and helps in narrowing down the list of individuals and putting together a demographic pattern.
The estimation of sex from osteological remains can be achieved using three major types of methods: morphological assessment (non-metric) of teeth and bone traits that exhibit dimorphic features, morphometric assessment (by measuring specific quantifiable features of bones and teeth) and biochemical analysis, such as DNA analysis [1][2][3][4] or Barr bodies analysis [5] (Figure 1). DNA analysis is by far the most accurate method, but it is also the most expensive and may not be suited for large numbers of specimens [6][7].
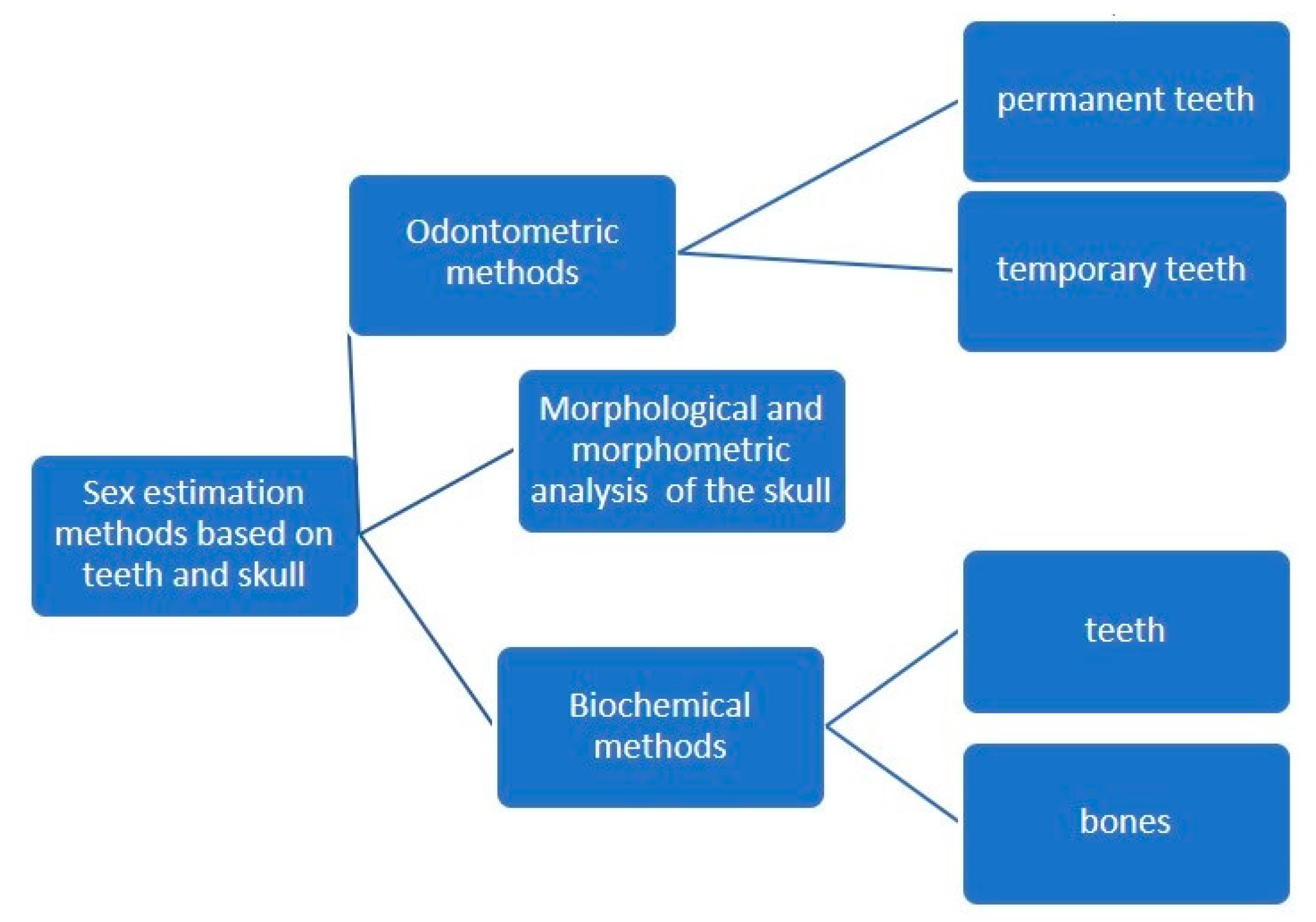
Figure 1. Odontological and cranial sex estimation methods (overview).
2. Morphological and Morphometric Methods
The morphological and morphometric assessment methods are both generally accepted techniques based on scientifically proven grounds, but they have limitations. For instance, morphological assessment (non-metric) is based on a certain subjective evaluation of the observer and also requires experience. Morphometric assessment (metric), on the other hand, is a laborious technique and depends on the exact determination of anatomical landmarks. Moreover, the population-specific variations in the skull make these methods almost impossible to generalize [8].
More recently, computer-aided techniques have facilitated the use of morphometric assessments, making them less subjective and time-consuming. Advances in three-dimensional image analysis have achieved rapid, automatic measurement of the entire outer surface of the craniofacial hard and soft tissue, as opposed to measurements of only limited distances and angles of the cranium. The digital analysis of the cranium and digital data storage have had a huge impact on sex estimation methods. The stored images, whether digital impressions or radiographic images, can be used time and time again for multiple analyses [9][10][11].
Almost all bones exhibit dimorphic features. Sex discrimination methods have proven successful in many bones, including the hyoid, ulna, sternal end of the rib, metacarpals and even metatarsals [12]. However, the pelvis shows the highest degree of dimorphism, followed by the skull [13], which has an accuracy for gender determination of up to 94% [14].
The anatomical structures of the skull used for the purpose of sex estimation are numerous: the frontal bone (position of squamous part, the appearance of the supraciliary arch, the sharpness and shape of the orbit, the frontal sinus—which remains stable and unchanged until old age and is, according to some studies, a unique structure, comparable to fingerprints) [15], the zygomatic bone (presence of marginal tubercle on the frontal process), the temporal bone (size and shape of the mastoid process, width of the zygomatic processes), the occipital bone (the nucal crest, the clivus), the mandible (angle between body and mandible ramus—angle of mandible, ramus height, base height), the shape of the nasal root, muscular insertions on bones, tooth size, face shape etc. [16] (Figure 2).
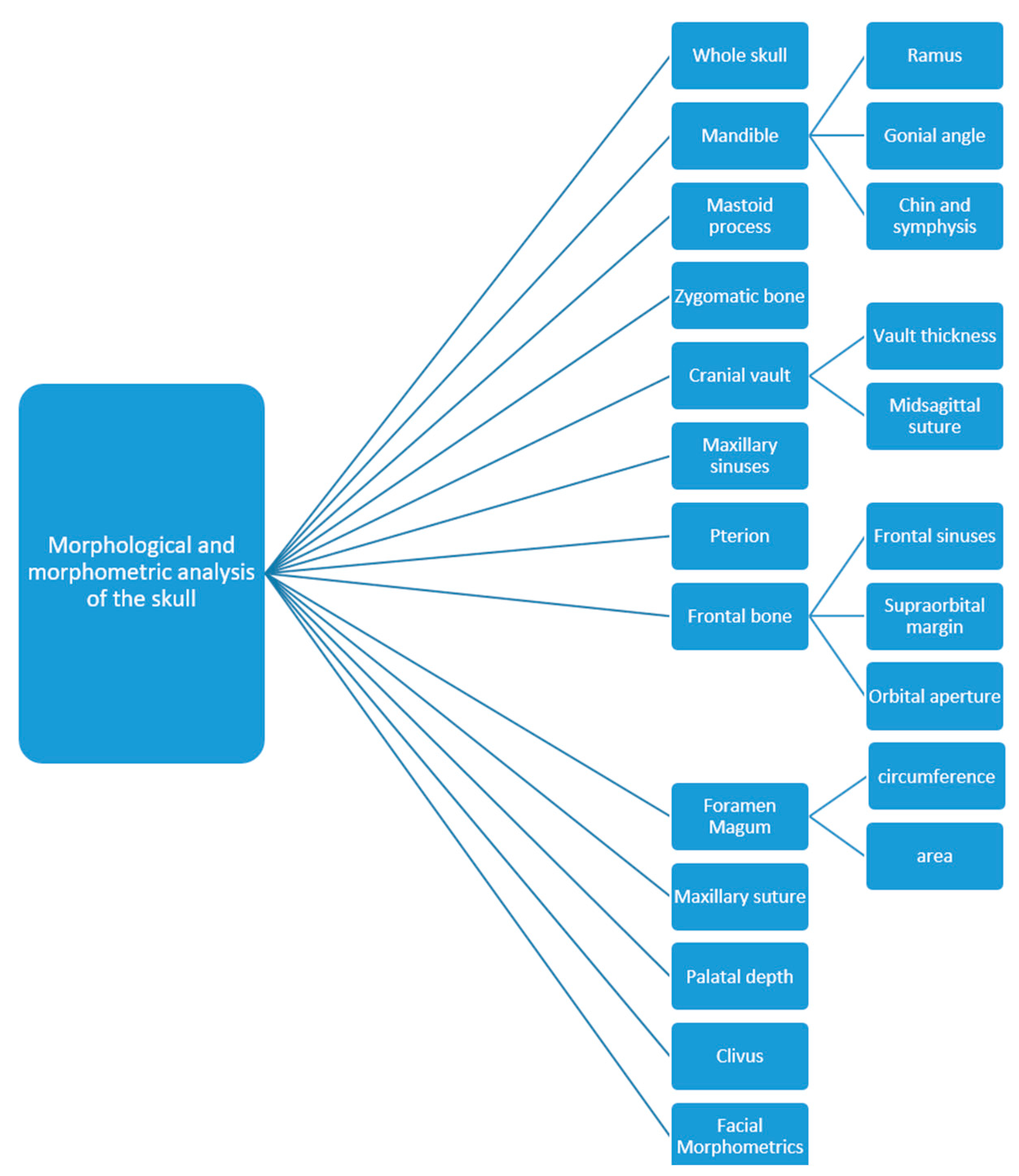
Figure 2. Parts of the skull used for sex estimation.
In many circumstances, whether in mass fatalities, explosions, mutilated bodies or poorly preserved archaeological records, the entire pelvis or skull cannot be retrieved and only fragmented parts of these bones are available for study. In these cases, the mandible plays a decisive role in sex estimation because it is the largest, strongest and one of the most dimorphic parts of the skull [17][18][19]. Dimorphism in the mandible is reflected in its shape and size; male bones are generally bigger and more robust than female bones. If only the mandible is available for assessment, gender determination has an accuracy of around 90% [16].
The mandible is usually one of the best preserved bones, along with the teeth, which are highly resistant to bacterial degradation, extreme heat and other types of aggressions and are therefore most likely to be preserved in fossil and archaeological records. Teeth can be heated to temperatures of 1600 °C without appreciable loss of microstructure [20] and, unlike skeletal bones, the human origin of teeth is rarely in doubt [21]. That is why the teeth form a highly valuable asset in estimating the sex of deceased individuals and are especially important in assessing children, where dimorphic aspects of the pelvis and other bones are not yet recognizable. In cases of fire or explosion, the thermal trauma causes major damage to the anatomical structures, leaving the teeth as the only way to establish the sex of the victims.
3. Biochemical Methods
Biochemical analyses for sex estimation purposes re based on DNA and Barr bodies from the dental pulp or from the hard tissue of the teeth. The DNA polymerase chain reaction (PCR) is more expensive and takes longer to obtain results, whereas the Barr bodies analysis is quicker and requires less equipment [5][22].
Due to their great tissue resistance, teeth can be considered as a reliable source of DNA, making them valuable in biochemical analysis methods as well. All structures of the tooth have proven value for extracting DNA material (enamel, cementum, dentine and pulp). The dental pulp contains fibroblasts, odontoblasts, endothelial cells, peripheral nerves, undifferentiated mesenchymal cells and nucleated components of blood, found in the coronal and radicular pulp, which are rich sources of DNA and free from contamination by external factors [23].
Amelogenin (AMEL) is the enamel-specific matrix formed during the first stages of tooth formation. It has been discovered that there are two types of AMEL genes, one found on the X chromosome and the other found on the Y chromosome. Hence, using PCR on the AMEL gene from DNA found in the dental pulp is a useful method to establish the sex of an individual [23]. PCR analyses that target regions of the amelogenin gene have become the method of choice for sex estimation of biological samples [24]. However, discrepancies have been noted with AMEL gene-based sex estimation, mostly due to X and Y deletion in the population and mutations in primer-binding sites. Some populations, such as Indians, appear to be affected by high frequencies of Y deletion. The presence of PCR inhibitors, degradation of the DNA samples and the presence of mixed DNA also contribute to inaccurate results obtained by amelogenin analysis and, therefore, other alternative techniques and markers have been suggested for sex estimation, such as STS, SRY, TSPY, DXYS156, SNPs, DYZ1 and next generation sequencing (NGS) [25].
Among the methods used to extract DNA from the dental pulp, the method using phenol chloroform appears to be quite cost-effective, but it is tedious and requires high precision. Newer extraction methods, such as Chelex 100TM (Medox Biotech, Chennai, India) and QIA cubeTM (Qiagen, Hilden, Germany), could be substituted for the traditional method [23]. Recently, another method, termed the loop-mediated amplification method (LAMP reaction), which can give results within an approximately half an hour time limit, has been recommended as an alternative to conventional PCR techniques. Another advantage of the LAMP method is that it works under isothermal conditions, which stops further denaturation of the DNA [24].
Other biochemical analysis methods include the use of a fluorescent body test. It has been shown that, when chromosomes are stained with quinacrine mustard, they fluoresce differentially along their length when viewed under ultraviolet light, and the human Y chromosome fluoresces more brightly than the other chromosomes [20]. The reason for the bright fluorescence of the Y chromosome is not entirely clear. This technique has been used in forensic science for sex estimation from dried blood stains, saliva and hair since the 1970s [20]. The fluorescent Y body test has shown to be a reliable, simple and cost-effective technique for gender determination in the immediate postmortem period of up to one month after death. Therefore, its limitation is related to the post-mortem interval, making it only relevant for recently deceased individuals and, hence, impossible to use in archaeological findings [20].
The estimation of sex in ancient archaeological remains and fossils is also possible through DNA extraction techniques. The dawn of ancient DNA (aDNA) techniques was in 1983 at Berkeley, California, when Higuchi et al. extracted and sequenced ancient mitochondrial DNA (mtDNA) from a 150-year-old specimen of the quagga, a zebra-like species [26]. Then, in 1985, Svante Pääbo successfully investigated 23 Egyptian mummies for DNA content [27] and, in 1997, aDNA from Neanderthal specimens from the Feldhofer Cave in Germany was also successfully extracted [28].
Even today, the retrieval of mtDNA from ancient human specimens is not always successful owing to DNA deterioration and contamination. Usually, only short DNA fragments can be retrieved from ancient specimens. Degradation and contamination in long-term preserved specimens still make analysis very difficult. This is due to the technical difficulties with extraction, amplification and sequencing of ancient mtDNA. In recent years, NGS has mainly been applied to ancient samples. It seems that this technique is suitable for aDNA research [29]. According to the literature, short tandem repeat (STR) typing could represent a time-saving and cost-effective solution for sex estimation in archaeological sites [30].
References
- Krishan, K.; Chatterjee, P.M.; Kanchan, T.; Kaur, S.; Baryah, N.; Singh, R.K. A review of sex estimation techniques during examination of skeletal remains in forensic anthropology casework. Forensic Sci. Int. 2016, 261, e1–e165.
- Bedalov, A.; Bašić, Ž.; Marelja, I.; Dolić, K.; Bukarica, K.; Missoni, S.; Šlaus, M.; Primorac, D.; Andjelinović, Š.; Kružić, I. Sex estimation of the sternum by automatic image processing of multi-slice computed tomography images in a Croatian population sample: A retrospective study. Croat. Med. J. 2019, 60, 237–245.
- Bubalo, P.; Baković, M.; Tkalčić, M.; Petrovečki, V.; Mayer, D. Acetabular osteometrie standards for sex estimation in contemporary Croatian population. Croat. Med. J. 2019, 60, 221–226.
- Bašić, Ž.; Kružić, I.; Jerković, I.; Andelinovic, D.; Andelinovic, Š. Sex estimation standards for medieval and contemporary Croats. Croat. Med. J. 2017, 58, 222–230.
- Gupta, M.; Mishra, P.; Shrivastava, K.; Singh, N. An Overview of age, sex and race determination from teeth and skull. Adv. Hum. Biol. 2015, 5, 20–31.
- Buonasera, T.; Eerkens, J.; de Flamingh, A.; Engbring, L.; Yip, J.; Li, H.; Haas, R.; DiGiuseppe, D.; Grant, D.; Salemi, M.; et al. A comparison of proteomic, genomic, and osteological methods of archaeological sex estimation. Sci. Rep. 2020, 10, 11897.
- Loreille, O.; Ratnayake, S.; Bazinet, A.L.; Stockwell, T.B.; Sommer, D.D.; Rohland, N.; Mallick, S.; Johnson, P.L.F.; Skoglund, P.; Onorato, A.J.; et al. Biological sexing of a 4000-year-old egyptian mummy head to assess the potential of nuclear DNA recovery from the most damaged and limited forensic specimens. Genes 2018, 9, 135.
- Arigbabu, O.A.; Liao, I.Y.; Abdullah, N.; Mohamad Noor, M.H. Computer vision methods for cranial sex estimation. IPSJ Trans. Comput. Vis. Appl. 2017, 9, 19.
- Gao, H.; Geng, G.; Yang, W. Sex Determination of 3D skull based on a novel unsupervised learning method. Comput. Math. Methods Med. 2018, 2018, 4567267.
- Chovalopoulou, M.E.; Valakos, E.D.; Manolis, S.K. Sex determination by three-dimensional geometric morphometrics of craniofacial form. Anthropol. Anzeiger. 2016, 73, 195–206.
- Agbolade, O.; Nazri, A.; Yaakob, R.; Ghani, A.A.; Cheah, Y.K. Morphometric approach to 3D soft-tissue craniofacial analysis and classification of ethnicity, sex, and age. PLoS ONE 2020, 15, e0228402.
- Cole, C.; Eliopoulos, C.; Zorba, E.; Borrini, M. An anthropometric method for sex determination from the mandible: Test on British medieval skeletal collections. J. Biol. Res. 2017, 90, 30–35.
- Tise, M.L.; Spradley, M.K.; Anderson, B.E. Postcranial sex estimation of individuals considered Hispanic. J. Forensic Sci. 2013, 58, S9–S14.
- Yang, W.; Zhou, M.; Zhang, P.; Geng, G.; Liu, X.; Zhang, H. skull sex estimation based on wavelet transform and Fourier transform. Biomed. Res. Int. 2020, 2020, 8608209.
- Nourbakhsh, R.; Razi, S.; Razi, T. Evaluation of relation of dimensional measurement of different anatomic skull structures to determine sexual dimorphism in cone beam CT images of an Iranian population. J. Res. Med. Dent. Sci. 2018, 6, 33–38.
- Sarač-Hadžihalilović, A.; Hojkurić, E.; Musić, M.; Hasanbegović, I.; Ajanović, Z.; Dervišević, L.; Brkić, S. Model “P” in gender prediction based on the mastoid process. Med. Glas. 2020, 17, 279–6284.
- Behl, A.; Grewal, S.; Bajaj, K.; Baweja, P.; Kaur, G.; Kataria, P. Mandibular ramus and gonial angle—Identification tool in age estimation and sex determination: A digital panoramic radiographic study in north indian population. J. Indian Acad. Oral. Med. Radiol. 2020, 32, 31–36.
- Kartheeki, B.; Nayyar, A.S.; Sindhu, Y.U.; Lakshmana, N. Accuracy of mandibular rami measurements in prediction of sex. Arch. Med. Health Sci. 2017, 5, 50–54.
- Sella Tunis, T.; Hershkovitz, I.; May, H.; Vardimon, A.D.; Sarig, R.; Shpack, N. Variation in chin and mandibular symphysis size and shape in males and females: A CT-Based study. Int. J. Environ. Res. Public Health 2020, 17, 4249.
- Veeraraghavan, G.; Lingappa, A.; Shankara, S.P.; Mamatha, G.P.; Sebastian, B.T.; Mujib, A. Determination of sex from tooth pulp tissue. Libyan J. Med. 2010, 5, 5084.
- Zagga, A.; Ahmed, H.; Ismail, S.; Tadros, A. Molecular sex identification of dry human teeth specimens from Sokoto, Northwestern Nigeria. J. Forensic Dent. Sci. 2014, 6, 132–138.
- Capitaneanu, C.; Willems, G.; Thevissen, P. A systematic review of odontological sex estimation methods. J. Forensic Odontostomatol. 2017, 35, 1–19.
- Chowdhury, R.; Singhvi, A.; Bagul, N.; Bhatia, S.; Singh, G.; Goswami, S. Sex determination by amplification of amelogenin gene from dental pulp tissue by polymerase chain reaction. Indian J. Dent. Res. 2018, 29, 470–476.
- Dutta, P.; Bhosale, S.; Singh, R.; Gubrellay, P.; Patil, J.; Sehdev, B.; Bhagat, S.; Bansal, T. Amelogenin gene—The pioneer in gender determination from forensic dental samples. J. Clin. Diagn. Res. 2017, 11, ZC56–ZC59.
- Dash, H.R.; Rawat, N.; Das, S. Alternatives to amelogenin markers for sex determination in humans and their forensic relevance. Mol. Biol. Rep. 2020, 47, 2347–2360.
- Higuchi, R.; Bowman, B.; Freiberger, M.; Ryder, O.A.; Wilson, A.C. DNA sequences from the quagga, an extinct member of the horse family. Nature 1984, 312, 282–284.
- Pääbo, S. Molecular cloning of ancient Egyptian mummy DNA. Nature 1985, 314, 644–645.
- Krings, M.; Stone, A.; Schmitz, R.W.; Krainitzki, H.; Stoneking, M.; Pääbo, S. Neandertal DNA sequences and the origin of modern humans. Cell 1997, 90, 19–30.
- Nesheva, D.V. Aspects of ancient mitochondrial dna analysis in different populations for understanding human evolution. Balk. J. Med. Genet. 2014, 17, 5–14.
- Pilli, E.; Vai, S.; Caruso, M.G.; D’Errico, G.; Berti, A.; Caramelli, D. Neither femur nor tooth: Petrous bone for identifying archaeological bone samples via forensic approach. Forensic Sci. Int. 2018, 283, 144–149.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
22 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




