
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Malaguti | -- | 3636 | 2022-09-21 09:04:02 | | | |
| 2 | Vivi Li | + 35 word(s) | 3671 | 2022-09-22 07:53:06 | | |
Video Upload Options
Endothelial damage is recognized as the initial step that precedes several cardiovascular diseases (CVD), such as atherosclerosis, hypertension, and coronary artery disease. It has been demonstrated that the best treatment for CVD is prevention, and, in the frame of a healthy lifestyle, the consumption of vegetables, rich in bioactive molecules, appears effective at reducing the risk of CVD. In this context, the large amount of agri-food industry waste, considered a global problem due to its environmental and economic impact, represents an unexplored source of bioactive compounds. The waste matrices of apple, pear, and sugar beet crops can represent promising starting material for producing “upcycled” products with functional applications, such as the prevention of endothelial dysfunction linked to cardiovascular diseases.
1. Introduction
2. Apple
2.1. Exploiting Apple Pomace
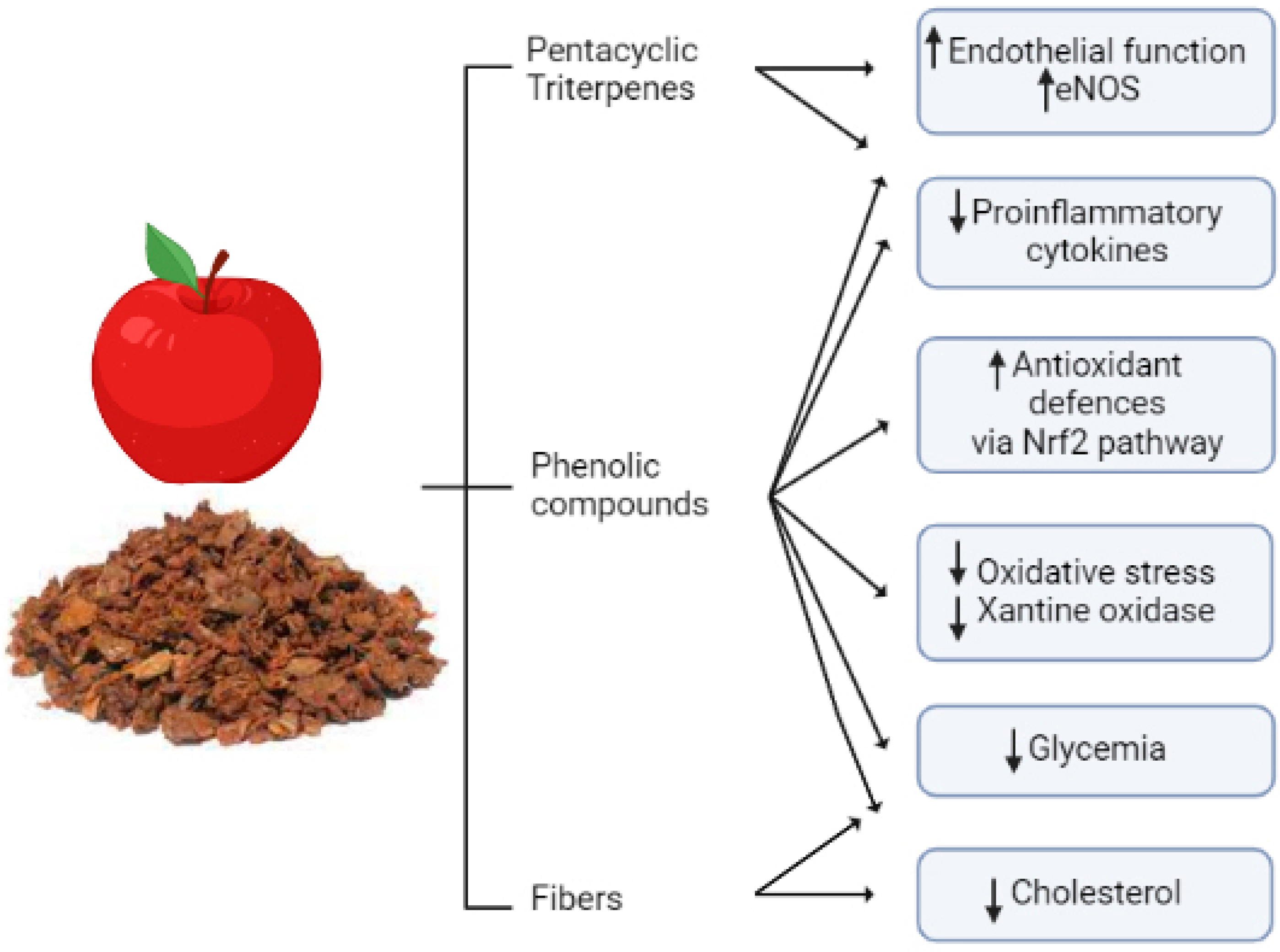
2.2. Cardiovascular Protective Effects of Bioactive Compounds from Apple By-Products
3. Pear
3.1. Exploiting Pear Pomaces
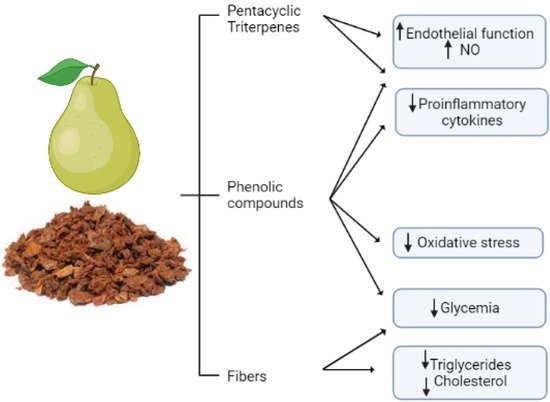
3.2. Cardiovascular Protective Effects of Bioactive Compounds from Pear By-Products
4. Sugar Beet
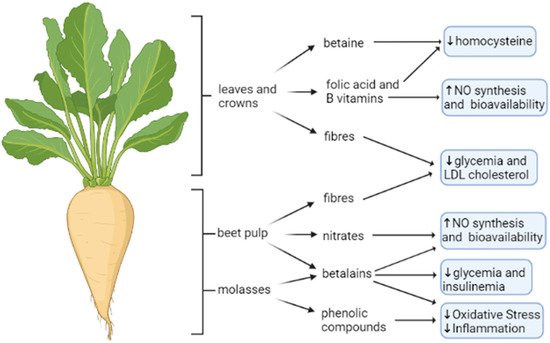
4.1. Sugar Beet Waste and By-Products
References
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; World Health Organization: Geneva, Switzerland, 2013.
- Morris, G.; Puri, B.K.; Olive, L.; Carvalho, A.; Berk, M.; Walder, K.; Gustad, L.T.; Maes, M. Endothelial dysfunction in neuroprogressive disorders—Causes and suggested treatments. BMC Med. 2020, 18, 305.
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308.
- He, F.J.; Nowson, C.A.; Lucas, M.; MacGregor, G.A. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens 2007, 21, 717–728.
- Cicero, A.F.G.; Caliceti, C.; Fogacci, F.; Giovannini, M.; Calabria, D.; Colletti, A.; Veronesi, M.; Roda, A.; Borghi, C. Effect of apple polyphenols on vascular oxidative stress and endothelium function: A translational study. Mol. Nutr. Food Res. 2017, 61, 1700373.
- Marracino, L.; Punzo, A.; Severi, P.; Nganwouo Tchoutang, R.; Vargas-De-la-Cruz, C.; Fortini, F.; Vieceli Dalla Sega, F.; Silla, A.; Porru, E.; Simoni, P.; et al. Fermentation of Vaccinium floribundum Berries with Lactiplantibacillus plantarum Reduces Oxidative Stress in Endothelial Cells and Modulates Macrophages Function. Nutrients 2022, 14, 1560.
- Caliceti, C.; Franco, P.; Spinozzi, S.; Roda, A.; Cicero, A.F.G. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016, 23, 1460–1476.
- Caliceti, C.; Rizzo, P.; Cicero, A.F.G. Potential Benefits of Berberine in the Management of Perimenopausal Syndrome. Oxidative Med. Cell. Longev. 2015, 2015, 723093.
- Stephen, J.; Livingstone, A.M. The salvage and utilization of food waste for animal feeding. J. R. Sanit. Inst. 1953, 73, 675–692.
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41.
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of food agro-industrial by-products: From the past to the present and perspectives. J. Environ. Manag. 2021, 299, 113571.
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181.
- Caliceti, C.; Urao, N.; Rizzo, P.; Giuliano, M. New Trends in Antioxidant Compounds: A Precise Nutraceutical in Cardiometabolic Disorders. Oxid. Med. Cell Longev. 2019, 2019, 4794563.
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From Agro-Industrial Waste to Food as Bioactive Molecules. Foods 2021, 10, 3152.
- Lenzi, M.; Malaguti, M.; Cocchi, V.; Hrelia, S.; Hrelia, P. Castanea sativa Mill. bark extract exhibits chemopreventive properties triggering extrinsic apoptotic pathway in Jurkat cells. BMC Complement. Altern. Med. 2017, 17, 251.
- Lenzi, M.; Cocchi, V.; Malaguti, M.; Barbalace, M.C.; Marchionni, S.; Hrelia, S.; Hrelia, P. 6-(Methylsulfonyl) hexyl isothiocyanate as potential chemopreventive agent: Molecular and cellular profile in leukaemia cell lines. Oncotarget 2017, 8, 111697–111714.
- ISTAT. Agricoltura, Dati e Microdati. Available online: https://www.istat.it/it/agricoltura?dati (accessed on 15 July 2022).
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042.
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols from Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508.
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46.
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062.
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and Spiny Burs of Castanea Sativa from an Experimental Chestnut Grove: Metabolomic Analysis and Anti-Neuroinflammatory Activity. Metabolites 2020, 10, 408.
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Gallina Toschi, T.; Comandini, P.; Hrelia, S. Sweet chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell. Longev. 2013, 2013, 471790.
- FAO. FAOSTAT Database. Available online: https://www.fao.org/faostat/en/ (accessed on 1 July 2022).
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842.
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple pomace: A versatile substrate for biotechnological applications. Crit. Rev. Biotechnol. 2008, 28, 1–12.
- Favela-Torres, E.; Volke-Sepulveda, T.; Viniegra-Gonzalvez, G. Production of hydrolytic depolymerising pectinases. Food Technol. Biotechnol. 2006, 44, 221–227.
- Gołębiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials 2022, 15, 1788.
- Hang, Y.D.; Lee, C.Y.; Woodams, E.E.; Cooley, H.J. Production of alcohol from apple pomace. Appl. Environ. Microbiol. 1981, 42, 1128–1129.
- Opriş, O.; Lung, I.; Soran, M.L.; Stegarescu, A.; Cesco, T.; Ghendov-Mosanu, A.; Podea, P.; Sturza, R. Efficient Extraction of Total Polyphenols from Apple and Investigation of Its SPF Properties. Molecules 2022, 27, 1679.
- Usman, M.; Ahmed, S.; Mehmood, A.; Bilal, M.; Patil, P.J.; Akram, K.; Farooq, U. Effect of apple pomace on nutrition, rheology of dough and cookies quality. J. Food Sci. Technol. 2020, 57, 3244–3251.
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top Med. Chem. 2021, 21, 1067–1095.
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869.
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909.
- Wang, L.; Huang, J.; Li, Z.; Liu, D.; Fan, J. A review of the polyphenols extraction from apple pomace: Novel technologies and techniques of cell disintegration. Crit. Rev. Food Sci. Nutr. 2022.
- Virot, M.; Tomao, V.; Le Bourvellec, C.; Renard, C.M.; Chemat, F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochem. 2010, 17, 1066–1074.
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18.
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129.
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758.
- Wang, L.; Bussetta, N.; Lebovka, N.; Vorobiev, E. Cell disintegration of apèple peels induced by pulsed electric field and efficiency bio-compound extraction. Food Bioprod. Processing 2020, 122, 13–21.
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-xylanase and endo-cellulase-assisted extraction of pectin from apple pomace. Carbohydr. Polym. 2016, 142, 199–205.
- Zhang, Z.; Manjunatha Poojary, M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of selected clean and green extraction technologies for biomolecules from apple pomace. Electrophoresis 2018, 39, 1934–1945.
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596.
- Kim, Y.; Je, Y. Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies. Clin. Nutr. ESPEN 2017, 20, 68–77.
- Chai, S.C.; Hooshmand, S.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Daily apple versus dried plum: Impact on cardiovascular disease risk factors in postmenopausal women. J. Acad. Nutr. Diet 2012, 112, 1158–1168.
- Bondonno, N.P.; Bondonno, C.P.; Warda, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–356.
- Gayer, B.A.; Avendano, E.E.; Edelson, E.; Nirmala, N.; Johnson, E.J.; Raman, G. Effects of Intake of Apples, Pears, or Their Products on Cardiometabolic Risk Factors and Clinical Outcomes: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2019, 3, nzz109.
- Birru, R.L.; Bein, K.; Bondarchuk, N.; Wells, H.; Lin, Q.; Di, Y.P.; Leikauf, G.D. Antimicrobial and Anti-Inflammatory Activity of Apple Polyphenol Phloretin on Respiratory Pathogens Associated with Chronic Obstructive Pulmonary Disease. Front. Cell Infect. Microbiol. 2021, 11, 652944.
- Waldbauer, K.; Seiringer, G.; Nguyen, D.L.; Winkler, J.; Blaschke, M.; McKinnon, R.; Urban, E.; Ladurner, A.; Dirsch, V.M.; Zehl, M.; et al. Triterpenoic Acids from Apple Pomace Enhance the Activity of the Endothelial Nitric Oxide Synthase (eNOS). J. Agric. Food Chem. 2016, 64, 185–194.
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2015, 95, 560–568.
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413.
- Su, Y.L.; Liu, D.; Liu, Y.J.; Ji, Y.L.; Liu, G.S.; Wang, J.L.; Wang, B.; Wang, H. Phlorizin alleviates cholinergic memory impairment and regulates gut microbiota in d-galactose induced mice. Exp. Gerontol. 2022, 165, 111863.
- Malaguti, M.; Angeloni, C.; Hrelia, S. Nutraceutical Bioactive Compounds Promote Healthspan Counteracting Cardiovascular Diseases. J. Am. Coll. Nutr. 2015, 34, 22–27.
- Angeloni, C.; Leoncini, E.; Malaguti, M.; Angelini, S.; Hrelia, P.; Hrelia, S. Role of quercetin in modulating rat cardiomyocyte gene expression profile. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1233–H1243.
- Angeloni, C.; Spencer, J.P.; Leoncini, E.; Biagi, P.L.; Hrelia, S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie 2007, 89, 73–82.
- Li, M.T.; Ke, J.; Guo, S.F.; Wu, Y.; Bian, Y.F.; Shan, L.L.; Liu, Q.Y.; Huo, Y.J.; Guo, C.; Liu, M.Y.; et al. The Protective Effect of Quercetin on Endothelial Cells Injured by Hypoxia and Reoxygenation. Front. Pharmacol. 2021, 12, 732874.
- Sharma, S.; Rana, S.; Patial, V.; Gupta, M.; Bhushan, S.; Padwad, Y.S. Antioxidant and hepatoprotective effect of polyphenols from apple pomace extract via apoptosis inhibition and Nrf2 activation in mice. Hum. Exp. Toxicol. 2016, 35, 1264–1275.
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. An apple a day keeps the doctor away: The potentials of apple bioactive constituents for chronic disease prevention. J. Food Sci. 2022, 87, 2291–2309.
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210.
- Liddle, D.M.; Lin, X.; Ward, E.M.; Cox, L.C.; Wright, A.J.; Robinson, L.E. Apple consumption reduces markers of postprandial inflammation following a high fat meal in overweight and obese adults: A randomized, crossover trial. Food Funct. 2021, 12, 6348–6362.
- Angeloni, C.; Hrelia, S. Quercetin reduces inflammatory responses in LPS-stimulated cardiomyoblasts. Oxid. Med. Cell Longev. 2012, 2012, 837104.
- Dehghani, F.; Sezavar Seyedi Jandaghi, S.H.; Janani, L.; Sarebanhassanabadi, M.; Emamat, H.; Vafa, M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: A double blind, placebo-controlled, randomized clinical trial. Phytother. Res. 2021, 35, 2085–2098.
- Wang, Y.L.; Sun, G.Y.; Zhang, Y.; He, J.J.; Zheng, S.; Lin, J.N. Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564.
- Erdmann, J.; Kujaciński, M.; Wiciński, M. Beneficial Effects of Ursolic Acid and Its Derivatives-Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2021, 13, 3900.
- Surampudi, P.; Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipid Lowering with Soluble Dietary Fiber. Curr. Atheroscler. Rep. 2016, 18, 75.
- Dongowski, G.; Lorenz, A. Intestinal steroids in rats are influenced by the structural parameters of pectin. J. Nutr. Biochem. 2004, 15, 196–205.
- Dongowski, G.; Lorenz, A.; Proll, J. The degree of methylation influences the degradation of pectin in the intestinal tract of rats and in vitro. J. Nutr. 2002, 132, 1935–1944.
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599.
- Titgemeyer, E.C.; Bourquin, L.D.; Fahey, G.C.; Garleb, K.A. Fermentability of various fiber sources by human fecal bacteria in vitro. Am. J. Clin. Nutr. 1991, 53, 1418–1424.
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013, 52, 1875–1889.
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 307–318.
- Osada, K.; Suzuki, T.; Kawakami, Y.; Senda, M.; Kasai, A.; Sami, M.; Ohta, Y.; Kanda, T.; Ikeda, M. Dose-dependent hypocholesterolemic actions of dietary apple polyphenol in rats fed cholesterol. Lipids 2006, 41, 133–139.
- Ma, X.; Yuan, H.; Wang, H.; Yu, H. Coproduction of bacterial cellulose and pear vinegar by fermentation of pear peel and pomace. Bioprocess Biosyst. Eng. 2021, 44, 2231–2244.
- Chang, S.; Cui, X.; Guo, M.; Tian, Y.; Xu, W.; Huang, K.; Zhang, Y. Insoluble Dietary Fiber from Pear Pomace Can Prevent High-Fat Diet-Induced Obesity in Rats Mainly by Improving the Structure of the Gut Microbiota. J. Microbiol. Biotechnol. 2017, 27, 856–867.
- You, M.K.; Rhuy, J.; Kim, H.A. Pear pomace water extract suppresses hepatic lipid peroxidation and protects against liver damage in rats fed a high fat/cholesterol diet. Food Sci. Biotechnol. 2017, 26, 801–806.
- Kolniak-Ostek, J.; Oszmiański, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163.
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133.
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538.
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16.
- Om, P.; Gopinath, M.S.; Madan Kumar, P.; Muthu Kumar, S.P.; Kudachikar, V.B. Ethanolic extract of Pyrus pashia buch ham ex. D. Don (Kainth): A bioaccessible source of polyphenols with anti-inflammatory activity in vitro and in vivo. J. Ethnopharmacol. 2022, 282, 114628.
- An, Q.; Hu, Q.; Wang, B.; Cui, W.; Wu, F.; Ding, Y. Oleanolic acid alleviates diabetic rat carotid artery injury through the inhibition of NLRP3 inflammasome signaling pathways. Mol. Med. Rep. 2017, 16, 8413–8419.
- Claro-Cala, C.M.; Quintela, J.C.; Pérez-Montero, M.; Miñano, J.; de Sotomayor, M.A.; Herrera, M.D.; Rodríguez-Rodríguez, A.R. Pomace Olive Oil Concentrated in Triterpenic Acids Restores Vascular Function, Glucose Tolerance and Obesity Progression in Mice. Nutrients 2020, 12, 323.
- Sivasangari, S.; Asaikumar, L.; Vennila, L. Arbutin prevents alterations in mitochondrial and lysosomal enzymes in isoproterenol-induced myocardial infarction: An in vivo study. Hum. Exp. Toxicol. 2021, 40, 100–112.
- Zhang, B.; Zeng, M.; Li, B.; Wang, Y.; Kan, Y.; Wang, S.; Meng, Y.; Gao, J.; Feng, W.; Zheng, X. Inhibition of oxidative stress and autophagy by arbutin in lipopolysaccharide-induced myocardial injury. Pharmacogn. Mag. 2019, 15, 507–513.
- Jiang, R.; Hodgson, J.M.; Mas, E.; Croft, K.D.; Ward, N.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J Nutr. Biochem. 2016, 27, 53–60.
- Sarkar, D.; Ankolekar, C.; Pinto, M.; Shetty, K. Dietary functional benefits of Bartlett and Starkrimson pears for potential management of hyperglycemia, hypertension and ulcer bacteria Helicobacter pylori while supporting beneficial probiotic bacterial response. Food Res. Int. 2015, 69, 80–90.
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J. Funct. Food 2015, 13, 276–288.
- Velmurugan, C.; Bhargava, A. Anti-diabetic and hypolipidemic activity of fruits of Pyrus Communis L. in hyperglycemic rats. Asian J Pharm. Clin. Res. 2013, 6, 108–111.
- European Commission. Sugar. Available online: https://agriculture.ec.europa.eu/data-and-analysis/markets/price-data/price-monitoring-sector/sugar_en (accessed on 27 July 2022).
- Mahapatra, C.K.; Bhadra, T.; Paul, K.S. Nutrient management in sugar beet: A review. Pak. Sugar J. 2020, 35, 31–44.
- Kaffka, S.R.; Grantz, D.A. Sugar crops. In Encyclopedia of Agriculture and Food Systems; Science, F., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 5, pp. 240–260.
- Kushwaha, R.; Kumar, V.; Vyas, G.; Kaur, J. Optimization of Different Variable for Eco-friendly Extraction of Betalains and Phytochemicals from Beetroot Pomace. Waste Biomass Valorization 2018, 9, 1485–1494.
- Shafiq, S.; Akram, N.A.; Ashraf, M.; Al-Harbi, M.S.; Samra, B.N. Sugar beet extract rich in glycine betaine modulates oxidative defense system and key physiological characteristics of maize under water-deficit stress. PLoS ONE 2021, 16, e0254906.
- Rivoira, L.; Studzińska, S.; Szultka-Młyńska, M.; Bruzzoniti, M.C.; Buszewski, B. New approaches for extraction and determination of betaine from Beta vulgaris samples by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5133–5141.
- Paroha, S.; Singh, S.; Gupta, A. Sugar beet-the potential feedstocks for alcohol production. Int. J. Agric. Sci. 2020, 12, 9778–9780.
- Sauthoff, S.; Musshoff, O.; Danne, M.; Anastassiadis, F. Sugar beet as a biogas substrate? A discrete choice experiment for the design of substrate supply contracts for German farmers. Biomass Bioenergy 2016, 90, 163–172.
- Battistella Lasta, H.F.; Lentz, L.; Gonçalves; Rodrigues, L.G.; Mezzomo, N.; Vitali, L.; Salvador Ferreira, S.R. Pressurized liquid extraction applied for the recovery of phenolic compounds from beetroot waste. Biocatal. Agric. Biotechnol. 2019, 21, 101353.
- Simić, S.; Petrović, J.; Rakić, D.; Pajin, B.; Lončarević, I.; Jozinović, A.; Fišteš, A.; Nikolić, S.; Blažić, M.; Miličević, B. The influence of extruded sugar beet pulp on cookies’ nutritional, physical and sensory characteristics. Sustainability 2021, 13, 5317.
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550.
- FAO. FAO—Agribusiness Handbook: White Sugar; FAO: Rome, Italy, 2009.





