Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephane Prange | -- | 3714 | 2022-09-20 17:33:17 | | | |
| 2 | Sirius Huang | Meta information modification | 3714 | 2022-09-21 02:48:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Prange, S.; Theis, H.; Banwinkler, M.; Eimeren, T.V. Neurotransmitter Imaging in Parkinsonian Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/27388 (accessed on 07 February 2026).
Prange S, Theis H, Banwinkler M, Eimeren TV. Neurotransmitter Imaging in Parkinsonian Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/27388. Accessed February 07, 2026.
Prange, Stéphane, Hendrik Theis, Magdalena Banwinkler, Thilo Van Eimeren. "Neurotransmitter Imaging in Parkinsonian Disorders" Encyclopedia, https://encyclopedia.pub/entry/27388 (accessed February 07, 2026).
Prange, S., Theis, H., Banwinkler, M., & Eimeren, T.V. (2022, September 20). Neurotransmitter Imaging in Parkinsonian Disorders. In Encyclopedia. https://encyclopedia.pub/entry/27388
Prange, Stéphane, et al. "Neurotransmitter Imaging in Parkinsonian Disorders." Encyclopedia. Web. 20 September, 2022.
Copy Citation
Neurodegenerative parkinsonian disorders are characterized by a great diversity of clinical symptoms and underlying neuropathology, yet differential diagnosis during lifetime remains probabilistic. Molecular imaging is a powerful method to detect pathological changes in vivo on a cellular and molecular level with high specificity. Thereby, molecular imaging enables to investigate functional changes and pathological hallmarks in neurodegenerative disorders, thus allowing to better differentiate between different forms of degenerative parkinsonism, improve the accuracy of the clinical diagnosis and disentangle the pathophysiology of disease-related symptoms.
PET
SPECT
Parkinson’s disease
atypical parkinsonism
neurodegeneration
monoaminergic neurotransmission
1. Introduction
Parkinson’s disease (PD) and related parkinsonian disorders are the most common neurodegenerative diseases, clinically characterized by distinctive motor symptoms and multisystem disorders. However, heterogeneity of clinical symptoms and pathology is the rule within the spectrum of neurodegenerative parkinsonian disorders. To date, post-mortem analysis enables to differentiate α-synucleinopathies, including PD, dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), from tauopathies, including progressive supranuclear palsy (PSP) and cortico-basal degeneration (CBD), whereas differential diagnosis during lifetime remains probabilistic. However, molecular imaging using radioactive tracers for single photon emission computed tomography (SPECT) and positron emission tomography (PET) now enables to detect pathological changes in vivo on a cellular or molecular level with high specificity and sensitivity, related to metabolism, inflammation, transporter/receptor availability, and protein aggregates. From the clinician’s point of view, nuclear molecular imaging offers not only the possibility to detect the degeneration of the dopaminergic system but can also differentiate between the different forms of degenerative parkinsonism based on imaging of brain metabolism and neurodegeneration [1]. From the researcher’s point of view, nuclear molecular imaging can help to disentangle the complexity of the underlying neuropathology, e.g., the spread of pathogenic proteins, neuroinflammation, and imaging of non-motor symptoms [2].
2. Neurotransmitter Imaging in Parkinsonian Disorders
2.1. Dopaminergic Imaging
2.1.1. Imaging the Dopaminergic System
Dysfunction of the dopaminergic system is central to the motor and nonmotor expression of parkinsonian disorders and predates the onset of overt clinical symptoms. As such, multiple investigations of the presynaptic compartment, using specific tracers for the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and aromatic-amino-acid decarboxylase were performed indicating progressive dysfunction of striatal dopaminergic terminals, besides imaging of presynaptic auto-receptors and postsynaptic dopamine receptors.
Multiple tracers have been developed for DAT using SPECT (123I-FP-CIT, 123I-beta-CIT, 99mTc-TRODAT, 123I-altropane, 123I-PE2I) and PET imaging (11C-RTI-32, 11C-CFT, 11C-methylphenidate, 11C-PE2I), of which 123I-FP-CIT and 99mTc-TRODAT are commercially available. Practice guidelines for acquisition and interpretation are available from the European Academy of Nuclear Medicine [3]. Validated tracers for the presynaptic dopaminergic (11C-PE2I), serotonergic (11C-DASB), and noradrenergic (11C-MeNER) transporters and their brain distribution in healthy individuals are presented in Figure 1. New to the field is the development and increasing use of fluorine-18-labeled PET tracers [3], next to 18F-DOPA. Indeed, longer radiological half-life relative to carbon-11 (110 min vs. 20 min) enables cost-effective distribution to imaging centers lacking on-site cyclotrons, besides advantages for new tracer design with improved affinity and selectivity, as in 18F-FP-CIT [4] and 18F-PR04-MZ for DAT [5] or 18F-FP-(+)-DTBZ for VMAT2 [6].
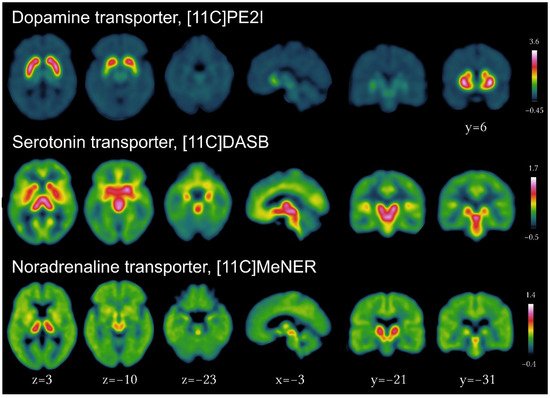
Figure 1. Imaging of the presynaptic dopaminergic, serotonergic and noradrenergic systems using PET tracers for the dopamine transporter ([11C]PE2I), serotonin transporter ([11C]DASB) and noradrenergic transporter ([11C]MeNER, courtesy of Christopher Doppler).
In particular, 18F-FE-PE2I represents a highly specific DAT tracer, enabling rapid static acquisitions as short as 10 min using high-resolution PET imaging, even 30 min after tracer injection (compared to 30 min scan time 3 to 6 h after injection for SPECT), without loss of diagnostic accuracy in comparison to standard 123I-FP-CIT scan [7]. Notably, DAT SPECT as opposed to PE2I PET also binds to the serotonin transporter, which, dependent on the research question, can be seen as an advantage or disadvantage. Importantly, it was recently shown that dopamine synthesis capacity, using 11C-β-LDOPA, and DAT availability, using 18F-FE-PE2I, were positively correlated in the putamen in healthy individuals, indicating regulated homeostasis of the synaptic dopamine concentration for normal functioning, whereas presynaptic D2 autoreceptors and post-synaptic D2 receptors striatal density were not determined by DAT or dopamine synthesis capacity [8].
2.1.2. Dopaminergic Imaging in PD
Important advances were recently achieved to disentangle the complex relationship between striatal dopaminergic loss and nigral degeneration using specific radiotracers combined with magnetic resonance imaging (MRI) of magnetic susceptibility differences related to iron and neuromelanin. Indeed, it was demonstrated that striatal dopaminergic denervation and changes in the substantia nigra pars compacta involving abnormalities of iron metabolism and neuromelanin followed a sequential progression in that order, sharing a similar but time-shifted pattern along the sensorimotor, associative, and limbic territories [9]. This temporal delay likely explains the weak or absent cross-sectional association of striatal and nigral degeneration [10], although pathology is strongly concordant and lateralized to the most affected hemisphere [11]. Using diffusion-weighted imaging, degeneration of striatal dopaminergic terminals measured with 11C-DTBZ was negatively correlated with increased free-water in the posterior part of the substantia nigra, while both contributed to the prediction of motor impairment [12].
Importantly, joint pattern analysis of multiple tracers represents a new analytical method to identify patterns reflecting functional similarities and differences provided by each tracer, as for 11C-methylphenydate (DAT) and 11C-DTBZ (VMAT2) labeling in early PD [13], revealing a shared pattern of asymmetry, rostro-caudal gradient, and progression of pathology in the least-affected striatum, while DAT appeared relatively more preserved in the posterior putamen.
Greater loss of striatal DAT in the (less-affected) hemisphere ipsilateral to the clinically more affected body side was also demonstrated in a longitudinal PET study using 11C-PE2I, while striatal asymmetry gradually became less prominent despite the persistence of clinical asymmetry [14]. In addition, integration of DAT decline rate in the putamen using SPECT enables to impute the start of dopaminergic degeneration to around 10 years before motor onset in the Parkinson’s Progression Markers Initiative (PPMI) cohort [15]. Moreover, DAT-SPECT may be used to improve the prediction of motor progression using machine learning [16][17][18], as well as for cognitive outcomes in addition to cerebrospinal fluid (CSF) amyloid and tau [19]. Interestingly, patients with higher baseline levels of neurofilament light chain in the serum had faster DAT decline in the PPMI cohort [20]. Contrastingly, no definitive delineation can be drawn for tremor-dominant patients due to mixed findings regarding increased striatal DAT tracer uptake [21].
Furthermore, dopaminergic imaging enables to investigate the individual susceptibility to dopaminergic degeneration in PD patients, which is conceptualized as motor reserve and corresponds to the discrepancy between the expected motor impairment given the level of striatal dopaminergic degeneration and the individual severity of motor symptoms [22]. Importantly, motor reserve was shown to be greater with educational attainment and premorbid physical activity, and with an increased degree of functional connectivity in a network involving the basal ganglia, inferior frontal cortex, insula, and cerebellar vermis, also associated with a slower increase in dopamine replacement therapy [22]. Notably, aerobic exercise may increase the release of endogenous dopamine in the striatum, as measured using 11C-raclopride PET for release evoked by repetitive transcranial magnetic stimulation [23].
2.1.3. Dopaminergic Imaging in Genetic PD
Although the prevalence of monogenic inherited PD is low, great efforts have been recently conducted to identify non-symptomatic mutation carriers of autosomic PD variants (SNCA; LRRK2) and high-risk variants (GBA). This has enabled to gain new insight into the neurodegenerative and compensatory processes at work during the premotor period and possibly underpinned by differential dysfunction of dopamine synthesis and DAT [24].
Importantly, overall striatal DAT availability using SPECT was found to be increased in GBA (mainly N370S) but not LRRK2 (mainly G2019S) non-manifesting carriers in comparison to healthy controls in the PPMI cohort [25], whereas lower DAT binding in the putamen was reported in 25 LRRK2 non-symptomatic mutation carriers using 11C-MP PET [26] and DAT SPECT [27], in line with previous findings [28]. Contrastingly, striatal dopamine synthesis capacity measured with 18F-FDOPA PET in LRRK2 [26] and heterozygous and homozygous GBA1 mutations carriers without PD [29] was similar to healthy controls. Interestingly, PD patients with GBA (N370S) mutations may exhibit a faster decline in striatal DAT availability during the premotor phase compared to patients with LRRK2 mutation or idiopathic PD using DAT SPECT in the PPMI cohort [15], while 18-FDOPA rate of change in GBA1 mutation carriers was similar to controls [29]. Moreover, a steady decline of DAT was previously reported in asymptomatic LRRK2 G2019S mutation carriers, comparable to the rate in patients converting to PD [30][31]. However, both patients with GBA or LRRK2 mutations and overt PD symptoms in the PPMI cohort may also have increased DAT availability restricted to the striatum contralateral to the more affected body side in early PD [32]. Considering GBA variants in PD patients (excluding GBA mutations causing Gaucher’s disease), Greuel et al. found lower striatal 18F-FDOPA binding [33], also consistent with increased PD-related pattern expression [34], overall compatible with a more malignant disease course in both GBA variants and mutation carriers.
Interestingly, recent studies of the PPMI cohort demonstrated that the genetic risk as determined from the polygenic load of single nucleotide polymorphisms (SNPs) associated with PD has no effect on DAT availability in healthy subjects [35] and on annual change in PD patients, although several SNPs may influence DAT decline and deserve further investigation in larger cohorts [15][36]. This is consistent with large-scale genome-wide association studies showing no single variant associated with motor progression [37]. Interestingly, the binding of 11C-(+)-PHNO to the D2 receptor in the ventral striatum was found to be modulated by SNPs of the DRD2 gene resulting in alternative splicing into long, mainly postsynaptic, and short, mainly presynaptic autoreceptor, variants in a study with 20 PD patients [38], although replication is critical for genetic association studies.
2.1.4. Dopaminergic Imaging in Prodromal PD
Due to the delay between the onset of dopaminergic pathology and motor symptoms in parkinsonian disorders, dopaminergic imaging represents the method of choice to detect early neurodegeneration at the premotor stage. It is now established that striatal (putaminal) DAT loss is the most reliable marker of prodromal PD [39] and predicts phenoconversion to symptomatic synucleinopathy within five years in patients with rapid eye movement sleep behavior disorder (RBD) [40][41][42][43] and hyposmia [44]. Inversely, normal dopamine imaging in patients with RBD has an important negative predictive value, although more than 70% of patients with RBD convert in the long term within 12 years, with an estimated annualized rate of 6% [45]. Multimodal imaging combining DAT SPECT with brain metabolic patterns and cardiac sympathetic denervation may further improve prediction for phenoconversion [46], as metabolic patterns become increasingly similar to PD [47].
Moreover, more than 25% of patients with RBD exhibit loss of the dorsal nigral hyperintensity using susceptibility-weighted MR imaging, associated with dopaminergic dysfunction, similar to PD patients [48]. Recently, dopaminergic deficit was also demonstrated in individuals with late-onset depression, exhibiting motor and nonmotor symptoms [49].
2.1.5. Dopaminergic Imaging in Atypical Parkinsonian Disorders
Dopaminergic dysfunction is also found in patients with non-PD parkinsonian disorders, most prominent in those with α-synucleinopathy such as DLB and MSA. However, differentiating patients with PD or DLB using dopaminergic imaging is notably difficult (see below). In particular, striatal DAT availability was similar between early-stage patients with PD and DLB [50]. Importantly, metabolic imaging with 18F-FDG considerably improved diagnostic accuracy, characterized by a hypermetabolic pattern involving the bilateral posterior putamen, vermis, and somato-motor cortex [51]. Contrastingly, the decline of striatal DAT SPECT was greater in patients with MSA of the parkinsonian subtype (MSA-P) and of the cerebellar subtype (MSA-C) compared to PD patients [52]. Overall, dopaminergic denervation in patients with MSA is an important diagnostic marker, improving diagnostic confidence for up to 43% of patients with possible MSA-C based on clinical criteria [53], although metabolic imaging may be superior (see below).
Moreover, subtle differences in dopaminergic degeneration and compensatory mechanisms may exist between degenerative parkinsonian disorders. In particular, lower striatal D2R binding was found in a meta-analysis for PSP and MSA-P patients in comparison to PD patients (14.2% and 21.8%, respectively), contrasting with an initial upregulation of striatal D2-receptors in PD patients up to 4 years after the onset of motor symptoms [54]. Overall, multiple studies indicate that dopaminergic degeneration is more symmetric in degenerative parkinsonian disorders other than PD, although this finding is not decisive for individual diagnosis and loses its importance as asymmetry is gradually reduced in moderate and advanced PD [14][55]. As such, greater regional dopaminergic asymmetry likely involved the nigrostriatal pathway in PD patients, whereas asymmetry was restricted to the bilateral caudate in patients with DLB and to the pallido-subthalamic pathway in patients with PSP [56]. In particular, PSP patients exhibit greater symmetric dopaminergic denervation [55]. Interestingly, in patients with DLB, striatal dopaminergic denervation shown using 18F-FP-CIT contributed to multi-domain cognitive dysfunction [51][57] and motor impairment [51]. Moreover, using 18F-florbetapen PET, dopaminergic loss was associated with increased β-amyloid deposition in the putamen, parietal, lateral temporal, cingular, and occipital cortices, the latter mediating visuo-spatial dysfunction [57].
2.1.6. Differential Diagnosis Using Dopaminergic Imaging and Use of Machine Learning
As such, imaging of the dopaminergic system is most sensitive to detect early striatal dopaminergic denervation useful for the differential diagnosis of parkinsonian disorders versus other degenerative conditions (AD, frontotemporal lobar degeneration, and other dementia) and non-degenerative parkinsonism (drug-induced, vascular, normal pressure hydrocephalus, psychogenic) and essential tremor. This has critical implications for accurate diagnosis and treatment in the vast majority of difficult cases [58]. Decision algorithm and practical issues have been previously reviewed [1][3][59][60]. Importantly, recent technical advances improved the reliability of semi-quantitative analysis of striatal binding, thanks to the better atlas-based delineation of the striatum and normative databases for age standardization [61][62].
Moreover, new automated classification methods are developed using machine-learning, including deep convolutional neural networks [63], robust to multi-site or multi-camera imaging characteristics [64], and support-vector-machine analysis [65], with performance similar to expert visual assessment across multiple centers [66]. Interestingly, extrastriatal DAT-SPECT signal greatly contributes to classification accuracy [67], in addition to the shape of the striatum [68], highlighting the need for explainable models to better depict salient features [69]. Increasingly, machine learning is also applied to metabolic imaging, highlighting the advantages of data dimensionality reduction methods such as scaled subprofile modeling [70] or LASSO regression [71].
2.1.7. Implications of Advances in Dopaminergic Imaging
Altogether, dopaminergic imaging represents an early and highly reliable marker of degenerative parkinsonian disorders, useful in clinical routine for the differential diagnosis of PD and related disorders. DAT SPECT/PET imaging is increasingly considered in the design of clinical trials, both as an enrichment biomarker in early PD [72][73], enabling to increase the confidence of clinical diagnosis [74], and to follow the rate of degeneration to evaluate the efficacy of disease-modifying therapeutic interventions to promote neuroprotection [75]. As such, novel PET tracers such as 11C and 18F-PE2I are most useful to follow the longitudinal progression of dopaminergic degeneration.
2.2. Imaging of Peripheral Neurotransmitter in Parkinsonian Disorders
In addition to brain imaging, multisystem disorder in prodromal and overt parkinsonian disorders can be assessed using PET or SPECT, as for sympathetic cardiac imaging using 123I-MIBG SPECT or 18F-FDOPA PET, and parasympathetic small intestine and colonic innervation using 11C-donepezil, as performed simultaneously in patients with suspected prodromal α-synucleinopathy [76][77]. In particular, combined in vivo imaging of brain dopaminergic and peripheral autonomous abnormalities has enabled to support that PD patients with RBD may represent a different subtype characterized by peripheral onset of α-synucleinopathy, summarized as the body-first hypothesis [78]. Indeed, using combined in vivo evaluation of cardiac sympathetic innervation with 123I-metaiodobenzylguanidine (MIBG) SPECT and digestive parasympathetic innervation with the cholinergic 11C-donepezil PET tracer besides 18F-DOPA PET, Horsager et al. demonstrated that de novo PD patients with RBD and patients with idiopathic RBD exhibiting alterations of the locus coeruleus shared a similar pattern combining cardiac sympathetic and digestive parasympathetic denervation [76]. As such, this peripheral pattern likely preceded striatal dopaminergic dysfunction and may represent a ‘body-first’ trajectory of α-synuclein propagation, in comparison to primary striatal dysfunction in PD patients without RBD, compatible with an opposite ‘brain-first’ trajectory. Furthermore, the authors subsequently showed that de novo PD patients with RBD and patients with idiopathic RBD had significantly more symmetric nigrostriatal dopaminergic degeneration, possibly reflecting more symmetric α-synuclein spreading in the brainstem related to overlapping vagal innervation [79]. Interestingly, no difference in presynaptic VMAT2 and postsynaptic D2R dopaminergic function, using 11C-DTBZ and 11C-FLB-457, respectively, was found in PD patients with or without probable RBD, with the extrastriatal decline of 11C-FLB-457 binding in the temporal cortex, involving the uncus parahippocampus, superior, lateral, and inferior temporal cortex, although group size was limited [80][81].
2.3. Serotonergic Imaging in PD and Parkinsonian Disorders
Characteristic of tracers derived from tropane analogs (CIT, TRODAT, altropane, RTI-32, CFT) is their affinity for serotonin and noradrenaline transporters, which importantly contributes to signal in extrastriatal regions with low-density DAT, as well as to noradrenaline transporters for methylphenydate and nomifensine.
Besides dopaminergic degeneration, prominent alterations of the serotonergic projections originating in the dorsal and median raphe nuclei are observed in degenerative parkinsonian disorders. Indeed, recent studies confirm the progressive loss of the serotonin transporter (SERT) in the brainstem in PD patients using DAT-SPECT [82], with significant dysfunction of raphe nuclei in 34% of patients 4 years after diagnosis, and possibly even earlier in SNCA A53T mutation carriers, starting in the premotor phase as measured with 11C-DASB [83]. Interestingly, raphe dysfunction is subjected to considerable variability, with similar rates in tremulous and non-tremulous patients [82]. Recent analysis of extrastriatal DAT-SPECT in the PPMI cohort indicates lower uptake in frontal, temporal, and posterior cortical regions, progressing over one year and correlated with the severity of motor symptoms, cognitive performance, and CSF α-synuclein levels [84].
Analysis of multimodal PET imaging of DAT using 11C-MP, VMAT2 using 11C-DTBZ, D2R using 11C-raclopride, and SERT using 11C-DASB revealed a pattern related to motor complications. This pattern is critically related to serotonergic denervation and associated with higher dopamine release and dopaminergic degeneration in the putamen, thus corresponding to abnormal dopamine turnover in early PD [85]. Furthermore, double-tracer PET studies of the dopamine and serotonin transporters demonstrated the prominent role of SERT dysfunction in the limbic system associated with apathy, depression, and anxiety in de novo [86] and moderate-to-advanced PD patients [87]. Moreover, longitudinal follow-up of de novo apathetic patients indicated a specific increase in serotonergic innervation in the ventral striatum and anterior cingulate cortex, associated with the reversal of apathy independently of dopamine replacement therapy [88]. Altogether, there is growing evidence that the serotonergic system might be subjected to compensatory changes in early PD, as in non-symptomatic LRRK2 mutations carriers [26]. Moreover, it was recently shown that changes in cerebral serotonin levels occur when DBS is turned off, with decreased 11C-AZ10419369 binding to 5HT1B receptors, possibly corresponding to increased serotonin levels in the temporal, limbic, and occipital cortices, dependent on regional receptor preservation [89].
In addition, patients with DLB have greater serotonergic degeneration in the thalamus [90] and hippocampus [50], likely more pronounced and involving the amygdala in patients with DLB and CSF AD biomarker profiles [91]. Furthermore, lower SERT binding of DAT SPECT was found in MSA-P and PSP in comparison to PD and MSA-C patients [92]. In addition, lower 18F-MPPF binding to 5HT1A receptors was shown in the raphe nuclei, caudate, and thalamus in patients with MSA when compared to healthy controls [93].
2.4. Noradrenergic Imaging in PD and RBD
Noradrenergic imaging is particularly challenging, given that current radiotracers derived from antidepressants are non-selective to the noradrenalin transporter (NET), but also bind to DAT and SERT [94]. However, new, more specific, PET tracers are developed, such as for alpha2-adrenoreceptors [95].
Using 11C-MeNER PET, Doppler et al. demonstrated that noradrenergic terminal loss exceeds cellular locus coeruleus degeneration measured using neuromelanin-sensitive MRI in PD [96], playing a critical role in RBD and disorganization of sleep microstructure [97]. Interestingly, a recent PET study using 11C-MeNER demonstrated that noradrenergic innervation is preserved in tremor-dominant PD patients, involving the locus coeruleus and thalamus [98]. Moreover, monoamine synthesis capacity in the putamen measured with 18F-FDOPA and noradrenergic dysfunction were correlated in patients with idiopathic RBD, who also had decreased 11C-MeNER binding in the primary sensorimotor cortex [99], likely reflecting early noradrenergic dysfunction in prodromal α-synucleinopathy.
In addition, cardiac sympathetic noradrenergic denervation can be assessed using 123I-MIBG SPECT and 18F-FDOPA PET. This provides information for the differential diagnosis of PD/DLB versus other parkinsonian disorders, and on the degree of autonomic dysfunction [100], which can be coupled to the assessment of brain dopamine synthesis in one session [101].
2.5. Cholinergic Imaging in PD
The development of novel cholinergic fluorine-based PET tracers, including 18F-Fluoroethoxybenzovesamicol (18F-FEOBV) binding to the vesicular acetylcholine transporter [102], has enabled rapid advances in the last five years to pinpoint the role of basal forebrain cholinergic dysfunction for falls and freezing of gait in PD [103], as well as multi-domain cognitive impairment [104], as reviewed in [105]. Indeed, in contrast with 11C-MP4A or 11C-donepezil, which indicates cholinesterase activity, but with low sensitivity in subcortical structures, 18F-FEOBV binds with high affinity to the vesicular transporter, notably in the striatum [106]. Notably, no tracer is currently available to image the neurotransmitter synthesis by the choline acetyltransferase. Most interestingly, van der Zee et al. demonstrated that PD patients without cognitive impairment had higher-than-normal binding in cerebellar, frontal, and subcortical regions possibly reflecting early compensatory cholinergic upregulation [107], although all patients had lower 18F-FEOBV in the posterior cortical region. In addition, patients with RBD exhibited increased 18F-FEOBV uptake in brainstem nuclei involved in muscle atonia, deep cerebellar nuclei, as well as in limbic territories of the thalamus, anterior cingulate, and orbitofrontal cortex [108]. Contrastingly, acetylcholinesterase levels assessed with 11C-donepezil PET were reduced in the superior temporal, occipital, cingulate, and dorsolateral prefrontal cortices [109].
In comparison, patients with DLB had a prominent reduction in 18F-FEOBV in the lateral geniculate nuclei, pulvinar, optic radiation, thalamus, fimbria, fornix, dedicated to visual attention and spatial navigation, but also the anterior and mid-cingulate cortex and insula in comparison to age-matched healthy controls [110], most likely involved in disabling and fluctuating symptoms in DLB.
Altogether, in vivo imaging of neurotransmitter abnormalities in the dopaminergic, serotonergic, noradrenergic, cholinergic, and autonomous systems has benefited from multiple advances in recent years, useful for the early diagnosis and the pathophysiology of nonmotor symptoms in parkinsonian disorders. In particular, PET tracers for DAT as 11C-PE2I and 18F-PE2I have demonstrated their utility to follow dopaminergic degeneration along time, which can be evaluated in neuroprotection trials. Furthermore, novel radiotracers are currently under development, such as dopaminergic D3 selective radiotracers [111], higher-affinity DAT tracers useful for extrastriatal DAT imaging such as 18F-PR04.MZ [5][112], but also cholinergic imaging such as for 18F-FDEX, a non-subtype selective tracer of muscarinic receptors evaluated in human [113][114] and A2A (adenosine) receptors with 18F-TOZ1 [115]. Most interesting is the development of radiotracers binding to LRRK2, including 18F-FMN3PA, 18F-FMN3PU, and 11C-GNE1023 [116][117]. PET imaging of the opioid system, using radiotracers selective to receptor subtypes [118], and of endocannabinoids, as with 11C-CURB binding to the endocannabinoid enzyme fatty acid amide hydrolase [119], may also prove critical for the understanding of non-motor symptoms in PD and related disorders.
References
- Peralta, C.; Strafella, A.P.; van Eimeren, T.; Ceravolo, R.; Seppi, K.; Kaasinen, V.; Arena, J.E.; Lehericy, S.; International Parkinson Movement Disorders Society-Neuroimaging Study Group. Pragmatic Approach on Neuroimaging Techniques for the Differential Diagnosis of Parkinsonisms. Mov. Disord. Clin. Pract. 2022, 9, 6–19.
- Strafella, A.P.; Bohnen, N.I.; Perlmutter, J.S.; Eidelberg, D.; Pavese, N.; Van Eimeren, T.; Piccini, P.; Politis, M.; Thobois, S.; Ceravolo, R.; et al. Molecular Imaging to Track Parkinson’s Disease and Atypical Parkinsonisms: New Imaging Frontiers. Mov. Disord. 2017, 32, 181–192.
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I.; Brooks, D.J.; Darcourt, J.; Dickson, J.C.; Douglas, D.; et al. EANM Practice Guideline/SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912.
- Kong, Y.; Zhang, C.; Liu, K.; Wagle Shukla, A.; Sun, B.; Guan, Y. Imaging of Dopamine Transporters in Parkinson Disease: A Meta-Analysis of 18F/123I-FP-CIT Studies. Ann. Clin. Transl. Neurol. 2020, 7, 1524–1534.
- Kramer, V.; Juri, C.; Riss, P.J.; Pruzzo, R.; Soza-Ried, C.; Flores, J.; Hurtado, A.; Rösch, F.; Chana-Cuevas, P.; Amaral, H. Pharmacokinetic Evaluation of PR04.MZ for PET/CT Imaging and Quantification of Dopamine Transporters in the Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1927–1937.
- Zhao, C.; Liu, C.; Tang, J.; Xu, Y.; Xie, M.; Chen, Z. An Efficient Automated Radiosynthesis and Bioactivity Confirmation of VMAT2 Tracer FP-(+)-DTBZ. Mol. Imaging Biol. 2020, 22, 265–273.
- Delva, A.; Van Weehaeghe, D.; van Aalst, J.; Ceccarini, J.; Koole, M.; Baete, K.; Nuyts, J.; Vandenberghe, W.; Van Laere, K. Quantification and Discriminative Power of 18F-FE-PE2I PET in Patients with Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1913–1926.
- Yamamoto, Y.; Takahata, K.; Kubota, M.; Takano, H.; Takeuchi, H.; Kimura, Y.; Sano, Y.; Kurose, S.; Ito, H.; Mimura, M.; et al. Differential Associations of Dopamine Synthesis Capacity with the Dopamine Transporter and D2 Receptor Availability as Assessed by PET in the Living Human Brain. NeuroImage 2021, 226, 117543.
- Biondetti, E.; Gaurav, R.; Yahia-Cherif, L.; Mangone, G.; Pyatigorskaya, N.; Valabrègue, R.; Ewenczyk, C.; Hutchison, M.; François, C.; Arnulf, I.; et al. Spatiotemporal Changes in Substantia Nigra Neuromelanin Content in Parkinson’s Disease. Brain 2020, 143, 2757–2770.
- Uchida, Y.; Kan, H.; Sakurai, K.; Inui, S.; Kobayashi, S.; Akagawa, Y.; Shibuya, K.; Ueki, Y.; Matsukawa, N. Magnetic Susceptibility Associates With Dopaminergic Deficits and Cognition in Parkinson’s Disease. Mov. Disord. 2020, 35, 1396–1405.
- Martín-Bastida, A.; Lao-Kaim, N.P.; Roussakis, A.A.; Searle, G.E.; Xing, Y.; Gunn, R.N.; Schwarz, S.T.; Barker, R.A.; Auer, D.P.; Piccini, P. Relationship between Neuromelanin and Dopamine Terminals within the Parkinson’s Nigrostriatal System. Brain 2019, 142, 2023–2036.
- Yang, J.; Archer, D.B.; Burciu, R.G.; Müller, M.L.T.M.; Roy, A.; Ofori, E.; Bohnen, N.I.; Albin, R.L.; Vaillancourt, D.E. Multimodal Dopaminergic and Free-Water Imaging in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 62, 10–15.
- Fu, J.F.; Klyuzhin, I.; McKenzie, J.; Neilson, N.; Shahinfard, E.; Dinelle, K.; McKeown, M.J.; Stoessl, A.J.; Sossi, V. Joint Pattern Analysis Applied to PET DAT and VMAT2 Imaging Reveals New Insights into Parkinson’s Disease Induced Presynaptic Alterations. NeuroImage Clin. 2019, 23, 101856.
- Roussakis, A.-A.; Zeng, Z.; Lao-Kaim, N.P.; Martin-Bastida, A.; Piccini, P.; Barker, R.A.; Farrell, K.; Guzman, N.V.; He, X.; Lazic, S.E.; et al. Parkinson’s Disease Laterality: A 11C-PE2I PET Imaging Study. J. Neurol. 2021, 268, 582–589.
- Lee, M.J.; Pak, K.; Kim, H.-K.; Nudelman, K.N.; Kim, J.H.; Kim, Y.H.; Kang, J.; Baek, M.S.; Lyoo, C.H. Genetic Factors Affecting Dopaminergic Deterioration during the Premotor Stage of Parkinson Disease. Npj Park. Dis. 2021, 7, 104.
- Adams, M.P.; Yang, B.; Rahmim, A.; Tang, J. Prediction of Outcome in Parkinson’s Disease Patients from DAT SPECT Images Using a Convolutional Neural Network. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, NSW, Australia, 10–17 November 2018; pp. 1–4.
- Salmanpour, M.R.; Shamsaei, M.; Saberi, A.; Klyuzhin, I.S.; Tang, J.; Sossi, V.; Rahmim, A. Machine Learning Methods for Optimal Prediction of Motor Outcome in Parkinson’s Disease. Phys. Med. 2020, 69, 233–240.
- Tang, J.; Yang, B.; Adams, M.P.; Shenkov, N.N.; Klyuzhin, I.S.; Fotouhi, S.; Davoodi-Bojd, E.; Lu, L.; Soltanian-Zadeh, H.; Sossi, V.; et al. Artificial Neural Network-Based Prediction of Outcome in Parkinson’s Disease Patients Using DaTscan SPECT Imaging Features. Mol. Imaging Biol. 2019, 21, 1165–1173.
- Yousaf, T.; Pagano, G.; Niccolini, F.; Politis, M. Predicting Cognitive Decline with Non-Clinical Markers in Parkinson’s Disease (PRECODE-2). J. Neurol. 2019, 266, 1203–1210.
- Ye, R.; Locascio, J.J.; Goodheart, A.E.; Quan, M.; Zhang, B.; Gomperts, S.N. Serum NFL Levels Predict Progression of Motor Impairment and Reduction in Putamen Dopamine Transporter Binding Ratios in de Novo Parkinson’s Disease: An 8-Year Longitudinal Study. Parkinsonism Relat. Disord. 2021, 85, 11–16.
- Boonstra, J.T.; Michielse, S.; Temel, Y.; Hoogland, G.; Jahanshahi, A. Neuroimaging Detectable Differences between Parkinson’s Disease Motor Subtypes: A Systematic Review. Mov. Disord. Clin. Pract. 2021, 8, 175–192.
- Chung, S.J.; Kim, H.-R.; Jung, J.H.; Lee, P.H.; Jeong, Y.; Sohn, Y.H. Identifying the Functional Brain Network of Motor Reserve in Early Parkinson’s Disease. Mov. Disord. 2020, 35, 577–586.
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise Increases Caudate Dopamine Release and Ventral Striatal Activation in Parkinson’s Disease. Mov. Disord. 2019, 34, 1891–1900.
- Sambin, S.; Lavisse, S.; Decaix, C.; Pyatigorskaya, N.; Mangone, G.; Valabrègue, R.; Arnulf, I.; Cormier, F.; Lesage, S.; Lehericy, S.; et al. Compensatory Mechanisms Nine Years Before Parkinson’s Disease Conversion in a LRRK2 R1441H Family. Mov. Disord. 2022, 37, 428–430.
- Simuni, T.; Uribe, L.; Cho, H.R.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; Singleton, A.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Non-Manifest LRRK2 and GBA Mutation Carriers in the Parkinson’s Progression Markers Initiative (PPMI): A Cross-Sectional Study. Lancet Neurol. 2020, 19, 71–80.
- Wile, D.J.; Agarwal, P.A.; Schulzer, M.; Mak, E.; Dinelle, K.; Shahinfard, E.; Vafai, N.; Hasegawa, K.; Zhang, J.; McKenzie, J.; et al. Serotonin and Dopamine Transporter PET Changes in the Premotor Phase of LRRK2 Parkinsonism: Cross-Sectional Studies. Lancet Neurol. 2017, 16, 351–359.
- Droby, A.; Artzi, M.; Lerman, H.; Hutchison, R.M.; Bashat, D.B.; Omer, N.; Gurevich, T.; Orr-Urtreger, A.; Cohen, B.; Cedarbaum, J.M.; et al. Aberrant Dopamine Transporter and Functional Connectivity Patterns in LRRK2 and GBA Mutation Carriers. Npj Park. Dis. 2022, 8, 20.
- Pont-Sunyer, C.; Tolosa, E.; Caspell-Garcia, C.; Coffey, C.; Alcalay, R.N.; Chan, P.; Duda, J.E.; Facheris, M.; Fernández-Santiago, R.; Marek, K.; et al. The Prodromal Phase of Leucine-Rich Repeat Kinase 2–Associated Parkinson Disease: Clinical and Imaging Studies. Mov. Disord. 2017, 32, 726–738.
- Lopez, G.; Eisenberg, D.P.; Gregory, M.D.; Ianni, A.M.; Grogans, S.E.; Masdeu, J.C.; Kim, J.; Groden, C.; Sidransky, E.; Berman, K.F. Longitudinal Positron Emission Tomography of Dopamine Synthesis in Subjects with GBA1 Mutations. Ann. Neurol. 2020, 87, 652–657.
- Sánchez-Rodríguez, A.; Martínez-Rodríguez, I.; Sánchez-Juan, P.; Sierra, M.; González-Aramburu, I.; Rivera-Sánchez, M.; Andrés-Pacheco, J.; Gutierrez-González, Á.; García-Hernández, A.; Madera, J.; et al. Serial DaT-SPECT Imaging in Asymptomatic Carriers of LRRK2 G2019S Mutation: 8 Years’ Follow-Up. Eur. J. Neurol. 2021, 28, 4204–4208.
- Sierra, M.; Martínez-Rodríguez, I.; Sánchez-Juan, P.; González-Aramburu, I.; Jiménez-Alonso, M.; Sánchez-Rodríguez, A.; Berciano, J.; Banzo, I.; Infante, J. Prospective Clinical and DaT-SPECT Imaging in Premotor LRRK2 G2019S-Associated Parkinson Disease. Neurology 2017, 89, 439–444.
- Simuni, T.; Brumm, M.C.; Uribe, L.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Alcalay, R.N.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Leucine Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s Disease Participants in the Parkinson’s Progression Markers Initiative: A Cross-Sectional Study. Mov. Disord. 2020, 35, 833–844.
- Greuel, A.; Trezzi, J.-P.; Glaab, E.; Ruppert, M.C.; Maier, F.; Jäger, C.; Hodak, Z.; Lohmann, K.; Ma, Y.; Eidelberg, D.; et al. GBA Variants in Parkinson’s Disease: Clinical, Metabolomic, and Multimodal Neuroimaging Phenotypes. Mov. Disord. 2020, 35, 2201–2210.
- Schindlbeck, K.A.; Vo, A.; Nguyen, N.; Tang, C.C.; Niethammer, M.; Dhawan, V.; Brandt, V.; Saunders-Pullman, R.; Bressman, S.B.; Eidelberg, D. LRRK2 and GBA Variants Exert Distinct Influences on Parkinson’s Disease-Specific Metabolic Networks. Cereb. Cortex 2020, 30, 2867–2878.
- Pak, K.; Lee, M.J.; Kim, K.; Kim, I.J. No Effect of Parkinson’s Disease-Polygenic Load on Striatal Density of Dopaminergic Neuron in Healthy Subjects. Ann. Nucl. Med. 2021, 35, 1187–1192.
- Shin, S.; Kim, K.; Lee, J.M.; Kim, E.J.; Kim, S.J.; Kim, I.J.; Pak, K.; Lee, M.J. Effect of Single-Nucleotide Polymorphisms on Decline of Dopamine Transporter Availability in Parkinson’s Disease. J. Clin. Neurol. Seoul Korea 2019, 15, 102–107.
- Tan, M.M.X.; Lawton, M.A.; Jabbari, E.; Reynolds, R.H.; Iwaki, H.; Blauwendraat, C.; Kanavou, S.; Pollard, M.I.; Hubbard, L.; Malek, N.; et al. Genome-Wide Association Studies of Cognitive and Motor Progression in Parkinson’s Disease. Mov. Disord. 2021, 36, 424–433.
- Valli, M.; Cho, S.S.; Masellis, M.; Chen, R.; Rusjan, P.; Kim, J.; Koshimori, Y.; Mihaescu, A.; Strafella, A.P. DRD2 Genotype-Based Variants Modulates D2 Receptor Distribution in Ventral Striatum. Mol. Neurobiol. 2019, 56, 6512–6520.
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B. Update of the MDS Research Criteria for Prodromal Parkinson’s Disease. Mov. Disord. 2019, 34, 1464–1470.
- Arnaldi, D.; Chincarini, A.; Hu, M.T.; Sonka, K.; Boeve, B.; Miyamoto, T.; Puligheddu, M.; De Cock, V.C.; Terzaghi, M.; Plazzi, G.; et al. Dopaminergic Imaging and Clinical Predictors for Phenoconversion of REM Sleep Behaviour Disorder. Brain 2021, 144, 278–287.
- Chahine, L.M.; Brumm, M.C.; Caspell-Garcia, C.; Oertel, W.; Mollenhauer, B.; Amara, A.; Fernandez-Arcos, A.; Tolosa, E.; Simonet, C.; Hogl, B.; et al. Dopamine Transporter Imaging Predicts Clinically-Defined α-Synucleinopathy in REM Sleep Behavior Disorder. Ann. Clin. Transl. Neurol. 2021, 8, 201–212.
- Ganapathy, S.R.; Levová, K.; Kotačková, L.; Trnka, J.; Zogala, D.; Rusz, J.; Zima, T.; Devos, D.; Šonka, K.; Růžička, E.; et al. Increased Transferrin Sialylation Predicts Phenoconversion in Isolated REM Sleep Behavior Disorder. Mov. Disord. 2022, 37, 983–992.
- Miyamoto, T.; Miyamoto, M.; Numahata, K.; Onoue, H.; Akaiwa, Y.; Sairenchi, T. Reduced Dopamine Transporter Binding Predicts Early Transition to Lewy Body Disease in Japanese Patients with Idiopathic Rapid Eye Movement Sleep Behavior Disorder. J. Neurol. Sci. 2020, 414, 116821.
- Siderowf, A.; Jennings, D.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K.; PARS Investigators. Clinical and Imaging Progression in the PARS Cohort: Long-Term Follow-Up. Mov. Disord. 2020, 35, 1550–1557.
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and Predictors of Dementia and Parkinsonism in Idiopathic REM Sleep Behaviour Disorder: A Multicentre Study. Brain 2019, 142, 744–759.
- Janzen, A.; Kogan, R.V.; Meles, S.K.; Sittig, E.; Renken, R.J.; Geibl, F.F.; Booij, J.; Stormezand, G.; Luster, M.; Mayer, G.; et al. Rapid Eye Movement Sleep Behavior Disorder: Abnormal Cardiac Image and Progressive Abnormal Metabolic Brain Pattern. Mov. Disord. 2022, 37, 624–629.
- Kogan, R.V.; Janzen, A.; Meles, S.K.; Sittig, E.; Renken, R.J.; Gurvits, V.; Mayer, G.; Leenders, K.L.; Oertel, W.H.; REMPET Working Group. Four-Year Follow-up of Fluorodeoxyglucose Positron Emission Tomography–Based Parkinson’s Disease–Related Pattern Expression in 20 Patients with Isolated Rapid Eye Movement Sleep Behavior Disorder Shows Prodromal Progression. Mov. Disord. 2021, 36, 230–235.
- Barber, T.R.; Griffanti, L.; Bradley, K.M.; McGowan, D.R.; Lo, C.; Mackay, C.E.; Hu, M.T.; Klein, J.C. Nigrosome 1 Imaging in REM Sleep Behavior Disorder and Its Association with Dopaminergic Decline. Ann. Clin. Transl. Neurol. 2020, 7, 26–35.
- Kazmi, H.; Walker, Z.; Booij, J.; Khan, F.; Shah, S.; Sudre, C.H.; Buckman, J.E.J.; Schrag, A.-E. Late Onset Depression: Dopaminergic Deficit and Clinical Features of Prodromal Parkinson’s Disease: A Cross-Sectional Study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 158–164.
- Joling, M.; Vriend, C.; Raijmakers, P.G.H.M.; van der Zande, J.J.; Lemstra, A.W.; Berendse, H.W.; Booij, J.; van den Heuvel, O.A. Striatal DAT and Extrastriatal SERT Binding in Early-Stage Parkinson’s Disease and Dementia with Lewy Bodies, Compared with Healthy Controls: An 123I-FP-CIT SPECT Study. NeuroImage Clin. 2019, 22, 101755.
- Kang, S.W.; Jeon, S.; Lee, Y.; Park, M.; Baik, K.; Jung, J.H.; Chung, S.J.; Yoo, H.S.; Jeong, S.H.; Yun, M.; et al. Implication of Metabolic and Dopamine Transporter PET in Dementia with Lewy Bodies. Sci. Rep. 2021, 11, 14394.
- Sakakibara, S.; Hashimoto, R.; Katayama, T.; Kenjyo, M.; Yokokawa, Y.; Saito, Y.; Hirakawa, A.; Ito, M.; Nakamura, T.; Hara, K.; et al. Longitudinal Change of DAT SPECT in Parkinson’s Disease and Multiple System Atrophy. J. Park. Dis. 2020, 10, 123–130.
- Vergnet, S.; Hives, F.; Foubert-Samier, A.; Payoux, P.; Fernandez, P.; Meyer, M.; Dupouy, J.; Brefel-Courbon, C.; Ory-Magne, F.; Rascol, O.; et al. Dopamine Transporter Imaging for the Diagnosis of Multiple System Atrophy Cerebellar Type. Parkinsonism Relat. Disord. 2019, 63, 199–203.
- Kaasinen, V.; Vahlberg, T.; Stoessl, A.J.; Strafella, A.P.; Antonini, A. Dopamine Receptors in Parkinson’s Disease: A Meta-Analysis of Imaging Studies. Mov. Disord. 2021, 36, 1781–1791.
- Shigekiyo, T.; Arawaka, S. Laterality of Specific Binding Ratios on DAT-SPECT for Differential Diagnosis of Degenerative Parkinsonian Syndromes. Sci. Rep. 2020, 10, 15761.
- Takahashi, R.; Ishii, K.; Sousa, K.; Marumoto, K.; Kashibayashi, T.; Fujita, J.; Yokoyama, K. Distinctive Regional Asymmetry in Dopaminergic and Serotoninergic Dysfunction in Degenerative Parkinsonisms. J. Neurol. Sci. 2021, 423, 117363.
- Yoo, H.S.; Lee, S.; Chung, S.J.; Lee, Y.H.; Lee, P.H.; Sohn, Y.H.; Lee, S.; Yun, M.; Ye, B.S. Dopaminergic Depletion, β-Amyloid Burden, and Cognition in Lewy Body Disease. Ann. Neurol. 2020, 87, 739–750.
- Isaacson, J.R.; Brillman, S.; Chhabria, N.; Isaacson, S.H. Impact of DaTscan Imaging on Clinical Decision Making in Clinically Uncertain Parkinson’s Disease. J. Park. Dis. 2021, 11, 885–889.
- Thobois, S.; Prange, S.; Scheiber, C.; Broussolle, E. What a Neurologist Should Know about PET and SPECT Functional Imaging for Parkinsonism: A Practical Perspective. Parkinsonism Relat. Disord. 2019, 59, 93–100.
- Verger, A.; Grimaldi, S.; Ribeiro, M.-J.; Frismand, S.; Guedj, E. Single Photon Emission Computed Tomography/Positron Emission Tomography Molecular Imaging for Parkinsonism: A Fast-Developing Field. Ann. Neurol. 2021, 90, 711–719.
- Schmitz-Steinkrüger, H.; Lange, C.; Apostolova, I.; Amthauer, H.; Lehnert, W.; Klutmann, S.; Buchert, R. Impact of the Size of the Normal Database on the Performance of the Specific Binding Ratio in Dopamine Transporter SPECT. EJNMMI Phys. 2020, 7, 34.
- Schmitz-Steinkrüger, H.; Lange, C.; Apostolova, I.; Mathies, F.L.; Frings, L.; Klutmann, S.; Hellwig, S.; Meyer, P.T.; Buchert, R. Impact of Age and Sex Correction on the Diagnostic Performance of Dopamine Transporter SPECT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1445–1459.
- Piccardo, A.; Cappuccio, R.; Bottoni, G.; Cecchin, D.; Mazzella, L.; Cirone, A.; Righi, S.; Ugolini, M.; Bianchi, P.; Bertolaccini, P.; et al. The Role of the Deep Convolutional Neural Network as an Aid to Interpreting Brain DOPA PET/CT in the Diagnosis of Parkinson’s Disease. Eur. Radiol. 2021, 31, 7003–7011.
- Wenzel, M.; Milletari, F.; Krüger, J.; Lange, C.; Schenk, M.; Apostolova, I.; Klutmann, S.; Ehrenburg, M.; Buchert, R. Automatic Classification of Dopamine Transporter SPECT: Deep Convolutional Neural Networks Can Be Trained to Be Robust with Respect to Variable Image Characteristics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2800–2811.
- Nicastro, N.; Wegrzyk, J.; Preti, M.G.; Fleury, V.; Van de Ville, D.; Garibotto, V.; Burkhard, P.R. Classification of Degenerative Parkinsonism Subtypes by Support-Vector-Machine Analysis and Striatal 123I-FP-CIT Indices. J. Neurol. 2019, 266, 1771–1781.
- Dotinga, M.; van Dijk, J.D.; Vendel, B.N.; Slump, C.H.; Portman, A.T.; van Dalen, J.A. Clinical Value of Machine Learning-Based Interpretation of I-123 FP-CIT Scans to Detect Parkinson’s Disease: A Two-Center Study. Ann. Nucl. Med. 2021, 35, 378–385.
- Nazari, M.; Kluge, A.; Apostolova, I.; Klutmann, S.; Kimiaei, S.; Schroeder, M.; Buchert, R. Data-Driven Identification of Diagnostically Useful Extrastriatal Signal in Dopamine Transporter SPECT Using Explainable AI. Sci. Rep. 2021, 11, 22932.
- Shiiba, T.; Arimura, Y.; Nagano, M.; Takahashi, T.; Takaki, A. Improvement of Classification Performance of Parkinson’s Disease Using Shape Features for Machine Learning on Dopamine Transporter Single Photon Emission Computed Tomography. PLoS ONE 2020, 15, e0228289.
- Magesh, P.R.; Myloth, R.D.; Tom, R.J. An Explainable Machine Learning Model for Early Detection of Parkinson’s Disease Using LIME on DaTSCAN Imagery. Comput. Biol. Med. 2020, 126, 104041.
- Manzanera, O.M.; Meles, S.K.; Leenders, K.L.; Renken, R.J.; Pagani, M.; Arnaldi, D.; Nobili, F.; Obeso, J.; Oroz, M.R.; Morbelli, S.; et al. Scaled Subprofile Modeling and Convolutional Neural Networks for the Identification of Parkinson’s Disease in 3D Nuclear Imaging Data. Int. J. Neural Syst. 2019, 29, 1950010.
- Shen, T.; Jiang, J.; Lin, W.; Ge, J.; Wu, P.; Zhou, Y.; Zuo, C.; Wang, J.; Yan, Z.; Shi, K. Use of Overlapping Group LASSO Sparse Deep Belief Network to Discriminate Parkinson’s Disease and Normal Control. Front. Neurosci. 2019, 13, 396.
- Arnaldi, D.; Mattioli, P.; Famà, F.; Girtler, N.; Brugnolo, A.; Pardini, M.; Donniaquio, A.; Massa, F.; Orso, B.; Raffa, S.; et al. Stratification Tools for Disease-Modifying Trials in Prodromal Synucleinopathy. Mov. Disord. 2022, 37, 52–61.
- Stephenson, D.; Hill, D.; Cedarbaum, J.M.; Tome, M.; Vamvakas, S.; Romero, K.; Conrado, D.J.; Dexter, D.T.; Seibyl, J.; Jennings, D.; et al. The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson’s Disease. J. Park. Dis. 2019, 9, 553–563.
- Massa, J.; Chahine, L.M. Revision of Diagnosis in Early Parkinsonism with Abnormal Dopamine Transporter Imaging. J. Park. Dis. 2019, 9, 327–334.
- Arnaldi, D.; Famà, F.; Girtler, N.; Brugnolo, A.; Pardini, M.; Mattioli, P.; Meli, R.; Massa, F.; Orso, B.; Sormani, M.P.; et al. Rapid Eye Movement Sleep Behavior Disorder: A Proof-of-Concept Neuroprotection Study for Prodromal Synucleinopathies. Eur. J. Neurol. 2021, 28, 1210–1217.
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088.
- Knudsen, K.; Fedorova, T.D.; Hansen, A.K.; Sommerauer, M.; Otto, M.; Svendsen, K.B.; Nahimi, A.; Stokholm, M.G.; Pavese, N.; Beier, C.P.; et al. In-Vivo Staging of Pathology in REM Sleep Behaviour Disorder: A Multimodality Imaging Case-Control Study. Lancet Neurol. 2018, 17, 618–628.
- Berg, D.; Borghammer, P.; Fereshtehnejad, S.-M.; Heinzel, S.; Horsager, J.; Schaeffer, E.; Postuma, R.B. Prodromal Parkinson Disease Subtypes—Key to Understanding Heterogeneity. Nat. Rev. Neurol. 2021, 17, 349–361.
- Knudsen, K.; Fedorova, T.D.; Horsager, J.; Andersen, K.B.; Skjærbæk, C.; Berg, D.; Schaeffer, E.; Brooks, D.J.; Pavese, N.; Van Den Berge, N.; et al. Asymmetric Dopaminergic Dysfunction in Brain-First versus Body-First Parkinson’s Disease Subtypes. J. Park. Dis. 2021, 11, 1677–1687.
- Valli, M.; Cho, S.S.; Uribe, C.; Masellis, M.; Chen, R.; Mihaescu, A.; Strafella, A.P. VMAT2 Availability in Parkinson’s Disease with Probable REM Sleep Behaviour Disorder. Mol. Brain 2021, 14, 165.
- Valli, M.; Cho, S.S.; Masellis, M.; Chen, R.; Koshimori, Y.; Diez-Cirarda, M.; Mihaescu, A.; Christopher, L.; Strafella, A.P. Extra-Striatal Dopamine in Parkinson’s Disease with Rapid Eye Movement Sleep Behavior Disorder. J. Neurosci. Res. 2021, 99, 1177–1187.
- Pasquini, J.; Ceravolo, R.; Brooks, D.J.; Bonuccelli, U.; Pavese, N. Progressive Loss of Raphe Nuclei Serotonin Transporter in Early Parkinson’s Disease: A Longitudinal 123I-FP-CIT SPECT Study. Parkinsonism Relat. Disord. 2020, 77, 170–175.
- Wilson, H.; Dervenoulas, G.; Pagano, G.; Koros, C.; Yousaf, T.; Picillo, M.; Polychronis, S.; Simitsi, A.; Giordano, B.; Chappell, Z.; et al. Serotonergic Pathology and Disease Burden in the Premotor and Motor Phase of A53T α-Synuclein Parkinsonism: A Cross-Sectional Study. Lancet Neurol. 2019, 18, 748–759.
- Sampedro, F.; Marín-Lahoz, J.; Martínez-Horta, S.; Camacho, V.; Lopez-Mora, D.-A.; Pagonabarraga, J.; Kulisevsky, J. Extrastriatal SPECT-DAT Uptake Correlates with Clinical and Biological Features of de Novo Parkinson’s Disease. Neurobiol. Aging 2021, 97, 120–128.
- Fu, J.F.; Matarazzo, M.; McKenzie, J.; Neilson, N.; Vafai, N.; Dinelle, K.; Felicio, A.C.; McKeown, M.J.; Stoessl, A.J.; Sossi, V. Serotonergic System Impacts Levodopa Response in Early Parkinson’s and Future Risk of Dyskinesia. Mov. Disord. 2021, 36, 389–397.
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Bars, D.L.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The Prominent Role of Serotonergic Degeneration in Apathy, Anxiety and Depression in de Novo Parkinson’s Disease. Brain 2016, 139, 2486–2502.
- Maillet, A.; Météreau, E.; Tremblay, L.; Favre, E.; Klinger, H.; Lhommée, E.; Le Bars, D.; Castrioto, A.; Prange, S.; Sgambato, V.; et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Mov Disord 2021, 36, 2888–2900.
- Prange, S.; Metereau, E.; Maillet, A.; Klinger, H.; Schmitt, E.; Lhommée, E.; Bichon, A.; Lancelot, S.; Meoni, S.; Broussolle, E.; et al. Limbic Serotonergic Plasticity Contributes to the Compensation of Apathy in Early Parkinson’s Disease. Mov. Disord. 2022, 37, 1211–1221.
- Jørgensen, L.M.; Henriksen, T.; Mardosiene, S.; Keller, S.H.; Stenbæk, D.S.; Hansen, H.D.; Jespersen, B.; Thomsen, C.; Weikop, P.; Svarer, C.; et al. Parkinson Patients Have a Presynaptic Serotonergic Deficit: A Dynamic Deep Brain Stimulation PET Study. J. Cereb. Blood Flow Metab. 2021, 41, 1954–1963.
- Pilotto, A.; Schiano di Cola, F.; Premi, E.; Grasso, R.; Turrone, R.; Gipponi, S.; Scalvini, A.; Cottini, E.; Paghera, B.; Garibotto, V.; et al. Extrastriatal Dopaminergic and Serotonergic Pathways in Parkinson’s Disease and in Dementia with Lewy Bodies: A 123I-FP-CIT SPECT Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1642–1651.
- van der Zande, J.J.; Joling, M.; Happach, I.G.; Vriend, C.; Scheltens, P.; Booij, J.; Lemstra, A.W. Serotonergic Deficits in Dementia with Lewy Bodies with Concomitant Alzheimer’s Disease Pathology: An 123I-FP-CIT SPECT Study. NeuroImage Clin. 2020, 25, 102062.
- Joling, M.; Vriend, C.; van den Heuvel, O.A.; Raijmakers, P.G.H.M.; Jones, P.A.; Berendse, H.W.; Booij, J. Analysis of Extrastriatal 123I-FP-CIT Binding Contributes to the Differential Diagnosis of Parkinsonian Diseases. J. Nucl. Med. 2017, 58, 1117–1123.
- Meyer, M.; Lamare, F.; Asselineau, J.; Foubert-Samier, A.; Mazère, J.; Zanotti-Fregonara, P.; Rizzo, G.; Delamarre, A.; Spampinato, U.; Rascol, O.; et al. Brain 5-HT1A Receptor Binding in Multiple System Atrophy: An -MPPF PET Study. Mov. Disord. 2021, 36, 246–251.
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent Advances in Radiotracers Targeting Norepinephrine Transporter: Structural Development and Radiolabeling Improvements. J. Neural Transm. Vienna Austria 1996 2020, 127, 851–873.
- Laurencin, C.; Lancelot, S.; Gobert, F.; Redouté, J.; Mérida, I.; Iecker, T.; Liger, F.; Irace, Z.; Greusard, E.; Lamberet, L.; et al. Modeling Yohimbine PET Human Brain Kinetics with Test-Retest Reliability, Competition Sensitivity Studies and Search for a Suitable Reference Region. NeuroImage 2021, 240, 118328.
- Doppler, C.E.J.; Kinnerup, M.B.; Brune, C.; Farrher, E.; Betts, M.; Fedorova, T.D.; Schaldemose, J.L.; Knudsen, K.; Ismail, R.; Seger, A.D.; et al. Regional Locus Coeruleus Degeneration Is Uncoupled from Noradrenergic Terminal Loss in Parkinson’s Disease. Brain 2021, 144, 2732–2744.
- Doppler, C.E.J.; Smit, J.A.M.; Hommelsen, M.; Seger, A.; Horsager, J.; Kinnerup, M.B.; Hansen, A.K.; Fedorova, T.D.; Knudsen, K.; Otto, M.; et al. Microsleep Disturbances Are Associated with Noradrenergic Dysfunction in Parkinson’s Disease. Sleep 2021, 44, zsab040.
- Kinnerup, M.B.; Sommerauer, M.; Damholdt, M.F.; Schaldemose, J.L.; Ismail, R.; Terkelsen, A.J.; Stær, K.; Hansen, A.; Fedorova, T.D.; Knudsen, K.; et al. Preserved Noradrenergic Function in Parkinson’s Disease Patients with Rest Tremor. Neurobiol. Dis. 2021, 152, 105295.
- Andersen, K.B.; Hansen, A.K.; Sommerauer, M.; Fedorova, T.D.; Knudsen, K.; Vang, K.; Van Den Berge, N.; Kinnerup, M.; Nahimi, A.; Pavese, N.; et al. Altered Sensorimotor Cortex Noradrenergic Function in Idiopathic REM Sleep Behaviour Disorder—A PET Study. Parkinsonism Relat. Disord. 2020, 75, 63–69.
- Lenka, A.; Lamotte, G.; Goldstein, D.S. Cardiac 18F-Dopamine PET Distinguishes PD with Orthostatic Hypotension from Parkinsonian MSA. Mov. Disord. Clin. Pract. 2021, 8, 582–586.
- Kuten, J.; Linevitz, A.; Lerman, H.; Freedman, N.; Kestenbaum, M.; Shiner, T.; Giladi, N.; Even-Sapir, E. FDOPA PET May Confirm the Clinical Diagnosis of Parkinson’s Disease by Imaging the Nigro-Striatal Pathway and the Sympathetic Cardiac Innervation: Proof-of-Concept Study. J. Integr. Neurosci. 2020, 19, 489–494.
- van der Zee, S.; Vállez García, D.; Elsinga, P.H.; Willemsen, A.T.M.; Boersma, H.H.; Gerritsen, M.J.J.; Spikman, J.M.; van Laar, T. Fluoroethoxybenzovesamicol in Parkinson’s Disease Patients: Quantification of a Novel Cholinergic Positron Emission Tomography Tracer. Mov. Disord. 2019, 34, 924–926.
- Bohnen, N.I.; Kanel, P.; Zhou, Z.; Koeppe, R.A.; Frey, K.A.; Dauer, W.T.; Albin, R.L.; Müller, M.L.T.M. Cholinergic System Changes of Falls and Freezing of Gait in Parkinson’s Disease. Ann. Neurol. 2019, 85, 538–549.
- van der Zee, S.; Müller, M.L.T.M.; Kanel, P.; van Laar, T.; Bohnen, N.I. Cholinergic Denervation Patterns Across Cognitive Domains in Parkinson’s Disease. Mov. Disord. 2021, 36, 642–650.
- Pasquini, J.; Brooks, D.J.; Pavese, N. The Cholinergic Brain in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 1012–1026.
- Bohnen, N.I.; Kanel, P.; Müller, M.L.T.M. Molecular Imaging of the Cholinergic System in Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 211–250. ISBN 978-0-12-815418-2.
- van der Zee, S.; Kanel, P.; Gerritsen, M.J.J.; Boertien, J.M.; Slomp, A.C.; Müller, M.L.T.M.; Bohnen, N.I.; Spikman, J.M.; van Laar, T. Altered Cholinergic Innervation in De Novo Parkinson’s Disease with and Without Cognitive Impairment. Mov. Disord. 2022, 37, 713–723.
- Bedard, M.-A.; Aghourian, M.; Legault-Denis, C.; Postuma, R.B.; Soucy, J.-P.; Gagnon, J.-F.; Pelletier, A.; Montplaisir, J. Brain Cholinergic Alterations in Idiopathic REM Sleep Behaviour Disorder: A PET Imaging Study with 18F-FEOBV. Sleep Med. 2019, 58, 35–41.
- Gersel Stokholm, M.; Iranzo, A.; Østergaard, K.; Serradell, M.; Otto, M.; Bacher Svendsen, K.; Garrido, A.; Vilas, D.; Fedorova, T.D.; Santamaria, J.; et al. Cholinergic Denervation in Patients with Idiopathic Rapid Eye Movement Sleep Behaviour Disorder. Eur. J. Neurol. 2020, 27, 644–652.
- Kanel, P.; Müller, M.L.T.M.; van der Zee, S.; Sanchez-Catasus, C.A.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Topography of Cholinergic Changes in Dementia With Lewy Bodies and Key Neural Network Hubs. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 370–375.
- Ji, L.; Fang, Y.; Tang, J.; Liu, C.; Huang, C.; Hu, Q.; Li, Q.; Chen, Z. Synthesis and Biological Evaluation of 18F-Labelled Dopamine D3 Receptor Selective Ligands. Bioorg. Med. Chem. Lett. 2022, 62, 128630.
- Lehnert, W.; Riss, P.J.; Hurtado de Mendoza, A.; Lopez, S.; Fernandez, G.; Ilheu, M.; Amaral, H.; Kramer, V. Whole-Body Biodistribution and Radiation Dosimetry of PR04.MZ: A New PET Radiotracer for Clinical Management of Patients with Movement Disorders. EJNMMI Res. 2022, 12, 1.
- Pain, C.D.; O’Keefe, G.J.; Ackermann, U.; Dore, V.; Villemagne, V.L.; Rowe, C.C. Human Biodistribution and Internal Dosimetry of 4-Fluorobenzyl-Dexetimide: A PET Radiopharmaceutical for Imaging Muscarinic Acetylcholine Receptors in the Brain and Heart. EJNMMI Res. 2020, 10, 61.
- Rowe, C.C.; Krishnadas, N.; Ackermann, U.; Doré, V.; Goh, R.Y.W.; Guzman, R.; Chong, L.; Bozinovski, S.; Mulligan, R.; Kanaan, R.; et al. PET Imaging of Brain Muscarinic Receptors with 18F-Fluorobenzyl-Dexetimide: A First in Human Study. Psychiatry Res. Neuroimaging 2021, 316, 111354.
- Lai, T.H.; Toussaint, M.; Teodoro, R.; Dukić-Stefanović, S.; Kranz, M.; Deuther-Conrad, W.; Moldovan, R.-P.; Brust, P. Synthesis and Biological Evaluation of a Novel 18F-Labeled Radiotracer for PET Imaging of the Adenosine A2A Receptor. Int. J. Mol. Sci. 2021, 22, 1182.
- Chen, X.; Zhang, Q.; Zhang, Y.; Fang, J.; Jiang, D.; Mou, Z.; Liu, H.; Su, R.; Wang, C.; He, F.; et al. 18F-Labelled Pyrrolopyrimidines Reveal Brain Leucine-Rich Repeat Kinase 2 Expression Implicated in Parkinson’s Disease. Eur. J. Med. Chem. 2021, 214, 113245.
- Malik, N.; Kornelsen, R.; McCormick, S.; Colpo, N.; Merkens, H.; Bendre, S.; Benard, F.; Sossi, V.; Schirrmacher, R.; Schaffer, P. Development and Biological Evaluation ofFMN3PA & FMN3PU for Leucine-Rich Repeat Kinase 2 (LRRK2) in Vivo PET Imaging. Eur. J. Med. Chem. 2021, 211, 113005.
- Thobois, S.; Brefel-Courbon, C.; Le Bars, D.; Sgambato-Faure, V. Molecular Imaging of Opioid System in Idiopathic Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 275–303. ISBN 978-0-12-815418-2.
- Green, D.G.J.; Kim, J.; Kish, S.J.; Tyndale, R.F.; Hill, M.N.; Strafella, A.P.; Tong, J.; McCluskey, T.; Westwood, D.J.; Houle, S.; et al. Fatty Acid Amide Hydrolase Binding Is Inversely Correlated with Amygdalar Functional Connectivity: A Combined Positron Emission Tomography and Magnetic Resonance Imaging Study in Healthy Individuals. J. Psychiatry Neurosci. 2021, 46, E238–E246.
More
Information
Subjects:
Neuroimaging
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
21 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




