Neurodegenerative parkinsonian disorders are characterized by a great diversity of clinical symptoms and underlying neuropathology, yet differential diagnosis during lifetime remains probabilistic. Molecular imaging is a powerful method to detect pathological changes in vivo on a cellular and molecular level with high specificity. Thereby, molecular imaging enables to investigate functional changes and pathological hallmarks in neurodegenerative disorders, thus allowing to better differentiate between different forms of degenerative parkinsonism, improve the accuracy of the clinical diagnosis and disentangle the pathophysiology of disease-related symptoms.
- PET
- SPECT
- Parkinson’s disease
- atypical parkinsonism
- neurodegeneration
- monoaminergic neurotransmission
1. Introduction
2. Neurotransmitter Imaging in Parkinsonian Disorders
2.1. Dopaminergic Imaging
2.1.1. Imaging the Dopaminergic System
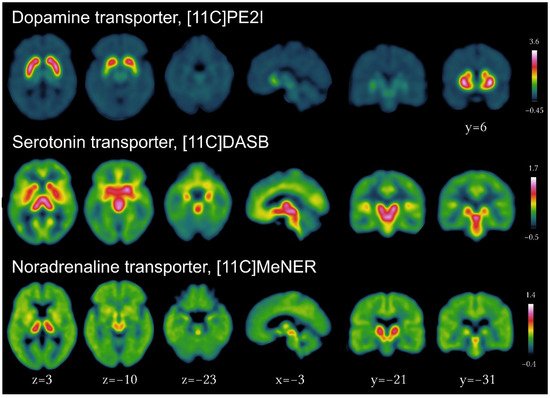
2.1.2. Dopaminergic Imaging in PD
2.1.3. Dopaminergic Imaging in Genetic PD
2.1.4. Dopaminergic Imaging in Prodromal PD
2.1.5. Dopaminergic Imaging in Atypical Parkinsonian Disorders
2.1.6. Differential Diagnosis Using Dopaminergic Imaging and Use of Machine Learning
2.1.7. Implications of Advances in Dopaminergic Imaging
2.2. Imaging of Peripheral Neurotransmitter in Parkinsonian Disorders
2.3. Serotonergic Imaging in PD and Parkinsonian Disorders
2.4. Noradrenergic Imaging in PD and RBD
2.5. Cholinergic Imaging in PD
This entry is adapted from the peer-reviewed paper 10.3390/brainsci12091146
References
- Peralta, C.; Strafella, A.P.; van Eimeren, T.; Ceravolo, R.; Seppi, K.; Kaasinen, V.; Arena, J.E.; Lehericy, S.; International Parkinson Movement Disorders Society-Neuroimaging Study Group. Pragmatic Approach on Neuroimaging Techniques for the Differential Diagnosis of Parkinsonisms. Mov. Disord. Clin. Pract. 2022, 9, 6–19.
- Strafella, A.P.; Bohnen, N.I.; Perlmutter, J.S.; Eidelberg, D.; Pavese, N.; Van Eimeren, T.; Piccini, P.; Politis, M.; Thobois, S.; Ceravolo, R.; et al. Molecular Imaging to Track Parkinson’s Disease and Atypical Parkinsonisms: New Imaging Frontiers. Mov. Disord. 2017, 32, 181–192.
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I.; Brooks, D.J.; Darcourt, J.; Dickson, J.C.; Douglas, D.; et al. EANM Practice Guideline/SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912.
- Kong, Y.; Zhang, C.; Liu, K.; Wagle Shukla, A.; Sun, B.; Guan, Y. Imaging of Dopamine Transporters in Parkinson Disease: A Meta-Analysis of 18F/123I-FP-CIT Studies. Ann. Clin. Transl. Neurol. 2020, 7, 1524–1534.
- Kramer, V.; Juri, C.; Riss, P.J.; Pruzzo, R.; Soza-Ried, C.; Flores, J.; Hurtado, A.; Rösch, F.; Chana-Cuevas, P.; Amaral, H. Pharmacokinetic Evaluation of PR04.MZ for PET/CT Imaging and Quantification of Dopamine Transporters in the Human Brain. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1927–1937.
- Zhao, C.; Liu, C.; Tang, J.; Xu, Y.; Xie, M.; Chen, Z. An Efficient Automated Radiosynthesis and Bioactivity Confirmation of VMAT2 Tracer FP-(+)-DTBZ. Mol. Imaging Biol. 2020, 22, 265–273.
- Delva, A.; Van Weehaeghe, D.; van Aalst, J.; Ceccarini, J.; Koole, M.; Baete, K.; Nuyts, J.; Vandenberghe, W.; Van Laere, K. Quantification and Discriminative Power of 18F-FE-PE2I PET in Patients with Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1913–1926.
- Yamamoto, Y.; Takahata, K.; Kubota, M.; Takano, H.; Takeuchi, H.; Kimura, Y.; Sano, Y.; Kurose, S.; Ito, H.; Mimura, M.; et al. Differential Associations of Dopamine Synthesis Capacity with the Dopamine Transporter and D2 Receptor Availability as Assessed by PET in the Living Human Brain. NeuroImage 2021, 226, 117543.
- Biondetti, E.; Gaurav, R.; Yahia-Cherif, L.; Mangone, G.; Pyatigorskaya, N.; Valabrègue, R.; Ewenczyk, C.; Hutchison, M.; François, C.; Arnulf, I.; et al. Spatiotemporal Changes in Substantia Nigra Neuromelanin Content in Parkinson’s Disease. Brain 2020, 143, 2757–2770.
- Uchida, Y.; Kan, H.; Sakurai, K.; Inui, S.; Kobayashi, S.; Akagawa, Y.; Shibuya, K.; Ueki, Y.; Matsukawa, N. Magnetic Susceptibility Associates With Dopaminergic Deficits and Cognition in Parkinson’s Disease. Mov. Disord. 2020, 35, 1396–1405.
- Martín-Bastida, A.; Lao-Kaim, N.P.; Roussakis, A.A.; Searle, G.E.; Xing, Y.; Gunn, R.N.; Schwarz, S.T.; Barker, R.A.; Auer, D.P.; Piccini, P. Relationship between Neuromelanin and Dopamine Terminals within the Parkinson’s Nigrostriatal System. Brain 2019, 142, 2023–2036.
- Yang, J.; Archer, D.B.; Burciu, R.G.; Müller, M.L.T.M.; Roy, A.; Ofori, E.; Bohnen, N.I.; Albin, R.L.; Vaillancourt, D.E. Multimodal Dopaminergic and Free-Water Imaging in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 62, 10–15.
- Fu, J.F.; Klyuzhin, I.; McKenzie, J.; Neilson, N.; Shahinfard, E.; Dinelle, K.; McKeown, M.J.; Stoessl, A.J.; Sossi, V. Joint Pattern Analysis Applied to PET DAT and VMAT2 Imaging Reveals New Insights into Parkinson’s Disease Induced Presynaptic Alterations. NeuroImage Clin. 2019, 23, 101856.
- Roussakis, A.-A.; Zeng, Z.; Lao-Kaim, N.P.; Martin-Bastida, A.; Piccini, P.; Barker, R.A.; Farrell, K.; Guzman, N.V.; He, X.; Lazic, S.E.; et al. Parkinson’s Disease Laterality: A 11C-PE2I PET Imaging Study. J. Neurol. 2021, 268, 582–589.
- Lee, M.J.; Pak, K.; Kim, H.-K.; Nudelman, K.N.; Kim, J.H.; Kim, Y.H.; Kang, J.; Baek, M.S.; Lyoo, C.H. Genetic Factors Affecting Dopaminergic Deterioration during the Premotor Stage of Parkinson Disease. Npj Park. Dis. 2021, 7, 104.
- Adams, M.P.; Yang, B.; Rahmim, A.; Tang, J. Prediction of Outcome in Parkinson’s Disease Patients from DAT SPECT Images Using a Convolutional Neural Network. In Proceedings of the 2018 IEEE Nuclear Science Symposium and Medical Imaging Conference Proceedings (NSS/MIC), Sydney, NSW, Australia, 10–17 November 2018; pp. 1–4.
- Salmanpour, M.R.; Shamsaei, M.; Saberi, A.; Klyuzhin, I.S.; Tang, J.; Sossi, V.; Rahmim, A. Machine Learning Methods for Optimal Prediction of Motor Outcome in Parkinson’s Disease. Phys. Med. 2020, 69, 233–240.
- Tang, J.; Yang, B.; Adams, M.P.; Shenkov, N.N.; Klyuzhin, I.S.; Fotouhi, S.; Davoodi-Bojd, E.; Lu, L.; Soltanian-Zadeh, H.; Sossi, V.; et al. Artificial Neural Network-Based Prediction of Outcome in Parkinson’s Disease Patients Using DaTscan SPECT Imaging Features. Mol. Imaging Biol. 2019, 21, 1165–1173.
- Yousaf, T.; Pagano, G.; Niccolini, F.; Politis, M. Predicting Cognitive Decline with Non-Clinical Markers in Parkinson’s Disease (PRECODE-2). J. Neurol. 2019, 266, 1203–1210.
- Ye, R.; Locascio, J.J.; Goodheart, A.E.; Quan, M.; Zhang, B.; Gomperts, S.N. Serum NFL Levels Predict Progression of Motor Impairment and Reduction in Putamen Dopamine Transporter Binding Ratios in de Novo Parkinson’s Disease: An 8-Year Longitudinal Study. Parkinsonism Relat. Disord. 2021, 85, 11–16.
- Boonstra, J.T.; Michielse, S.; Temel, Y.; Hoogland, G.; Jahanshahi, A. Neuroimaging Detectable Differences between Parkinson’s Disease Motor Subtypes: A Systematic Review. Mov. Disord. Clin. Pract. 2021, 8, 175–192.
- Chung, S.J.; Kim, H.-R.; Jung, J.H.; Lee, P.H.; Jeong, Y.; Sohn, Y.H. Identifying the Functional Brain Network of Motor Reserve in Early Parkinson’s Disease. Mov. Disord. 2020, 35, 577–586.
- Sacheli, M.A.; Neva, J.L.; Lakhani, B.; Murray, D.K.; Vafai, N.; Shahinfard, E.; English, C.; McCormick, S.; Dinelle, K.; Neilson, N.; et al. Exercise Increases Caudate Dopamine Release and Ventral Striatal Activation in Parkinson’s Disease. Mov. Disord. 2019, 34, 1891–1900.
- Sambin, S.; Lavisse, S.; Decaix, C.; Pyatigorskaya, N.; Mangone, G.; Valabrègue, R.; Arnulf, I.; Cormier, F.; Lesage, S.; Lehericy, S.; et al. Compensatory Mechanisms Nine Years Before Parkinson’s Disease Conversion in a LRRK2 R1441H Family. Mov. Disord. 2022, 37, 428–430.
- Simuni, T.; Uribe, L.; Cho, H.R.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; Singleton, A.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Non-Manifest LRRK2 and GBA Mutation Carriers in the Parkinson’s Progression Markers Initiative (PPMI): A Cross-Sectional Study. Lancet Neurol. 2020, 19, 71–80.
- Wile, D.J.; Agarwal, P.A.; Schulzer, M.; Mak, E.; Dinelle, K.; Shahinfard, E.; Vafai, N.; Hasegawa, K.; Zhang, J.; McKenzie, J.; et al. Serotonin and Dopamine Transporter PET Changes in the Premotor Phase of LRRK2 Parkinsonism: Cross-Sectional Studies. Lancet Neurol. 2017, 16, 351–359.
- Droby, A.; Artzi, M.; Lerman, H.; Hutchison, R.M.; Bashat, D.B.; Omer, N.; Gurevich, T.; Orr-Urtreger, A.; Cohen, B.; Cedarbaum, J.M.; et al. Aberrant Dopamine Transporter and Functional Connectivity Patterns in LRRK2 and GBA Mutation Carriers. Npj Park. Dis. 2022, 8, 20.
- Pont-Sunyer, C.; Tolosa, E.; Caspell-Garcia, C.; Coffey, C.; Alcalay, R.N.; Chan, P.; Duda, J.E.; Facheris, M.; Fernández-Santiago, R.; Marek, K.; et al. The Prodromal Phase of Leucine-Rich Repeat Kinase 2–Associated Parkinson Disease: Clinical and Imaging Studies. Mov. Disord. 2017, 32, 726–738.
- Lopez, G.; Eisenberg, D.P.; Gregory, M.D.; Ianni, A.M.; Grogans, S.E.; Masdeu, J.C.; Kim, J.; Groden, C.; Sidransky, E.; Berman, K.F. Longitudinal Positron Emission Tomography of Dopamine Synthesis in Subjects with GBA1 Mutations. Ann. Neurol. 2020, 87, 652–657.
- Sánchez-Rodríguez, A.; Martínez-Rodríguez, I.; Sánchez-Juan, P.; Sierra, M.; González-Aramburu, I.; Rivera-Sánchez, M.; Andrés-Pacheco, J.; Gutierrez-González, Á.; García-Hernández, A.; Madera, J.; et al. Serial DaT-SPECT Imaging in Asymptomatic Carriers of LRRK2 G2019S Mutation: 8 Years’ Follow-Up. Eur. J. Neurol. 2021, 28, 4204–4208.
- Sierra, M.; Martínez-Rodríguez, I.; Sánchez-Juan, P.; González-Aramburu, I.; Jiménez-Alonso, M.; Sánchez-Rodríguez, A.; Berciano, J.; Banzo, I.; Infante, J. Prospective Clinical and DaT-SPECT Imaging in Premotor LRRK2 G2019S-Associated Parkinson Disease. Neurology 2017, 89, 439–444.
- Simuni, T.; Brumm, M.C.; Uribe, L.; Caspell-Garcia, C.; Coffey, C.S.; Siderowf, A.; Alcalay, R.N.; Trojanowski, J.Q.; Shaw, L.M.; Seibyl, J.; et al. Clinical and Dopamine Transporter Imaging Characteristics of Leucine Rich Repeat Kinase 2 (LRRK2) and Glucosylceramidase Beta (GBA) Parkinson’s Disease Participants in the Parkinson’s Progression Markers Initiative: A Cross-Sectional Study. Mov. Disord. 2020, 35, 833–844.
- Greuel, A.; Trezzi, J.-P.; Glaab, E.; Ruppert, M.C.; Maier, F.; Jäger, C.; Hodak, Z.; Lohmann, K.; Ma, Y.; Eidelberg, D.; et al. GBA Variants in Parkinson’s Disease: Clinical, Metabolomic, and Multimodal Neuroimaging Phenotypes. Mov. Disord. 2020, 35, 2201–2210.
- Schindlbeck, K.A.; Vo, A.; Nguyen, N.; Tang, C.C.; Niethammer, M.; Dhawan, V.; Brandt, V.; Saunders-Pullman, R.; Bressman, S.B.; Eidelberg, D. LRRK2 and GBA Variants Exert Distinct Influences on Parkinson’s Disease-Specific Metabolic Networks. Cereb. Cortex 2020, 30, 2867–2878.
- Pak, K.; Lee, M.J.; Kim, K.; Kim, I.J. No Effect of Parkinson’s Disease-Polygenic Load on Striatal Density of Dopaminergic Neuron in Healthy Subjects. Ann. Nucl. Med. 2021, 35, 1187–1192.
- Shin, S.; Kim, K.; Lee, J.M.; Kim, E.J.; Kim, S.J.; Kim, I.J.; Pak, K.; Lee, M.J. Effect of Single-Nucleotide Polymorphisms on Decline of Dopamine Transporter Availability in Parkinson’s Disease. J. Clin. Neurol. Seoul Korea 2019, 15, 102–107.
- Tan, M.M.X.; Lawton, M.A.; Jabbari, E.; Reynolds, R.H.; Iwaki, H.; Blauwendraat, C.; Kanavou, S.; Pollard, M.I.; Hubbard, L.; Malek, N.; et al. Genome-Wide Association Studies of Cognitive and Motor Progression in Parkinson’s Disease. Mov. Disord. 2021, 36, 424–433.
- Valli, M.; Cho, S.S.; Masellis, M.; Chen, R.; Rusjan, P.; Kim, J.; Koshimori, Y.; Mihaescu, A.; Strafella, A.P. DRD2 Genotype-Based Variants Modulates D2 Receptor Distribution in Ventral Striatum. Mol. Neurobiol. 2019, 56, 6512–6520.
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B. Update of the MDS Research Criteria for Prodromal Parkinson’s Disease. Mov. Disord. 2019, 34, 1464–1470.
- Arnaldi, D.; Chincarini, A.; Hu, M.T.; Sonka, K.; Boeve, B.; Miyamoto, T.; Puligheddu, M.; De Cock, V.C.; Terzaghi, M.; Plazzi, G.; et al. Dopaminergic Imaging and Clinical Predictors for Phenoconversion of REM Sleep Behaviour Disorder. Brain 2021, 144, 278–287.
- Chahine, L.M.; Brumm, M.C.; Caspell-Garcia, C.; Oertel, W.; Mollenhauer, B.; Amara, A.; Fernandez-Arcos, A.; Tolosa, E.; Simonet, C.; Hogl, B.; et al. Dopamine Transporter Imaging Predicts Clinically-Defined α-Synucleinopathy in REM Sleep Behavior Disorder. Ann. Clin. Transl. Neurol. 2021, 8, 201–212.
- Ganapathy, S.R.; Levová, K.; Kotačková, L.; Trnka, J.; Zogala, D.; Rusz, J.; Zima, T.; Devos, D.; Šonka, K.; Růžička, E.; et al. Increased Transferrin Sialylation Predicts Phenoconversion in Isolated REM Sleep Behavior Disorder. Mov. Disord. 2022, 37, 983–992.
- Miyamoto, T.; Miyamoto, M.; Numahata, K.; Onoue, H.; Akaiwa, Y.; Sairenchi, T. Reduced Dopamine Transporter Binding Predicts Early Transition to Lewy Body Disease in Japanese Patients with Idiopathic Rapid Eye Movement Sleep Behavior Disorder. J. Neurol. Sci. 2020, 414, 116821.
- Siderowf, A.; Jennings, D.; Stern, M.; Seibyl, J.; Eberly, S.; Oakes, D.; Marek, K.; PARS Investigators. Clinical and Imaging Progression in the PARS Cohort: Long-Term Follow-Up. Mov. Disord. 2020, 35, 1550–1557.
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and Predictors of Dementia and Parkinsonism in Idiopathic REM Sleep Behaviour Disorder: A Multicentre Study. Brain 2019, 142, 744–759.
- Janzen, A.; Kogan, R.V.; Meles, S.K.; Sittig, E.; Renken, R.J.; Geibl, F.F.; Booij, J.; Stormezand, G.; Luster, M.; Mayer, G.; et al. Rapid Eye Movement Sleep Behavior Disorder: Abnormal Cardiac Image and Progressive Abnormal Metabolic Brain Pattern. Mov. Disord. 2022, 37, 624–629.
- Kogan, R.V.; Janzen, A.; Meles, S.K.; Sittig, E.; Renken, R.J.; Gurvits, V.; Mayer, G.; Leenders, K.L.; Oertel, W.H.; REMPET Working Group. Four-Year Follow-up of Fluorodeoxyglucose Positron Emission Tomography–Based Parkinson’s Disease–Related Pattern Expression in 20 Patients with Isolated Rapid Eye Movement Sleep Behavior Disorder Shows Prodromal Progression. Mov. Disord. 2021, 36, 230–235.
- Barber, T.R.; Griffanti, L.; Bradley, K.M.; McGowan, D.R.; Lo, C.; Mackay, C.E.; Hu, M.T.; Klein, J.C. Nigrosome 1 Imaging in REM Sleep Behavior Disorder and Its Association with Dopaminergic Decline. Ann. Clin. Transl. Neurol. 2020, 7, 26–35.
- Kazmi, H.; Walker, Z.; Booij, J.; Khan, F.; Shah, S.; Sudre, C.H.; Buckman, J.E.J.; Schrag, A.-E. Late Onset Depression: Dopaminergic Deficit and Clinical Features of Prodromal Parkinson’s Disease: A Cross-Sectional Study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 158–164.
- Joling, M.; Vriend, C.; Raijmakers, P.G.H.M.; van der Zande, J.J.; Lemstra, A.W.; Berendse, H.W.; Booij, J.; van den Heuvel, O.A. Striatal DAT and Extrastriatal SERT Binding in Early-Stage Parkinson’s Disease and Dementia with Lewy Bodies, Compared with Healthy Controls: An 123I-FP-CIT SPECT Study. NeuroImage Clin. 2019, 22, 101755.
- Kang, S.W.; Jeon, S.; Lee, Y.; Park, M.; Baik, K.; Jung, J.H.; Chung, S.J.; Yoo, H.S.; Jeong, S.H.; Yun, M.; et al. Implication of Metabolic and Dopamine Transporter PET in Dementia with Lewy Bodies. Sci. Rep. 2021, 11, 14394.
- Sakakibara, S.; Hashimoto, R.; Katayama, T.; Kenjyo, M.; Yokokawa, Y.; Saito, Y.; Hirakawa, A.; Ito, M.; Nakamura, T.; Hara, K.; et al. Longitudinal Change of DAT SPECT in Parkinson’s Disease and Multiple System Atrophy. J. Park. Dis. 2020, 10, 123–130.
- Vergnet, S.; Hives, F.; Foubert-Samier, A.; Payoux, P.; Fernandez, P.; Meyer, M.; Dupouy, J.; Brefel-Courbon, C.; Ory-Magne, F.; Rascol, O.; et al. Dopamine Transporter Imaging for the Diagnosis of Multiple System Atrophy Cerebellar Type. Parkinsonism Relat. Disord. 2019, 63, 199–203.
- Kaasinen, V.; Vahlberg, T.; Stoessl, A.J.; Strafella, A.P.; Antonini, A. Dopamine Receptors in Parkinson’s Disease: A Meta-Analysis of Imaging Studies. Mov. Disord. 2021, 36, 1781–1791.
- Shigekiyo, T.; Arawaka, S. Laterality of Specific Binding Ratios on DAT-SPECT for Differential Diagnosis of Degenerative Parkinsonian Syndromes. Sci. Rep. 2020, 10, 15761.
- Takahashi, R.; Ishii, K.; Sousa, K.; Marumoto, K.; Kashibayashi, T.; Fujita, J.; Yokoyama, K. Distinctive Regional Asymmetry in Dopaminergic and Serotoninergic Dysfunction in Degenerative Parkinsonisms. J. Neurol. Sci. 2021, 423, 117363.
- Yoo, H.S.; Lee, S.; Chung, S.J.; Lee, Y.H.; Lee, P.H.; Sohn, Y.H.; Lee, S.; Yun, M.; Ye, B.S. Dopaminergic Depletion, β-Amyloid Burden, and Cognition in Lewy Body Disease. Ann. Neurol. 2020, 87, 739–750.
- Isaacson, J.R.; Brillman, S.; Chhabria, N.; Isaacson, S.H. Impact of DaTscan Imaging on Clinical Decision Making in Clinically Uncertain Parkinson’s Disease. J. Park. Dis. 2021, 11, 885–889.
- Thobois, S.; Prange, S.; Scheiber, C.; Broussolle, E. What a Neurologist Should Know about PET and SPECT Functional Imaging for Parkinsonism: A Practical Perspective. Parkinsonism Relat. Disord. 2019, 59, 93–100.
- Verger, A.; Grimaldi, S.; Ribeiro, M.-J.; Frismand, S.; Guedj, E. Single Photon Emission Computed Tomography/Positron Emission Tomography Molecular Imaging for Parkinsonism: A Fast-Developing Field. Ann. Neurol. 2021, 90, 711–719.
- Schmitz-Steinkrüger, H.; Lange, C.; Apostolova, I.; Amthauer, H.; Lehnert, W.; Klutmann, S.; Buchert, R. Impact of the Size of the Normal Database on the Performance of the Specific Binding Ratio in Dopamine Transporter SPECT. EJNMMI Phys. 2020, 7, 34.
- Schmitz-Steinkrüger, H.; Lange, C.; Apostolova, I.; Mathies, F.L.; Frings, L.; Klutmann, S.; Hellwig, S.; Meyer, P.T.; Buchert, R. Impact of Age and Sex Correction on the Diagnostic Performance of Dopamine Transporter SPECT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1445–1459.
- Piccardo, A.; Cappuccio, R.; Bottoni, G.; Cecchin, D.; Mazzella, L.; Cirone, A.; Righi, S.; Ugolini, M.; Bianchi, P.; Bertolaccini, P.; et al. The Role of the Deep Convolutional Neural Network as an Aid to Interpreting Brain DOPA PET/CT in the Diagnosis of Parkinson’s Disease. Eur. Radiol. 2021, 31, 7003–7011.
- Wenzel, M.; Milletari, F.; Krüger, J.; Lange, C.; Schenk, M.; Apostolova, I.; Klutmann, S.; Ehrenburg, M.; Buchert, R. Automatic Classification of Dopamine Transporter SPECT: Deep Convolutional Neural Networks Can Be Trained to Be Robust with Respect to Variable Image Characteristics. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2800–2811.
- Nicastro, N.; Wegrzyk, J.; Preti, M.G.; Fleury, V.; Van de Ville, D.; Garibotto, V.; Burkhard, P.R. Classification of Degenerative Parkinsonism Subtypes by Support-Vector-Machine Analysis and Striatal 123I-FP-CIT Indices. J. Neurol. 2019, 266, 1771–1781.
- Dotinga, M.; van Dijk, J.D.; Vendel, B.N.; Slump, C.H.; Portman, A.T.; van Dalen, J.A. Clinical Value of Machine Learning-Based Interpretation of I-123 FP-CIT Scans to Detect Parkinson’s Disease: A Two-Center Study. Ann. Nucl. Med. 2021, 35, 378–385.
- Nazari, M.; Kluge, A.; Apostolova, I.; Klutmann, S.; Kimiaei, S.; Schroeder, M.; Buchert, R. Data-Driven Identification of Diagnostically Useful Extrastriatal Signal in Dopamine Transporter SPECT Using Explainable AI. Sci. Rep. 2021, 11, 22932.
- Shiiba, T.; Arimura, Y.; Nagano, M.; Takahashi, T.; Takaki, A. Improvement of Classification Performance of Parkinson’s Disease Using Shape Features for Machine Learning on Dopamine Transporter Single Photon Emission Computed Tomography. PLoS ONE 2020, 15, e0228289.
- Magesh, P.R.; Myloth, R.D.; Tom, R.J. An Explainable Machine Learning Model for Early Detection of Parkinson’s Disease Using LIME on DaTSCAN Imagery. Comput. Biol. Med. 2020, 126, 104041.
- Manzanera, O.M.; Meles, S.K.; Leenders, K.L.; Renken, R.J.; Pagani, M.; Arnaldi, D.; Nobili, F.; Obeso, J.; Oroz, M.R.; Morbelli, S.; et al. Scaled Subprofile Modeling and Convolutional Neural Networks for the Identification of Parkinson’s Disease in 3D Nuclear Imaging Data. Int. J. Neural Syst. 2019, 29, 1950010.
- Shen, T.; Jiang, J.; Lin, W.; Ge, J.; Wu, P.; Zhou, Y.; Zuo, C.; Wang, J.; Yan, Z.; Shi, K. Use of Overlapping Group LASSO Sparse Deep Belief Network to Discriminate Parkinson’s Disease and Normal Control. Front. Neurosci. 2019, 13, 396.
- Arnaldi, D.; Mattioli, P.; Famà, F.; Girtler, N.; Brugnolo, A.; Pardini, M.; Donniaquio, A.; Massa, F.; Orso, B.; Raffa, S.; et al. Stratification Tools for Disease-Modifying Trials in Prodromal Synucleinopathy. Mov. Disord. 2022, 37, 52–61.
- Stephenson, D.; Hill, D.; Cedarbaum, J.M.; Tome, M.; Vamvakas, S.; Romero, K.; Conrado, D.J.; Dexter, D.T.; Seibyl, J.; Jennings, D.; et al. The Qualification of an Enrichment Biomarker for Clinical Trials Targeting Early Stages of Parkinson’s Disease. J. Park. Dis. 2019, 9, 553–563.
- Massa, J.; Chahine, L.M. Revision of Diagnosis in Early Parkinsonism with Abnormal Dopamine Transporter Imaging. J. Park. Dis. 2019, 9, 327–334.
- Arnaldi, D.; Famà, F.; Girtler, N.; Brugnolo, A.; Pardini, M.; Mattioli, P.; Meli, R.; Massa, F.; Orso, B.; Sormani, M.P.; et al. Rapid Eye Movement Sleep Behavior Disorder: A Proof-of-Concept Neuroprotection Study for Prodromal Synucleinopathies. Eur. J. Neurol. 2021, 28, 1210–1217.
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-First versus Body-First Parkinson’s Disease: A Multimodal Imaging Case-Control Study. Brain 2020, 143, 3077–3088.
- Knudsen, K.; Fedorova, T.D.; Hansen, A.K.; Sommerauer, M.; Otto, M.; Svendsen, K.B.; Nahimi, A.; Stokholm, M.G.; Pavese, N.; Beier, C.P.; et al. In-Vivo Staging of Pathology in REM Sleep Behaviour Disorder: A Multimodality Imaging Case-Control Study. Lancet Neurol. 2018, 17, 618–628.
- Berg, D.; Borghammer, P.; Fereshtehnejad, S.-M.; Heinzel, S.; Horsager, J.; Schaeffer, E.; Postuma, R.B. Prodromal Parkinson Disease Subtypes—Key to Understanding Heterogeneity. Nat. Rev. Neurol. 2021, 17, 349–361.
- Knudsen, K.; Fedorova, T.D.; Horsager, J.; Andersen, K.B.; Skjærbæk, C.; Berg, D.; Schaeffer, E.; Brooks, D.J.; Pavese, N.; Van Den Berge, N.; et al. Asymmetric Dopaminergic Dysfunction in Brain-First versus Body-First Parkinson’s Disease Subtypes. J. Park. Dis. 2021, 11, 1677–1687.
- Valli, M.; Cho, S.S.; Uribe, C.; Masellis, M.; Chen, R.; Mihaescu, A.; Strafella, A.P. VMAT2 Availability in Parkinson’s Disease with Probable REM Sleep Behaviour Disorder. Mol. Brain 2021, 14, 165.
- Valli, M.; Cho, S.S.; Masellis, M.; Chen, R.; Koshimori, Y.; Diez-Cirarda, M.; Mihaescu, A.; Christopher, L.; Strafella, A.P. Extra-Striatal Dopamine in Parkinson’s Disease with Rapid Eye Movement Sleep Behavior Disorder. J. Neurosci. Res. 2021, 99, 1177–1187.
- Pasquini, J.; Ceravolo, R.; Brooks, D.J.; Bonuccelli, U.; Pavese, N. Progressive Loss of Raphe Nuclei Serotonin Transporter in Early Parkinson’s Disease: A Longitudinal 123I-FP-CIT SPECT Study. Parkinsonism Relat. Disord. 2020, 77, 170–175.
- Wilson, H.; Dervenoulas, G.; Pagano, G.; Koros, C.; Yousaf, T.; Picillo, M.; Polychronis, S.; Simitsi, A.; Giordano, B.; Chappell, Z.; et al. Serotonergic Pathology and Disease Burden in the Premotor and Motor Phase of A53T α-Synuclein Parkinsonism: A Cross-Sectional Study. Lancet Neurol. 2019, 18, 748–759.
- Sampedro, F.; Marín-Lahoz, J.; Martínez-Horta, S.; Camacho, V.; Lopez-Mora, D.-A.; Pagonabarraga, J.; Kulisevsky, J. Extrastriatal SPECT-DAT Uptake Correlates with Clinical and Biological Features of de Novo Parkinson’s Disease. Neurobiol. Aging 2021, 97, 120–128.
- Fu, J.F.; Matarazzo, M.; McKenzie, J.; Neilson, N.; Vafai, N.; Dinelle, K.; Felicio, A.C.; McKeown, M.J.; Stoessl, A.J.; Sossi, V. Serotonergic System Impacts Levodopa Response in Early Parkinson’s and Future Risk of Dyskinesia. Mov. Disord. 2021, 36, 389–397.
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Bars, D.L.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The Prominent Role of Serotonergic Degeneration in Apathy, Anxiety and Depression in de Novo Parkinson’s Disease. Brain 2016, 139, 2486–2502.
- Maillet, A.; Météreau, E.; Tremblay, L.; Favre, E.; Klinger, H.; Lhommée, E.; Le Bars, D.; Castrioto, A.; Prange, S.; Sgambato, V.; et al. Serotonergic and Dopaminergic Lesions Underlying Parkinsonian Neuropsychiatric Signs. Mov Disord 2021, 36, 2888–2900.
- Prange, S.; Metereau, E.; Maillet, A.; Klinger, H.; Schmitt, E.; Lhommée, E.; Bichon, A.; Lancelot, S.; Meoni, S.; Broussolle, E.; et al. Limbic Serotonergic Plasticity Contributes to the Compensation of Apathy in Early Parkinson’s Disease. Mov. Disord. 2022, 37, 1211–1221.
- Jørgensen, L.M.; Henriksen, T.; Mardosiene, S.; Keller, S.H.; Stenbæk, D.S.; Hansen, H.D.; Jespersen, B.; Thomsen, C.; Weikop, P.; Svarer, C.; et al. Parkinson Patients Have a Presynaptic Serotonergic Deficit: A Dynamic Deep Brain Stimulation PET Study. J. Cereb. Blood Flow Metab. 2021, 41, 1954–1963.
- Pilotto, A.; Schiano di Cola, F.; Premi, E.; Grasso, R.; Turrone, R.; Gipponi, S.; Scalvini, A.; Cottini, E.; Paghera, B.; Garibotto, V.; et al. Extrastriatal Dopaminergic and Serotonergic Pathways in Parkinson’s Disease and in Dementia with Lewy Bodies: A 123I-FP-CIT SPECT Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1642–1651.
- van der Zande, J.J.; Joling, M.; Happach, I.G.; Vriend, C.; Scheltens, P.; Booij, J.; Lemstra, A.W. Serotonergic Deficits in Dementia with Lewy Bodies with Concomitant Alzheimer’s Disease Pathology: An 123I-FP-CIT SPECT Study. NeuroImage Clin. 2020, 25, 102062.
- Joling, M.; Vriend, C.; van den Heuvel, O.A.; Raijmakers, P.G.H.M.; Jones, P.A.; Berendse, H.W.; Booij, J. Analysis of Extrastriatal 123I-FP-CIT Binding Contributes to the Differential Diagnosis of Parkinsonian Diseases. J. Nucl. Med. 2017, 58, 1117–1123.
- Meyer, M.; Lamare, F.; Asselineau, J.; Foubert-Samier, A.; Mazère, J.; Zanotti-Fregonara, P.; Rizzo, G.; Delamarre, A.; Spampinato, U.; Rascol, O.; et al. Brain 5-HT1A Receptor Binding in Multiple System Atrophy: An -MPPF PET Study. Mov. Disord. 2021, 36, 246–251.
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent Advances in Radiotracers Targeting Norepinephrine Transporter: Structural Development and Radiolabeling Improvements. J. Neural Transm. Vienna Austria 1996 2020, 127, 851–873.
- Laurencin, C.; Lancelot, S.; Gobert, F.; Redouté, J.; Mérida, I.; Iecker, T.; Liger, F.; Irace, Z.; Greusard, E.; Lamberet, L.; et al. Modeling Yohimbine PET Human Brain Kinetics with Test-Retest Reliability, Competition Sensitivity Studies and Search for a Suitable Reference Region. NeuroImage 2021, 240, 118328.
- Doppler, C.E.J.; Kinnerup, M.B.; Brune, C.; Farrher, E.; Betts, M.; Fedorova, T.D.; Schaldemose, J.L.; Knudsen, K.; Ismail, R.; Seger, A.D.; et al. Regional Locus Coeruleus Degeneration Is Uncoupled from Noradrenergic Terminal Loss in Parkinson’s Disease. Brain 2021, 144, 2732–2744.
- Doppler, C.E.J.; Smit, J.A.M.; Hommelsen, M.; Seger, A.; Horsager, J.; Kinnerup, M.B.; Hansen, A.K.; Fedorova, T.D.; Knudsen, K.; Otto, M.; et al. Microsleep Disturbances Are Associated with Noradrenergic Dysfunction in Parkinson’s Disease. Sleep 2021, 44, zsab040.
- Kinnerup, M.B.; Sommerauer, M.; Damholdt, M.F.; Schaldemose, J.L.; Ismail, R.; Terkelsen, A.J.; Stær, K.; Hansen, A.; Fedorova, T.D.; Knudsen, K.; et al. Preserved Noradrenergic Function in Parkinson’s Disease Patients with Rest Tremor. Neurobiol. Dis. 2021, 152, 105295.
- Andersen, K.B.; Hansen, A.K.; Sommerauer, M.; Fedorova, T.D.; Knudsen, K.; Vang, K.; Van Den Berge, N.; Kinnerup, M.; Nahimi, A.; Pavese, N.; et al. Altered Sensorimotor Cortex Noradrenergic Function in Idiopathic REM Sleep Behaviour Disorder—A PET Study. Parkinsonism Relat. Disord. 2020, 75, 63–69.
- Lenka, A.; Lamotte, G.; Goldstein, D.S. Cardiac 18F-Dopamine PET Distinguishes PD with Orthostatic Hypotension from Parkinsonian MSA. Mov. Disord. Clin. Pract. 2021, 8, 582–586.
- Kuten, J.; Linevitz, A.; Lerman, H.; Freedman, N.; Kestenbaum, M.; Shiner, T.; Giladi, N.; Even-Sapir, E. FDOPA PET May Confirm the Clinical Diagnosis of Parkinson’s Disease by Imaging the Nigro-Striatal Pathway and the Sympathetic Cardiac Innervation: Proof-of-Concept Study. J. Integr. Neurosci. 2020, 19, 489–494.
- van der Zee, S.; Vállez García, D.; Elsinga, P.H.; Willemsen, A.T.M.; Boersma, H.H.; Gerritsen, M.J.J.; Spikman, J.M.; van Laar, T. Fluoroethoxybenzovesamicol in Parkinson’s Disease Patients: Quantification of a Novel Cholinergic Positron Emission Tomography Tracer. Mov. Disord. 2019, 34, 924–926.
- Bohnen, N.I.; Kanel, P.; Zhou, Z.; Koeppe, R.A.; Frey, K.A.; Dauer, W.T.; Albin, R.L.; Müller, M.L.T.M. Cholinergic System Changes of Falls and Freezing of Gait in Parkinson’s Disease. Ann. Neurol. 2019, 85, 538–549.
- van der Zee, S.; Müller, M.L.T.M.; Kanel, P.; van Laar, T.; Bohnen, N.I. Cholinergic Denervation Patterns Across Cognitive Domains in Parkinson’s Disease. Mov. Disord. 2021, 36, 642–650.
- Pasquini, J.; Brooks, D.J.; Pavese, N. The Cholinergic Brain in Parkinson’s Disease. Mov. Disord. Clin. Pract. 2021, 8, 1012–1026.
- Bohnen, N.I.; Kanel, P.; Müller, M.L.T.M. Molecular Imaging of the Cholinergic System in Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 211–250. ISBN 978-0-12-815418-2.
- van der Zee, S.; Kanel, P.; Gerritsen, M.J.J.; Boertien, J.M.; Slomp, A.C.; Müller, M.L.T.M.; Bohnen, N.I.; Spikman, J.M.; van Laar, T. Altered Cholinergic Innervation in De Novo Parkinson’s Disease with and Without Cognitive Impairment. Mov. Disord. 2022, 37, 713–723.
- Bedard, M.-A.; Aghourian, M.; Legault-Denis, C.; Postuma, R.B.; Soucy, J.-P.; Gagnon, J.-F.; Pelletier, A.; Montplaisir, J. Brain Cholinergic Alterations in Idiopathic REM Sleep Behaviour Disorder: A PET Imaging Study with 18F-FEOBV. Sleep Med. 2019, 58, 35–41.
- Gersel Stokholm, M.; Iranzo, A.; Østergaard, K.; Serradell, M.; Otto, M.; Bacher Svendsen, K.; Garrido, A.; Vilas, D.; Fedorova, T.D.; Santamaria, J.; et al. Cholinergic Denervation in Patients with Idiopathic Rapid Eye Movement Sleep Behaviour Disorder. Eur. J. Neurol. 2020, 27, 644–652.
- Kanel, P.; Müller, M.L.T.M.; van der Zee, S.; Sanchez-Catasus, C.A.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I. Topography of Cholinergic Changes in Dementia With Lewy Bodies and Key Neural Network Hubs. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 370–375.
- Ji, L.; Fang, Y.; Tang, J.; Liu, C.; Huang, C.; Hu, Q.; Li, Q.; Chen, Z. Synthesis and Biological Evaluation of 18F-Labelled Dopamine D3 Receptor Selective Ligands. Bioorg. Med. Chem. Lett. 2022, 62, 128630.
- Lehnert, W.; Riss, P.J.; Hurtado de Mendoza, A.; Lopez, S.; Fernandez, G.; Ilheu, M.; Amaral, H.; Kramer, V. Whole-Body Biodistribution and Radiation Dosimetry of PR04.MZ: A New PET Radiotracer for Clinical Management of Patients with Movement Disorders. EJNMMI Res. 2022, 12, 1.
- Pain, C.D.; O’Keefe, G.J.; Ackermann, U.; Dore, V.; Villemagne, V.L.; Rowe, C.C. Human Biodistribution and Internal Dosimetry of 4-Fluorobenzyl-Dexetimide: A PET Radiopharmaceutical for Imaging Muscarinic Acetylcholine Receptors in the Brain and Heart. EJNMMI Res. 2020, 10, 61.
- Rowe, C.C.; Krishnadas, N.; Ackermann, U.; Doré, V.; Goh, R.Y.W.; Guzman, R.; Chong, L.; Bozinovski, S.; Mulligan, R.; Kanaan, R.; et al. PET Imaging of Brain Muscarinic Receptors with 18F-Fluorobenzyl-Dexetimide: A First in Human Study. Psychiatry Res. Neuroimaging 2021, 316, 111354.
- Lai, T.H.; Toussaint, M.; Teodoro, R.; Dukić-Stefanović, S.; Kranz, M.; Deuther-Conrad, W.; Moldovan, R.-P.; Brust, P. Synthesis and Biological Evaluation of a Novel 18F-Labeled Radiotracer for PET Imaging of the Adenosine A2A Receptor. Int. J. Mol. Sci. 2021, 22, 1182.
- Chen, X.; Zhang, Q.; Zhang, Y.; Fang, J.; Jiang, D.; Mou, Z.; Liu, H.; Su, R.; Wang, C.; He, F.; et al. 18F-Labelled Pyrrolopyrimidines Reveal Brain Leucine-Rich Repeat Kinase 2 Expression Implicated in Parkinson’s Disease. Eur. J. Med. Chem. 2021, 214, 113245.
- Malik, N.; Kornelsen, R.; McCormick, S.; Colpo, N.; Merkens, H.; Bendre, S.; Benard, F.; Sossi, V.; Schirrmacher, R.; Schaffer, P. Development and Biological Evaluation ofFMN3PA & FMN3PU for Leucine-Rich Repeat Kinase 2 (LRRK2) in Vivo PET Imaging. Eur. J. Med. Chem. 2021, 211, 113005.
- Thobois, S.; Brefel-Courbon, C.; Le Bars, D.; Sgambato-Faure, V. Molecular Imaging of Opioid System in Idiopathic Parkinson’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 141, pp. 275–303. ISBN 978-0-12-815418-2.
- Green, D.G.J.; Kim, J.; Kish, S.J.; Tyndale, R.F.; Hill, M.N.; Strafella, A.P.; Tong, J.; McCluskey, T.; Westwood, D.J.; Houle, S.; et al. Fatty Acid Amide Hydrolase Binding Is Inversely Correlated with Amygdalar Functional Connectivity: A Combined Positron Emission Tomography and Magnetic Resonance Imaging Study in Healthy Individuals. J. Psychiatry Neurosci. 2021, 46, E238–E246.
