Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gonçalo Justino | -- | 2645 | 2022-09-20 13:12:45 | | | |
| 2 | Conner Chen | Meta information modification | 2645 | 2022-09-21 10:20:13 | | | | |
| 3 | Conner Chen | + 16 word(s) | 2661 | 2022-09-21 10:22:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marques, C.F.; Marques, M.M.; Justino, G.C. Cysteinyl Leukotrienes as Multifunctional Inflammation Mediators. Encyclopedia. Available online: https://encyclopedia.pub/entry/27375 (accessed on 06 March 2026).
Marques CF, Marques MM, Justino GC. Cysteinyl Leukotrienes as Multifunctional Inflammation Mediators. Encyclopedia. Available at: https://encyclopedia.pub/entry/27375. Accessed March 06, 2026.
Marques, Cátia F., Maria Matilde Marques, Gonçalo C. Justino. "Cysteinyl Leukotrienes as Multifunctional Inflammation Mediators" Encyclopedia, https://encyclopedia.pub/entry/27375 (accessed March 06, 2026).
Marques, C.F., Marques, M.M., & Justino, G.C. (2022, September 20). Cysteinyl Leukotrienes as Multifunctional Inflammation Mediators. In Encyclopedia. https://encyclopedia.pub/entry/27375
Marques, Cátia F., et al. "Cysteinyl Leukotrienes as Multifunctional Inflammation Mediators." Encyclopedia. Web. 20 September, 2022.
Copy Citation
Montelukast (MTK)—an antagonist of the cysteinyl leukotrienes receptor 1—is widely used in the management of symptoms among adults and children. Initially described as the slow-reacting substances of anaphylaxis, leukotrienes (LTs) are pro-inflammatory lipid mediators derived from arachidonic acid. Its systemic anti-inflammatory actions, which are particularly important in the brain tissues, are at the onset of various clinical studies focused on the repurposing of this drug for various other diseases, aimed particularly at Alzheimer’s and Parkinson’s diseases.
montelukast
leukotrienes
adverse drug reactions
1. Introduction
Montelukast (MTK) is an antagonist of the cysteinyl leukotrienes receptor 1 and is routinely used in the management of asthma symptoms among adults and children. Its systemic anti-inflammatory actions, which are particularly important in the brain tissues, are at the onset of various clinical studies focused on the repurposing of this drug for various other diseases, aimed particularly at Alzheimer’s and Parkinson’s diseases. However, this repurposing clashes with neuropsychiatric adverse drug reactions elicited by the drug.
2. Cysteinyl Leukotrienes—Multifunctional Inflammation Mediators
2.1. Cysteinyl Leukotrienes and Their Receptors
Initially described as the slow-reacting substances of anaphylaxis, leukotrienes (LTs) are pro-inflammatory lipid mediators derived from arachidonic acid [1][2]. These mediators are synthesized mainly in cells from the innate immune system (e.g., polymorphonuclear leukocytes, macrophages, mast cells, and brain microglia) following activation by immune and non-immune stimuli such as infection, tissue injury, allergens, and exercise. Upon cell activation, the cytosolic calcium concentration increases, and the cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LOX) enzymes are activated and translocated to the nuclear envelope. There, cPLA2 cleaves glycerophospholipids, releasing arachidonic acid (AA), which is converted to the acyclic hydroperoxide 5(S)-hydroperoxyeicosatetraenoic acid (5-HpETE) by 5-LOX-mediated oxidation upon LOX activation by 5-LOX activating protein (FLAP); 5-HpETE, in turn, undergoes dehydration to the unstable conjugated triene epoxide leukotriene A4 (LTA4), the first metabolite in the leukotriene pathway. LTA4 is a short-lived intermediate that can undergo conjugate addition of water to form leukotriene B4 (LTB4) or conjugation with glutathione by LTC4 synthase to form leukotriene C4 (LTC4, an S-glutathionyl LT). LTB4 and LTC4 are transported to the extracellular space mainly by multidrug resistance proteins, namely through MRP4 (LTB4) and MRP1 (LTC4) [3][4], where cleavage of LTC4 to leukotriene D4 (LTD4) and subsequently to leukotriene E4 (LTE4) takes place. LTD4, an S-cysteinyl LT, is synthesised from LTC4 by a γ-glutamyl transpeptidase (GGT)-mediated cleavage, whereas LTE4 results from the cleavage of LTD4 by a membrane-bound dipeptidase [5][6][7][8][9][10][11][12][13][14][15][16][17][18].
LTB4 is a pro-inflammatory LT that acts on human polymorphonuclear leukocytes (PMNLs) such as neutrophils, via G protein-coupled receptors B-LT1 or B-LT2, triggering chemotaxis and the subsequent activation of the inflammatory response. LTC4, LTD4, and LTE4 constitute a group of cysteinyl leukotrienes (CysLTs) that act through G protein-coupled cell surface receptors, of which the two classical receptors are the cysteinyl leukotriene receptors 1 (CysLTR1) and 2 (CysLTR2). LTC4 is an agonist of CysLTR1 whereas LTD4 binds CysLTR1 and CysLTR2. LTE4 is described as an agonist of CysLTR3 (also known as GPR99 receptor) and of the purinergic receptors GPR17 and P2Y12 [5][6][7][8][9][10][11][12][13][14][15][16][17][18].
Cysteinyl leukotriene receptors (CysLTRs) are involved in the pathophysiology of various respiratory allergic diseases, including bronchial asthma, exercise- and aspirin-induced asthma, and allergic rhinitis, as well as atopic dermatitis, allergic conjunctivitis, and anaphylaxis, exhibiting a large overlap with the B-LT receptors, but allowing a finely tuned immune response [11][12][13][19][20]. Receptor engagement by CysLTs promotes bronchoconstriction, vascular leakage, and neutrophil extravasation to inflammation sites [7]. CysLTR1 is expressed in most human tissues, particularly in the appendix, oesophagus, gall bladder, lung, lymph nodes, spleen, and urinary bladder. The affinity of leukotrienes to this receptor varies in the order LTD4 > LTC4 > LTE4. This receptor is sensitive to classical antagonists (Figure 1) such as montelukast (MTK, Singulair®), zafirlukast (Accolate®), pranlukast (Onon®, Azlaire®), pobilukast, and MK571, all members of the Lukast group (cysteinyl leukotriene receptor antagonists).
CysLTR2 is predominantly expressed in the spleen, heart, brain, and adrenal gland, and its affinity strength is LTC4 = LTD4 > LTE4. HAMI3379 (Figure 1) was identified as a potent and selective CysLTR2 receptor antagonist [21]. Only two dual inhibitors of both CysTR1 and CysLTR2 are reported—BAY-u9773 and gemilukast (Figure 1). However, BAY-u9773 is neither very potent nor selective for human CysLTs [11][12][19][20][22] and gemilukast did not show outcome differences when compared with MTK [23][24]. Figure 1 also shows the experimental IC50 values available for these compounds.
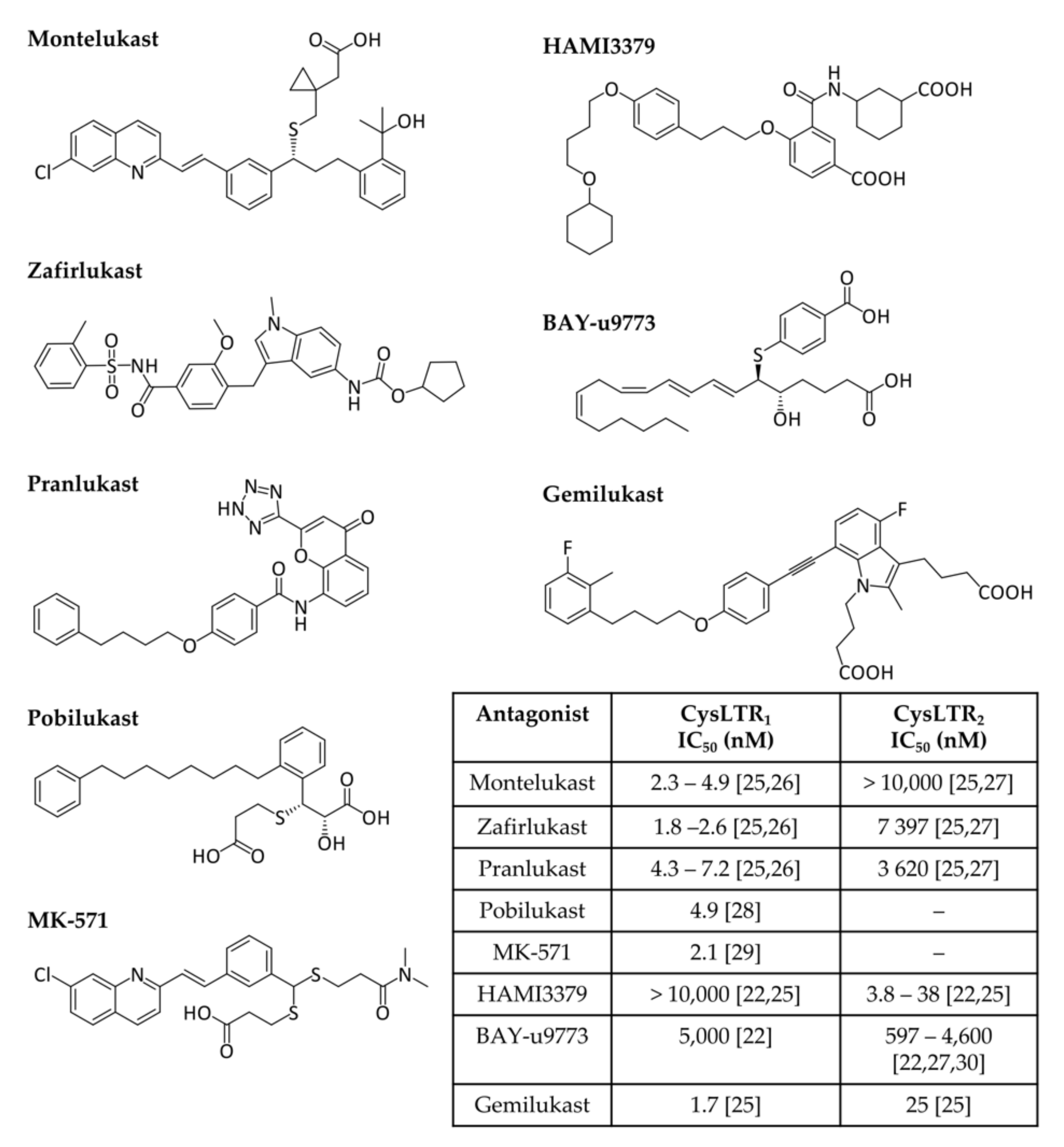
Figure 1. Cysteinyl leukotrienes receptor antagonists. CysLTR1 antagonists (montelukast, zafirlukast, pranlukast, pobilukast, and MK571), CysLTR2 antagonists (HAMI3379) and dual antagonists (BAY-u9773 and gemilukast) are shown. Experimental IC50 values available from the literature are also given in the inset table [21][24][25][26][27][28][29].
Besides these classical receptors, three other receptors are associated with the leukotriene cascade—GPR99, P2Y12, and GPR17.
GPR99, or OXGR1, is an α-ketoglutarate receptor that was originally thought to be a P2Y receptor [30]. This receptor is expressed in the kidney, placenta, trachea, salivary glands, lungs, and smooth muscle cells, as well as in some brain regions; in addition to its effects on acid–base homeostasis, it is also involved in axon growth [31][32][33][34]. GPR99 is considered the third CysLT receptor (CysLTR3) due to its high affinity for LTE4. No antagonists are currently available for this receptor [31][35].
P2Y12 is an adenosine diphosphate receptor that also mediates LTE4-dependent pulmonary inflammation (but not the LTD4 response) [36]. This receptor is mainly expressed in platelets and microglia, where it triggers platelet activation and blood clotting, and induces microglial chemotaxis in situations of central nervous system (CNS) injury [37][38][39][40]. P2Y12 is also associated with some asthma symptoms, namely with eosinophilic inflammation and airway hyper-responsiveness [41][42]. The P2Y12 receptor is blocked by anti-platelet drugs such as clopidogrel, prasugrel, and ticagrelor [43].
Lastly, GPR17 is a uracil nucleotide P2Y receptor expressed in the brain that also binds CysLTs [12][14][44][45][46][47][48][49]. This receptor is described as a sensor of neuronal damage, being activated by nucleotides and CysLTs released in the damaged area and plays a dual role depending on its surroundings: under physiological conditions, GPR17 contributes to the differentiation and maturation of oligodendrocytes, whereas under pathological conditions it mediates demyelination and apoptosis [50][51][52][53][54][55]. GPR17 is described as a putative negative regulator of CysLTR1 [56]. The CysLTR1 inhibitors pranlukast and montelukast are also antagonists of this receptor [45][57][58].
2.2. Leukotrienes in the Brain
The potential of leukotrienes as pro-inflammatory lipid mediators, described above, together with the pattern of expression of their receptors in different organs, has led to the suggestion that LTs play an important role in the central nervous system. In fact, recent advances have associated inflammation with some brain pathologies such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, brain ischemia, and epilepsy, among others, and leukotrienes are thought to play a role in this process [59][60].
Despite having been originally found in leukocytes, leukotrienes are also present in the brain. Not only is the 5-LOX enzyme widely distributed in various brain regions (e.g., cortex, hippocampus, and cerebellum), but CysLTs are also produced by vascular endothelial cells, neurons, and glial cells upon LTA4 expression by activated neutrophils [46]. CysLTR1 is widely expressed in the cortex, hippocampus, and nigrostriatum, as well as in cerebrovascular endothelial cells, astrocytes, microglia, and several types of neurons. On the other hand, CysLTR2 is expressed in the cortex, hippocampus, substantia nigra, astrocytes, microglia, and neurons [61][62][63][64][65]. These receptors are usually weakly expressed unless activated by pathological stimuli [65]. Some studies have shown that the exposure of neurons to acute neuronal injury is associated with upregulated levels of CysLTR1 and CysLTR2, and with increased blood–brain barrier (BBB) permeability. Once activated, CysLT receptors will trigger an inflammatory cascade, activating pro-inflammatory cytokines and inflammation, ultimately leading to neuronal damage [61][63][64][66].
2.2.1. Leukotrienes: Role in Neuroinflammation
Neuroinflammation is a complex biological response of the brain and spinal cord mediated by the production of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), chemokines (CCL2, CCL5, and CXCL1), reactive oxygen species (ROS), and other mediators (NO, prostaglandins, and leukotrienes) [67][68][69]. This biological response is associated with restoration of homeostatic balance, in order to eliminate and repair the initial cause of cell injury, and can be classified as acute (seconds to days) or chronic [67][70].
An acute inflammatory response is an adaptive response, usually beneficial, meant to protect tissues from a specific injury as trauma or infection [70]. In situations of acute inflammation, the immune system priorities are neuroprotection, tissue repair, and neuroplasticity. When the brain is exposed to immune signals after any infection, microglia and astrocytes are activated and neuroinflammatory cytokines such as IL-1β, TNF-α, and IL-6 are expressed to sustain the inflammatory response. This response is short and transient, and no severe effects take place [67]. Brain development and plasticity are other positive aspects of neuroinflammation. Neurons, astrocytes, and glia cells are involved in neurotransmission through the modulatory effect of cytokines and neuromodulators such as IL-1β, IL-6, TNF-α, NF-κB, and glutamate [67][71]. Brain tissue repair can also be stimulated through the activation of macrophages, lymphocytes, and microglia, which promotes angiogenesis, axon regeneration, myelin clearance, and oligodendrocyte regeneration [67][72][73][74]. Lastly, immune system training through immune pre-conditioning or euflammation allows modulation of the microglia response against hyper-inflammatory conditions, protecting the brain from CNS injuries [67][75][76].
However, if the acute inflammation response fails and the inflammation process persists, chronic inflammation ensues with a long-lasting maladaptive or defective response that could destroy tissues and compromise the immune response [69][70]. Characteristically, there is an increased production of cytokines (IL-1 and TNF-α), reactive oxygen species (ROS), and other inflammatory mediators (e.g., inducible nitric oxide synthase, iNOS), associated with the activation of microglia cells, and consequent expression of more pro-inflammatory cytokines and chemokines in the brain [67]. This activation could be caused by noradrenergic signalling, inflammasome activation, and ATP release [77][78][79]. Microglia activation is also involved in the recruitment of monocytes from the bone marrow to the brain and is linked to anxiety-like behaviour and to the development of mood disorders [67][80].
The normal ageing process is one example of the disruption of the communication pathways between the brain and the immune system, leading to chronic neuroinflammation. During ageing, there is an increase in inflammatory (e.g., IL-1β and IL-6) and a decrease in anti-inflammatory (e.g., IL-10 and IL-4) cytokines that results in damage to the nervous system and the onset of neurodegenerative diseases [67].
It has been shown that the leukotriene receptors CysLTR1 and CysLTR2 in different brain cells, namely microglia (known as the brain’s immune system), astrocytes, and several types of neurons, are upregulated in response to brain injury such as brain ischemia, Alzheimer’s disease, and Parkinson’s disease [61][62][63][64][81][82][83][84][85][86][87]. The modulation of these receptors is associated not only with the outcome of acute inflammation but also with the restoring of homeostasis during chronic inflammation [61][62][63][64][81][82][83][84][85][86][87].
Although the mechanisms of action are still poorly understood, evidence supports the relationship between leukotrienes and neuroinflammation, suggesting the use of leukotriene antagonists as a possible therapeutic strategy in neuroinflammation, given that antagonists of either CysLTR1 or CysLTR2 display wide multi-target anti-inflammatory activity [65]. Both receptors are expressed at low levels in multiple brain regions, but are upregulated following injury, as observed in various experimental models of ischemia and Alzheimer’s and Parkinson’s diseases [64][81][82][83][88]. Interestingly, silencing the expression of the genes coding for these two receptors leads to in vivo protection against lipopolysaccharide- and ischemia-induced brain inflammation and injury [86][87]. Although this strategy needs to be further explored, it could be a very promising therapeutic approach to the improvement of symptoms (or even disease treatment) in patients who suffer from neurodegenerative disorders and have no alternative therapy to manage the debilitating symptoms characteristic of neurodegeneration.
2.2.2. Leukotrienes in Neuro-Signalling Pathways
Message transmission between neurons results from an electrical impulse (action potential) that causes the release of neurotransmitters into the synaptic cleft. After crossing the synaptic cleft, neurotransmitters will reach their receptors on the postsynaptic side to excite or inhibit the target neuron. Excitatory synaptic transmission is mainly assured by L-glutamate, whereas γ-aminobutyric acid (GABA) is the major neurotransmitter involved in the inhibitory synaptic response. In addition to these neurotransmitters, there are other molecules involved in signalling and neuromodulation, such as acetylcholine, monoamines (e.g., dopamine, adrenaline, serotonin, and histamine), purines (e.g., adenosine), and neuropeptides [88].
A close relationship between neuroinflammation and neuro-signalling pathways has been proposed. One example is the involvement of excitotoxicity in neuroinflammation: an exacerbated or prolonged activation of glutamate receptors, particularly the N-methyl-D-aspartic acid receptors (NMDA), causes an increase in calcium influx into the neurons. This increase of intracellular calcium levels leads to a neurotoxic response, including the activation of the AA pathway, that can lead to the loss of neuronal function and, ultimately, cell death [89]. Studies involving CysLTR antagonists showed that pranlukast was able to inhibit NMDA-induced CysLTR1 expression, leading to a decrease in excitotoxic cell death [90]. Montelukast also presented a strong anti-excitotoxicity effect, as well as anti-inflammatory and neuroprotective properties [82].
Dopamine reuptake is also associated with the leukotriene pathway. Inhibition of the 5-LOX activating protein (FLAP) is associated with the improved integrity of dopaminergic neurons [91].
2.2.3. The Leukotriene Link between Stress and Depression
Depression can result from chronic neuroinflammation. Not only pro-inflammatory cytokines (e.g., IL-1β and TNF-α) were found to be dysregulated in depression patients, but also IL-1β, IL-6, TNF-α, or lipopolysaccharide (LPS) administration in animal models led to depression- and anxiety-like behaviours [92][93][94][95].
Stress stimuli led to an increase in calcium concentration, releasing AA after cPLA2 activation by phosphorylation [96]. Once released, AA is used to synthesise leukotrienes and prostaglandins. A study using mice in which the cysltr1 gene was silenced in the hippocampus suggested that the absence of CysLTR1 prevents the development of neuroinflammation and of a depressive-like phenotype [97]. The effects observed upon blocking the same receptors in a mouse lipopolysaccharide-induced neuroinflammation model support those previous results [98]. Inhibition of the 5-LOX enzyme has also been associated with a relief of depression-like behaviour [99].
2.2.4. The Role of Leukotrienes in Neurodegenerative Diseases
Besides their role in inflammation, leukotrienes are also involved in some of the most characteristic hallmarks of neurodegenerative disorders: neuronal cell death, neuroinflammation, altered neurogenesis, and disrupted blood–brain barrier and vascular system, among others.
The clear association between neuroinflammation and Alzheimer’s and/or Parkinson’s disease led to the study of the role of CysLTs pathways and receptors in these diseases.
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by memory loss and dementia. There is evidence for CysLTR1 involvement in AD, leading to amyloidogenesis and neuroinflammation. In particular:
(1) In an AD mouse model (APP/PS1 double transgenic, overexpressing mutated forms of human amyloid precursor protein, APP, and presenilin 1), the expression of CysLTR1 was found to increase with ageing, and to correlate with Aβ deposits and behaviour deficits [83][100];
(2) LTD4 upregulates APP, β-, and γ-secretase levels, and facilitates Aβ amyloid accumulation via the CysLTR1-mediated NF-κB pathway [101][102][103].
Aggregated Aβ1–42 is known to cause AD-like neurotoxicity and cognitive deficiency, associated with pro-inflammatory cytokine production (TNF-α, IL-1β) and increased cell apoptosis [83][104][105]. Additional studies also revealed that Aβ plaques are associated with an increased oxidative stress status. Oxidative stress is known to upregulate cPLA2 activity, leading to an increased release of arachidonic acid metabolites [65]. These responses are inhibited by Lukast drugs (pranlukast, montelukast, and zafirlukast), suggesting that CysLTR1 is a pro-inflammatory regulator and is involved in AD initiation and progression [65][83][104][105].
Parkinson’s disease (PD) is also a neurodegenerative disorder characterised by the progressive degeneration and loss of dopaminergic neurons. Inflammation induction in PD models (with rotenone or lipopolysaccharide) leads to microglia activation, increasing the production of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, and brain inflammation, leading to dopaminergic neuronal loss [46][65][106][107][108][109]. This action was inhibited by montelukast via the CysLTR1-mediated p38 MAPK/NF-κB pathway [81][106][110], and also by selective inhibition or knockout of CysLTR2 [85], suggesting that CysLTR1 and CysLTR2 could be strategic targets against PD. CysLTR1, as well as 5-LOX, are found to be upregulated in mouse PD models [91], further strengthening the hypothesis that the LT pathway contributes to the progression of PD.
In conclusion, leukotrienes play an important role in the progression of neurodegenerative disorders. Receptors involved in the different steps of the LT cascade interfere with the inflammatory process, which is partially responsible for the development of the characteristic hallmarks of AD and PD. For this reason, targeting the CysLT pathway seems to be a promising strategy to delay the progression of these disorders.
References
- Bäck, M. Leukotriene signaling in atherosclerosis and ischemia. Cardiovasc. Drugs Ther. 2009, 23, 41–48.
- Brocklehurst, W.E. The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock. J. Physiol. 1960, 151, 416–435.
- Rius, M.; Hummel-Eisenbeiss, J.; Keppler, D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). J. Pharmacol. Exp. Ther. 2008, 324, 86–94.
- Johnson, Z.L.; Chen, J. Structural Basis of Substrate Recognition by the Multidrug Resistance Protein MRP1. Cell 2017, 168, 1075–1085.e9.
- Samuelsson, B.; Dáhlen, S.E.; Lindgren, J.A.; Rouzer, C.A.; Serhan, C.N. Leukotrienes and Lipoxins—Structures, Biosynthesis, and Biological Effects. Science 1987, 237, 1171–1176.
- Heidenreich, K.A.; Corser-Jensen, C.E. Chapter 12-5-Lipoxygenase-Activating Protein Inhibitors: Promising Drugs for Treating Acute and Chronic Neuroinflammation Following Brain Injury. In New Therapeutics for Traumatic Brain Injury; Heidenreich, K.A., Ed.; Academic Press: San Diego, CA, USA, 2017; pp. 199–210.
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523.
- Massoumi, R.; Sjölander, A. The role of leukotriene receptor signaling in inflammation and cancer. Sci. World J. 2007, 7, 1413–1421.
- Kanaoka, Y.; Boyce, J.A. Cysteinyl leukotrienes and their receptors: Cellular distribution and function in immune and inflammatory responses. J. Immunol. 2004, 173, 1503–1510.
- Capra, V. Molecular and functional aspects of human cysteinyl leukotriene receptors. Pharm. Res. 2004, 50, 1–11.
- Singh, R.K.; Gupta, S.; Dastidar, S.; Ray, A. Cysteinyl leukotrienes and their receptors: Molecular and functional characteristics. Pharmacology 2010, 85, 336–349.
- Singh, R.K.; Tandon, R.; Dastidar, S.G.; Ray, A. A review on leukotrienes and their receptors with reference to asthma. J. Asthma. 2013, 50, 922–931.
- Liu, F.; Ouyang, J.; Sharma, A.N.; Liu, S.; Yang, B.; Xiong, W.; Xu, R. Leukotriene inhibitors for bronchiolitis in infants and young children. Cochrane Database Syst. Rev. 2015.
- Yokomizo, T.; Nakamura, M.; Shimizu, T. Leukotriene receptors as potential therapeutic targets. J. Clin. Investig. 2018, 128, 2691–2701.
- Smith, W.L.; Murphy, R.C. The Eicosanoids. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 259–296.
- Bäck, M. Leukotrienes: Potential therapeutic targets in cardiovascular diseases. Bull. Acad. Natl. Med. 2006, 190, 1511–1521.
- Bäck, M. Inhibitors of the 5-lipoxygenase pathway in atherosclerosis. Curr. Pharm. Des. 2009, 15, 3116–3132.
- Nakamura, M.; Shimizu, T. Leukotriene receptors. Chem. Rev. 2011, 111, 6231–6298.
- Capra, V.; Accomazzo, M.R.; Gardoni, F.; Barbieri, S.; Rovati, G.E. A role for inflammatory mediators in heterologous desensitization of CysLT1 receptor in human monocytes. J. Lipid. Res. 2010, 51, 1075–1084.
- Liu, M.; Yokomizo, T. The role of leukotrienes in allergic diseases. Allergol. Int. 2015, 64, 17–26.
- Wunder, F.; Tinel, H.; Kast, R.; Geerts, A.; Becker, E.M.; Kolkhof, P.; Hutter, J.; Erguden, J.; Härter, M. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT2) receptor. Br. J. Pharmacol. 2010, 160, 399–409.
- Poff, C.D.; Balazy, M. Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer. Curr. Drug Targets Inflamm. Allergy 2004, 3, 19–33.
- Gauvreau, G.M.; Boulet, L.P.; FitzGerald, J.M.; Cockcroft, D.W.; Davis, B.E.; Leigh, R.; Tanaka, M.; Fourre, J.A.; Tanaka, M.; Nabata, T.; et al. A dual CysLT1/2 antagonist attenuates allergen-induced airway responses in subjects with mild allergic asthma. Allergy 2016, 71, 1721–1727.
- Itadani, S.; Yashiro, K.; Aratani, Y.; Sekiguchi, T.; Kinoshita, A.; Moriguchi, H.; Ohta, N.; Takahashi, S.; Ishida, A.; Tajima, Y.; et al. Discovery of Gemilukast (ONO-6950), a Dual CysLT1 and CysLT2 Antagonist As a Therapeutic Agent for Asthma. J. Med. Chem. 2015, 58, 6093–6113.
- Lynch, K.R.; O'Neill, G.P.; Liu, Q.; Im, D.S.; Sawyer, N.; Metters, K.M.; Coulombe, N.; Abramovitz, M.; Figueroa, D.J.; Zeng, Z.; et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature 1999, 399, 789–793.
- Heise, C.E.; O'Dowd, B.F.; Figueroa, D.J.; Sawyer, N.; Nguyen, T.; Im, D.S.; Stocco, R.; Bellefeuille, J.N.; Abramovitz, M.; Cheng, R.; et al. Characterization of the human cysteinyl leukotriene 2 receptor. J. Biol. Chem. 2000, 275, 30531–30536.
- Sarau, H.M.; Ames, R.S.; Chambers, J.; Ellis, C.; Elshourbagy, N.; Foley, J.J.; Schmidt, D.B.; Muccitelli, R.M.; Jenkins, O.; Murdock, P.R.; et al. Identification, molecular cloning, expression, and characterization of a cysteinyl leukotriene receptor. Mol. Pharmacol. 1999, 56, 657–663.
- J Jones, T.R.; Zamboni, R.; Belley, M.; Champion, E.; Charette, L.; Ford-Hutchinson, A.W.; Frenette, R.; Gauthier, J.Y.; Leger, S.; Masson, P.; et al. Pharmacology of L-660,711 (MK-571): A novel potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1989, 67, 17–28.
- Ni, N.C.; Yan, D.; Ballantyne, L.L.; Barajas-Espinosa, A.; St Amand, T.; Pratt, D.A.; Funk, C.D. A selective cysteinyl leukotriene receptor 2 antagonist blocks myocardial ischemia/reperfusion injury and vascular permeability in mice. J. Pharmacol. Exp. Ther. 2011, 339, 768–778.
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Miras-Portugal, M.T.; King, B.F.; Gachet, C.; Jacobson, K.A.; et al. The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor. Trends Pharmacol. Sci. 2005, 26, 8–9.
- Kanaoka, Y.; Maekawa, A.; Austen, K.F. Identification of GPR99 protein as a potential third cysteinyl leukotriene receptor with a preference for leukotriene E4 ligand. J. Biol. Chem. 2013, 288, 10967–10972.
- Cherif, H.; Duhamel, F.; Cécyre, B.; Bouchard, A.; Quintal, A.; Chemtob, S.; Bouchard, J.F. Receptors of intermediates of carbohydrate metabolism, GPR91 and GPR99, mediate axon growth. PLoS Biol. 2018, 16, e2003619.
- Rajkumar, P.; Pluznick, J.L. Unsung renal receptors: Orphan G-protein-coupled receptors play essential roles in renal development and homeostasis. Acta Physiol. (Oxf) 2017, 220, 189–200.
- Wittenberger, T.; Hellebrand, S.; Munck, A.; Kreienkamp, H.J.; Schaller, H.C.; Hampe, W. GPR99, a new G protein-coupled receptor with homology to a new subgroup of nucleotide receptors. BMC Genomics. 2002, 3, 17.
- Peebles, R.S. Antileukotriene Therapy in Asthma. In Middleton’s Allergy: Principles and Practice, 9th ed.; Burks, A.W., Holgate, S.T., O'Hehir, R.E., Bacharier, L.B., Broide, D.H., Hershey, G.K.K., Peebles, S., Eds.; Elsevier: Edinburgh, UK, 2020; pp. 1584–1598.
- Paruchuri, S.; Tashimo, H.; Feng, C.; Maekawa, A.; Xing, W.; Jiang, Y.; Kanaoka, Y.; Conley, P.; Boyce, J.A. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J. Exp. Med. 2009, 206, 2543–2555.
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519.
- Moore, C.S.; Ase, A.R.; Kinsara, A.; Rao, V.T.; Michell-Robinson, M.; Leong, S.Y.; Butovsky, O.; Ludwin, S.K.; Séguéla, P.; Bar-Or, A.; et al. P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e80.
- Hollopeter, G.; Jantzen, H.M.; Vincent, D.; Li, G.; England, L.; Ramakrishnan, V.; Yang, R.B.; Nurden, P.; Nurden, A.; Julius, D.; et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001, 409, 202–207.
- Gómez Morillas, A.; Besson, V.C.; Lerouet, D. Microglia and Neuroinflammation: What Place for P2RY12? Int. J. Mol. Sci. 2021, 22, 1636.
- Neves, J.S.; Radke, A.L.; Weller, P.F. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J. Allergy Clin. Immunol. 2010, 125, 477–482.
- Suh, D.H.; Trinh, H.K.T.; Liu, J.N.; Pham, L.D.; Park, S.M.; Park, H.S.; Shin, Y.S. P2Y12 antagonist attenuates eosinophilic inflammation and airway hyperresponsiveness in a mouse model of asthma. J. Cell Mol. Med. 2016, 20, 333–341.
- Collet, J.P.; O’Connor, S. Clinical effects and outcomes with new P2Y12 inhibitors in ACS. Fund. Clin. Pharmacol. 2012, 26, 16–18.
- Fumagalli, M.; Daniele, S.; Lecca, D.; Lee, P.R.; Parravicini, C.; Fields, R.D.; Rosa, P.; Antonucci, F.; Verderio, C.; Trincavelli, M.L.; et al. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J. Biol. Chem. 2011, 286, 10593–10604.
- Ciana, P.; Fumagalli, M.; Trincavelli, M.L.; Verderio, C.; Rosa, P.; Lecca, D.; Ferrario, S.; Parravicini, C.; Capra, V.; Gelosa, P.; et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006, 25, 4615–4627.
- Marschallinger, J.; Schäffner, I.; Klein, B.; Gelfert, R.; Rivera, F.J.; Illes, S.; Grassner, L.; Janssen, M.; Rotheneichner, P.; Schmuckermair, C.; et al. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat. Commun. 2015, 6, 8466.
- Fumagalli, M.; Lecca, D.; Coppolino, G.T.; Parravicini, C.; Abbracchio, M.P. Pharmacological Properties and Biological Functions of the GPR17 Receptor, a Potential Target for Neuro-Regenerative Medicine. Adv. Exp. Med. Biol. 2017, 1051, 169–192.
- Maekawa, A.; Xing, W.; Austen, K.F.; Kanaoka, Y. GPR17 regulates immune pulmonary inflammation induced by house dust mites. J. Immunol. 2010, 185, 1846–1854.
- Zhao, B.; Zhao, C.Z.; Zhang, X.Y.; Huang, X.Q.; Shi, W.Z.; Fang, S.H.; Lu, Y.B.; Zhang, W.P.; Xia, Q.; Wei, E.Q. The new P2Y-like receptor G protein-coupled receptor 17 mediates acute neuronal injury and late microgliosis after focal cerebral ischemia in rats. Neuroscience 2012, 202, 42–57.
- Ceruti, S.; Villa, G.; Genovese, T.; Mazzon, E.; Longhi, R.; Rosa, P.; Bramanti, P.; Cuzzocrea, S.; Abbracchio, M.P. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain 2009, 132, 2206–2218.
- Burnstock, G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 2016, 104, 4–17.
- Franke, H.; Parravicini, C.; Lecca, D.; Zanier, E.R.; Heine, C.; Bremicker, K.; Fumagalli, M.; Rosa, P.; Longhi, L.; Stocchetti, N.; et al. Changes of the GPR17 receptor, a new target for neurorepair, in neurons and glial cells in patients with traumatic brain injury. Purinergic. Signal. 2013, 9, 451–462.
- Lecca, D.; Trincavelli, M.L.; Gelosa, P.; Sironi, L.; Ciana, P.; Fumagalli, M.; Villa, G.; Verderio, C.; Grumelli, C.; Guerrini, U.; et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 2008, 3, e3579.
- Dziedzic, A.; Miller, E.; Saluk-Bijak, J.; Bijak, M. The GPR17 Receptor-A Promising Goal for Therapy and a Potential Marker of the Neurodegenerative Process in Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 1852.
- Ou, Z.; Ma, Y.; Sun, Y.; Zheng, G.; Wang, S.; Xing, R.; Chen, X.; Han, Y.; Wang, J.; Lu, Q.R.; et al. A GPR17-cAMP-Lactate Signaling Axis in Oligodendrocytes Regulates Whole-Body Metabolism. Cell Rep. 2019, 26, 2984–2997.e4.
- Maekawa, A.; Balestrieri, B.; Austen, K.F.; Kanaoka, Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc. Natl. Acad. Sci. USA 2009, 106, 11685–11690.
- Pugliese, A.M.; Trincavelli, M.L.; Lecca, D.; Coppi, E.; Fumagalli, M.; Ferrario, S.; Failli, P.; Daniele, S.; Martini, C.; Pedata, F.; et al. Functional characterization of two isoforms of the P2Y-like receptor GPR17: GTPγS binding and electrophysiological studies in 1321N1 cells. Am. J. Physiol. Cell Physiol. 2009, 297, C1028–C1040.
- Hennen, S.; Wang, H.; Peters, L.; Merten, N.; Simon, K.; Spinrath, A.; Blättermann, S.; Akkari, R.; Schrage, R.; Schröder, R.; et al. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci. Signal. 2013, 6, ra93.
- Ghosh, A.; Chen, F.; Thakur, A.; Hong, H. Cysteinyl Leukotrienes and Their Receptors: Emerging Therapeutic Targets in Central Nervous System Disorders. CNS Neurosci. Ther. 2016, 22, 943–951.
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases. Mediat. Inflamm. 2017, 2017, 3454212.
- Zhao, C.Z.; Zhao, B.; Zhang, X.Y.; Huang, X.Q.; Shi, W.Z.; Liu, H.L.; Fang, S.H.; Lu, Y.B.; Zhang, W.P.; Tang, F.D.; et al. Cysteinyl leukotriene receptor 2 is spatiotemporally involved in neuron injury, astrocytosis and microgliosis after focal cerebral ischemia in rats. Neuroscience 2011, 189, 1–11.
- Zhang, W.P.; Hu, H.; Zhang, L.; Ding, W.; Yao, H.T.; Chen, K.D.; Sheng, W.W.; Chen, Z.; Wei, E.Q. Expression of cysteinyl leukotriene receptor 1 in human traumatic brain injury and brain tumors. Neurosci. Lett. 2004, 363, 247–251.
- Zhang, Y.-j.; Zhang, L.; Ye, Y.-l.; Fang, S.-h.; Zhou, Y.; Zhang, W.-p.; Lu, Y.-b.; Wei, E.-q. Cysteinyl leukotriene receptors CysLT1 and CysLT2 are upregulated in acute neuronal injury after focal cerebral ischemia in mice. Acta Pharmacol. Sin. 2006, 27, 1553–1560.
- Fang, S.H.; Wei, E.Q.; Zhou, Y.; Wang, M.L.; Zhang, W.P.; Yu, G.L.; Chu, L.S.; Chen, Z. Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience 2006, 140, 969–979.
- Wang, Y.; Yang, Y.; Zhang, S.; Li, C.; Zhang, L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 2020, 87, 1–10.
- Michael, J.; Marschallinger, J.; Aigner, L. The leukotriene signaling pathway: A druggable target in Alzheimer’s disease. Drug Discov. Today 2019, 24, 505–516.
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153.
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633.
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell Neurosci. 2018, 12, 72.
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435.
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194.
- Schonberg, D.L.; Popovich, P.G.; McTigue, D.M. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J. Neuropathol. Exp. Neurol. 2007, 66, 1124–1135.
- Sica, A.; Schioppa, T.; Mantovani, A.; Allavena, P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur. J. Cancer 2006, 42, 717–727.
- Kotter, M.R.; Setzu, A.; Sim, F.J.; Van Rooijen, N.; Franklin, R.J. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia 2001, 35, 204–212.
- Tarr, A.J.; Liu, X.; Reed, N.S.; Quan, N. Kinetic characteristics of euflammation: The induction of controlled inflammation without overt sickness behavior. Brain Behav. Immun. 2014, 42, 96–108.
- Liu, X.; Nemeth, D.P.; Tarr, A.J.; Belevych, N.; Syed, Z.W.; Wang, Y.; Ismail, A.S.; Reed, N.S.; Sheridan, J.F.; Yajnik, A.R.; et al. Euflammation attenuates peripheral inflammation-induced neuroinflammation and mitigates immune-to-brain signaling. Brain Behav. Immun. 2016, 54, 140–148.
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302.
- Iwata, M.; Ota, K.T.; Duman, R.S. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013, 31, 105–114.
- Yoshida, H.; Goedert, M. Molecular cloning and functional characterization of chicken brain tau: Isoforms with up to five tandem repeats. Biochemistry-Us 2002, 41, 15203–15211.
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 2013, 33, 13820–13833.
- Mansour, R.M.; Ahmed, M.A.E.; El-Sahar, A.E.; El Sayed, N.S. Montelukast attenuates rotenone-induced microglial activation/p38 MAPK expression in rats: Possible role of its antioxidant, anti-inflammatory and antiapoptotic effects. Toxicol. Appl. Pharmacol. 2018, 358, 76–85.
- Saad, M.A.; Abdelsalam, R.M.; Kenawy, S.A.; Attia, A.S. Montelukast, a cysteinyl leukotriene receptor-1 antagonist protects against hippocampal injury induced by transient global cerebral ischemia and reperfusion in rats. Neurochem. Res. 2015, 40, 139–150.
- Tang, S.S.; Hong, H.; Chen, L.; Mei, Z.L.; Ji, M.J.; Xiang, G.Q.; Li, N.; Ji, H. Involvement of cysteinyl leukotriene receptor 1 in Aβ1-42-induced neurotoxicity in vitro and in vivo. Neurobiol. Aging 2014, 35, 590–599.
- Hu, H.; Chen, G.; Zhang, J.M.; Zhang, W.P.; Zhang, L.; Ge, Q.F.; Yao, H.T.; Ding, W.; Chen, Z.; Wei, E.Q. Distribution of cysteinyl leukotriene receptor 2 in human traumatic brain injury and brain tumors. Acta Pharmacol Sin. 2005, 26, 685–690.
- Chen, L.; Yang, Y.; Li, C.T.; Zhang, S.R.; Zheng, W.; Wei, E.Q.; Zhang, L.H. CysLT2 receptor mediates lipopolysaccharide-induced microglial inflammation and consequent neurotoxicity in vitro. Brain Res. 2015, 1624, 433–445.
- Chen, F.; Ghosh, A.; Wu, F.; Tang, S.; Hu, M.; Sun, H.; Kong, L.; Hong, H. Preventive effect of genetic knockdown and pharmacological blockade of CysLT1R on lipopolysaccharide (LPS)-induced memory deficit and neurotoxicity in vivo. Brain Behav. Immun. 2017, 60, 255–269.
- Shi, Q.J.; Wang, H.; Liu, Z.X.; Fang, S.H.; Song, X.M.; Lu, Y.B.; Zhang, W.P.; Sa, X.Y.; Ying, H.Z.; Wei, E.Q. HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience 2015, 291, 53–69.
- Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 3rd ed.; Brunton, L.L.; Knollmann, B.r.C.; Hilal-Dandan, R. (Eds.) McGraw Hill Medical: New York, NY, USA, 2018.
- Armada-Moreira, A.; Gomes, J.I.; Pina, C.C.; Savchak, O.K.; Gonçalves-Ribeiro, J.; Rei, N.; Pinto, S.; Morais, T.P.; Martins, R.S.; Ribeiro, F.F.; et al. Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 90.
- Ding, Q.; Wei, E.Q.; Zhang, Y.J.; Zhang, W.P.; Chen, Z. Cysteinyl leukotriene receptor 1 is involved in N-methyl-D-aspartate-mediated neuronal injury in mice. Acta Pharmacol. Sin. 2006, 27, 1526–1536.
- Kang, K.-H.; Liou, H.-H.; Hour, M.-J.; Liou, H.-C.; Fu, W.-M. Protection of dopaminergic neurons by 5-lipoxygenase inhibitor. Neuropharmacology 2013, 73, 380–387.
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31.
- Sukoff Rizzo, S.J.; Neal, S.J.; Hughes, Z.A.; Beyna, M.; Rosenzweig-Lipson, S.; Moss, S.J.; Brandon, N.J. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl. Psychiatry 2012, 2, e199.
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56.
- Bluthé, R.M.; Layé, S.; Michaud, B.; Combe, C.; Dantzer, R.; Parnet, P. Role of interleukin-1β and tumour necrosis factor-α in lipopolysaccharide-induced sickness behaviour: A study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 2000, 12, 4447–4456.
- Buschbeck, M.; Ghomashchi, F.; Gelb, M.H.; Watson, S.P.; Börsch-Haubold, A.G. Stress stimuli increase calcium-induced arachidonic acid release through phosphorylation of cytosolic phospholipase A2. Biochem. J. 1999, 344 Pt 2, 359–366.
- Yu, X.B.; Dong, R.R.; Wang, H.; Lin, J.R.; An, Y.Q.; Du, Y.; Tang, S.S.; Hu, M.; Long, Y.; Sun, H.B.; et al. Knockdown of hippocampal cysteinyl leukotriene receptor 1 prevents depressive behavior and neuroinflammation induced by chronic mild stress in mice. Psychopharmacology 2016, 233, 1739–1749.
- Lin, J.R.; Fang, S.C.; Tang, S.S.; Hu, M.; Long, Y.; Ghosh, A.; Sun, H.B.; Kong, L.Y.; Hong, H. Hippocampal CysLT1R knockdown or blockade represses LPS-induced depressive behaviors and neuroinflammatory response in mice. Acta Pharmacol. Sin. 2017, 38, 477–487.
- Uz, T.; Dimitrijevic, N.; Imbesi, M.; Manev, H.; Manev, R. Effects of MK-886, a 5-lipoxygenase activating protein (FLAP) inhibitor, and 5-lipoxygenase deficiency on the forced swimming behavior of mice. Neurosci. Lett. 2008, 436, 269–272.
- Na, J.Y.; Song, K.; Lee, J.W.; Kim, S.; Kwon, J. 6-Shogaol has anti-amyloidogenic activity and ameliorates Alzheimer’s disease via CysLT1R-mediated inhibition of cathepsin B. Biochem. Biophys. Res. Commun. 2016, 477, 96–102.
- Wang, X.Y.; Tang, S.S.; Hu, M.; Long, Y.; Li, Y.Q.; Liao, M.X.; Ji, H.; Hong, H. Leukotriene D4 induces amyloid-β generation via CysLT1R-mediated NF-κB pathways in primary neurons. Neurochem. Int. 2013, 62, 340–347.
- Tang, S.S.; Wang, X.Y.; Hong, H.; Long, Y.; Li, Y.Q.; Xiang, G.Q.; Jiang, L.Y.; Zhang, H.T.; Liu, L.P.; Miao, M.X.; et al. Leukotriene D4 induces cognitive impairment through enhancement of CysLT1R-mediated amyloid-β generation in mice. Neuropharmacology 2013, 65, 182–192.
- Herbst-Robinson, K.J.; Liu, L.; James, M.; Yao, Y.; Xie, S.X.; Brunden, K.R. Inflammatory Eicosanoids Increase Amyloid Precursor Protein Expression via Activation of Multiple Neuronal Receptors. Sci. Rep. 2015, 5, 18286.
- Lai, J.; Hu, M.; Wang, H.; Hu, M.; Long, Y.; Miao, M.X.; Li, J.C.; Wang, X.B.; Kong, L.Y.; Hong, H. Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice. Neuropharmacology 2014, 79, 707–714.
- Kalra, J.; Kumar, P.; Majeed, A.B.; Prakash, A. Modulation of LOX and COX pathways via inhibition of amyloidogenesis contributes to mitoprotection against β-amyloid oligomer-induced toxicity in an animal model of Alzheimer's disease in rats. Pharmacol. Biochem. Behav. 2016, 146–147, 1–12.
- Jang, H.; Kim, S.; Lee, J.M.; Oh, Y.-S.; Park, S.M.; Kim, S.R. Montelukast treatment protects nigral dopaminergic neurons against microglial activation in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neuroreport 2017, 28, 242–249.
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.G. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid. Med. Cell Longev. 2012, 2012, 921941.
- Chung, Y.C.; Kim, S.R.; Park, J.Y.; Chung, E.S.; Park, K.W.; Won, S.Y.; Bok, E.; Jin, M.; Park, E.S.; Yoon, S.H.; et al. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 2011, 60, 963–974.
- Sherer, T.B.; Betarbet, R.; Kim, J.H.; Greenamyre, J.T. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci. Lett. 2003, 341, 87–90.
- Nagarajan, V.B.; Marathe, P.A. Effect of montelukast in experimental model of Parkinson’s disease. Neurosci. Lett. 2018, 682, 100–105.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
867
Revisions:
3 times
(View History)
Update Date:
21 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





