
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jun Guo | -- | 2203 | 2022-09-20 07:29:28 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 2205 | 2022-09-21 06:08:26 | | |
Video Upload Options
Food is a necessity in people’s lives. Equally importantly, alcoholic beverages are also highly demanded globally due to the indispensable role they play in cultural, social, and ritual events. However, the production of food and alcoholic beverages suffers from a variety of contaminants, such as toxins, pesticides, antibiotic residues, and heavy metals, which are seriously harmful to human beings. These urgent threats have raised the awareness of the need to improve product quality and safety via developing effective, rapid, and economical monitoring and detecting methods. Fortunately, due to their numerous advantages, including high sensitivity, short response time, low cost, and easy portability, electrochemistry sensors have made huge contributions to ensuring the quality of food and alcoholic beverages.
1. Introduction
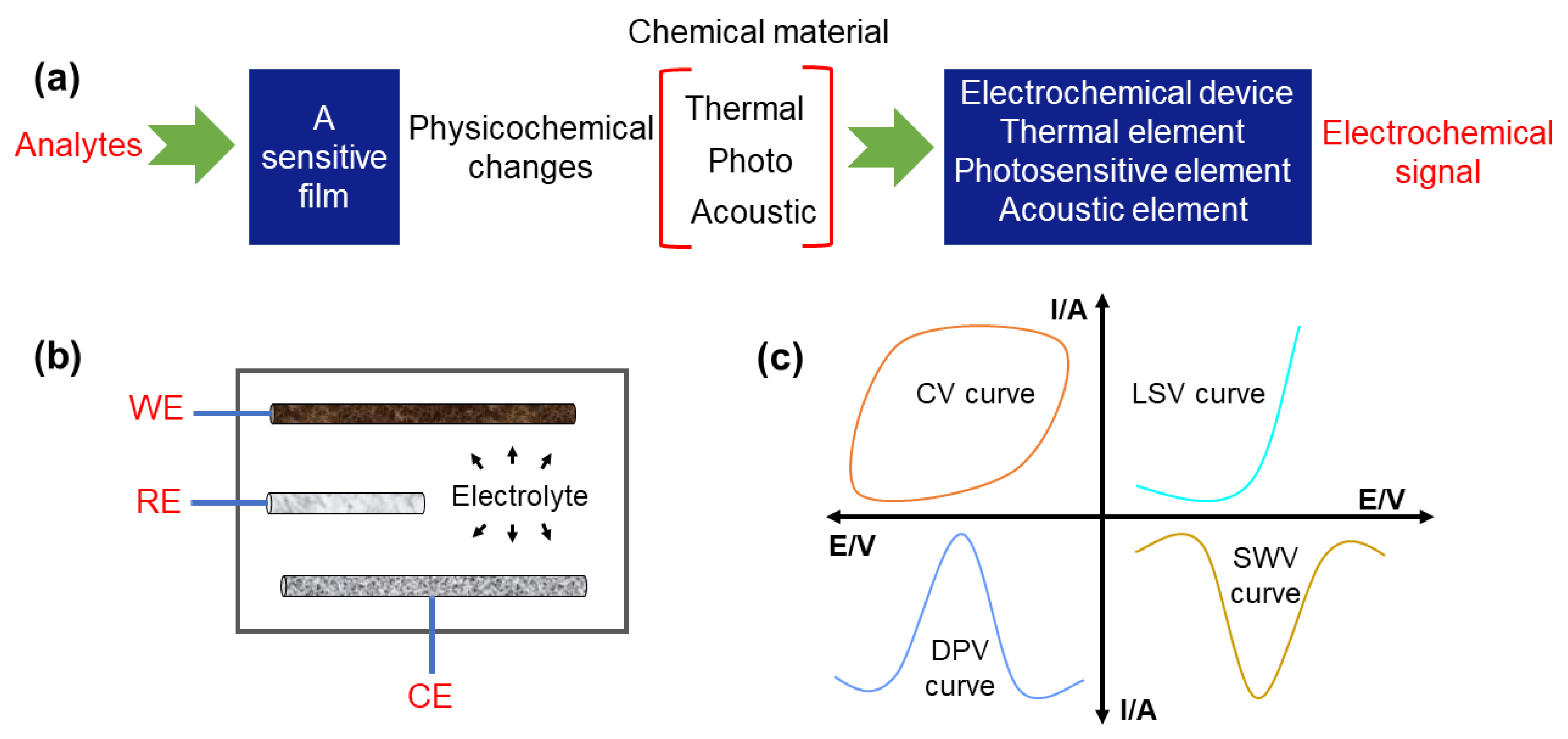
2. Electrochemical Techniques
3. Carbon Nanomaterial-Based Electrochemical Sensors
3.1. CDs
3.2. CNTs
3.3. Graphene
4. Conclusions
In summary, electrochemical sensors constructed by carbon-based composites can acquire a wide linear response range and low detection limit in the detection of food and alcoholic beverage contaminants. CV, LSV, SWV, and DPV are the most widely used electrochemical sensing methods because they can discern the possible intermediates with an extremely low detection limit, decode the nature of coupled chemical reactions, explore the weak adsorption phenomenon, study the mechanism of complex electrode reactions, and suppress the background current, respectively. Due to the good electrical conductivity, wide potential window, and easy chemical modifiability, carbon material (i.e., CDs, CNTs, and graphene)-based composite electrodes have been built with the introduction of metal nanoparticles, polymers, organic acids, doped heteroatoms, metal-oxide nanoparticles, and 2D sulfides to offer high sensitivity, unique selectivity, good durability, and repeatability. The presented electrochemical sensors have been widely used for detecting and monitoring chemical contaminants including dopamine, acetaminophen, H2O2, GA, methyl parathion, Cu2+ and Fe3+, Bisphenol A, sunset yellow FCF, Aflatoxin B1, and amino acids in foods and alcoholic beverages. Furthermore, electrochemical sensors have been practically applied for the detection of chemical contaminants in white wine, red wine, beer, edible oils, water, milk, and medicines.
References
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415.
- Yáñez, L.; Ortiz, D.; Calderón, J.; Batres, L.; Carrizales, L.; Mejía, J.; Martínez, L.; García-Nieto, E.; Díaz-Barriga, F. Overview of human health and chemical mixtures: Problems facing developing countries. Env. Health Persp. 2002, 110, 901–909.
- Shenashen, M.A.; Emran, M.Y.; El Sabagh, A.; Selim, M.M.; Elmarakbi, A.; El-Safty, S.A. Progress in sensory devices of pesticides, pathogens, coronavirus, and chemical additives and hazards in food assessment: Food safety concerns. Prog. Mater. Sci. 2021, 124, 100866.
- Xu, Y.; Li, X.; Zeng, X.; Cao, J.; Jiang, W. Application of blockchain technology in food safety control: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2020, 62, 2800–2819.
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2019, 19, 124–148.
- Wiwanitkit, V. Alcoholic Beverage Production in Indochina: Local Wisdom, Safety, Quality, and Legal Control. Prod. Mana. Bevera. 2019, 1, 381–407.
- Anagnostopoulos, C.; Miliadis, G. Development and validation of an easy multiresidue method for the determination of mul-ticlass pesticide residues using GC–MS/MS and LC–MS/MS in olive oil and olives. Talanta 2013, 112, 1–10.
- Xiao, Z.; Yang, Y.; Li, Y.; Fan, X.; Ding, S. Determination of neonicotinoid insecticides residues in eels using subcritical water extraction and ultra-performance liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2013, 777, 32–40.
- Vilchez, J.; El-Khattabi, R.; Fernández, J.; González-Casado, A.; Navalón, A. Determination of imidacloprid in water and soil samples by gas chromatography-mass spectrometry. J. Chromatogr. A 1996, 746, 289–294.
- Fang, Q.; Wang, L.; Cheng, Q.; Cai, J.; Wang, Y.; Yang, M.; Hua, X.; Liu, F. A bare-eye based one-step signal amplified semi-quantitative immunochromatographic assay for the detection of imidacloprid in Chinese cabbage samples. Anal. Chim. Acta 2015, 881, 82–89.
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2019, 20, 682–692.
- Qian, L.T.; Durairaj, S.; Prins, S. Aicheng ChenNanomaterial-based electrochemical sensors and biosensors for the detection of pharmaceutical compounds. Biosens. Bioelectro. 2021, 175, 112836.
- Adley, C.C. Past, Present and Future of Sensors in Food Production. Foods 2014, 3, 491–510.
- Jia, J.; Wang, B.; Wu, A.; Cheng, G.; Li, Z.; Dong, S. A Method to Construct a Third-Generation Horseradish Peroxidase Biosensor: Self-Assembling Gold Nanoparticles to Three-Dimensional Sol−Gel Network. Anal. Chem. 2002, 74, 2217–2223.
- Privett, B.; Shin, J.; Schoenfisch, M. Electrochemical sensors. Anal. Chem. 2010, 78, 3965.
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967.
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J. Carbon dots and graphene quantum dots in electrochemical biosensing. Nanomaterials 2019, 9, 634.
- Xiang, Q.; Huang, J.; Huang, H.; Mao, W.; Ye, Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv. 2018, 8, 1820–1825.
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.-H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556.
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134.
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554.
- Rajeshwar, K.; Ibanez, J.G.; Swain, G.M. Electrochemistry and the environment. J. Appl. Electrochem. 1994, 24, 1077–1091.
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458.
- Arrigan, D. Bioelectrochemistry. Fundamentals, Experimental Techniques and Applications. Chromatographia 2010, 72, 585.
- Moses, P.R.; Wier, L.; Murray, W.R. Chemically modified tin oxide electrode. Anal. Chem. 1975, 47, 1882–1886.
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613.
- Bonizzoni, M.; Anslyn, E.V. Combinatorial Methods for Chemical and Biological Sensors. J. Am. Chem. Soc. 2009, 131, 14597–14598.
- Ouyang, J. Application of intrinsically conducting polymers in flexible electronics. SmartMat 2021, 2, 263–285.
- Menon, S.; Jesny, S.; Kumar, K. A voltammetric sensor for acetaminophen based on electropolymerized molecular-ly imprinted poly (oaminophenol) modified gold electrode. Talanta 2018, 179, 668–675.
- Guiseppi-Elie, A.; Lingerfelt, L. Immobilisation of DNA on Chips I; Wittmann, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 161–186.
- Mirsky, V.M.; Riepl, M.; Wolfbeis, O.S. Capacitive monitoring of protein immobilization and antigen–antibody reactions on monomolecular alkylthiol films on gold electrodes. Biosens. Bioelectron. 1997, 12, 977–989.
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195.
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447.
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon Nanofiber-Based Functional Nanomaterials for Sensor Applications. Nanomaterials 2019, 9, 1045.
- Fan, Z.; Zhao, Q.; Li, T.; Yan, J.; Ren, Y.; Feng, J.; Wei, T. Easy synthesis of porous graphene nanosheets and their use in supercapacitors. Carbon 2012, 50, 1699–1703.
- Ito, Y.; Shen, Y.H.; Hojo, D.; Itagaki, Y.; Fujita, T.; Chen, L.H.; Aida, T.; Tang, Z.; Adschiri, T.; Chen, M.W. Correlation be-tween chemical dopants and topological defects in catalytically active nanoporous graphene. Adv. Mater. 2016, 28, 10644–10651.
- Zhang, X.F.; Liu, H.T.; Shi, Y.N.; Han, J.Y.; Yang, Z.J.; Zhang, Y.; Long, C.; Guo, J.; Zhu, Y.F.; Qiu, X.Y.; et al. Boosting CO2 conversion with terminal alkynes by molecular architecture of gra-phene oxide-supported Ag nanoparticles. Matter 2020, 3, 558–570.
- Cao, Y.; Rodan-Legrain, D.; Rubies-Bigorda, O.; Park, J.M.; Watanabe, K.; Taniguchi, T.; Jarillo-Herrero, P. Tunable corre-lated states and spin-polarized phases in twisted bilayer-bilayer graphene. Nature 2020, 583, 215–220.
- The different dimensions of nanotechnology. Nat. Nanotechnol. 2009, 4, 135.
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737.
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable Photoluminescence Across the Entire Visible Spectrum from Carbon Dots Excited by White Light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974.
- Teradal, N.; Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574.
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822.
- Atkin, P.; Daeneke, T.; Wang, Y.; Careya, B.; Berean, K.; Clark, R.; Ou, J.; Trinchi, A.; Cole, I.; Kalantar-zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13563–13571.
- Vercelli, B.; Donnini, R.; Ghezzi, F.; Sansonetti, A.; Giovanella, U.; La Ferla, B. Nitrogen-doped carbon quantum dots obtained hydrothermally from citric acid and urea: The role of the specific nitrogen centers in their electrochemical and optical responses. Electrochim. Acta 2021, 387, 138557.
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939.
- Stachurski, C.D.; Click, S.M.; Wolfe, K.D.; Dervishogullari, D.; Rosenthal, S.J.; Jennings, G.K.; Cliffel, D.E. Optical and electrochemical tuning of hydrothermally synthesized nitrogen-doped carbon dots. Nanoscale Adv. 2020, 2, 3375–3383.
- Rigodanza, F.; Đorđević, L.; Arcudi, F.; Prato, M. Customizing the electrochemical properties of carbon nanodots with quinones in bottom-up syntheses. Angew. Chem. Int. Ed. 2018, 130, 5156–5161.
- Ji, X.; Palui, G.; Avellini, T.; Na, H.; Yi, C.K.; Knappenberger, L.; Mattoussi, H. On the pH-dependent quenching of quantum dot photoluminescence by redox active dopamine. J. Am. Chem. Soc. 2012, 134, 6006–6017.
- Morgan, L.D.; Baker, H.; Yeoman, M.S.; Patel, B.A. Chromatographic assay to study the activity of multiple enzymes involved in the synthesis and metabolism of dopamine and serotonin. Analyst 2012, 137, 1409–1415.
- Jiang, Y.; Wang, B.; Meng, F. Microwave-assisted preparation of N-doped carbon dots as a biosensor for electrochemical do-pamine detection. J. Colloid Interface Sci. 2015, 452, 199–202.
- Huang, Q.; Zhang, H.; Hu, S.; Li, F.; Weng, W.; Chen, J.; Wang, Q.; He, Y.; Zhang, W.; Bao, X. A sensitive and reliable dopamine biosensor was developed based on the Au carbon dots–chitosan composite film. Biosens. Bioelectron. 2013, 52, 277–280.
- Dai, H.; Xu, G.; Gong, L.; Yang, C.; Lin, Y.; Tong, Y.; Chen, J.; Chen, G. Electrochemical detection of triclosan at a glassy carbon electrode modifies with carbon nanodots and chitosan. Electrochim. Acta 2012, 80, 362–367.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Henifehpour, Y.; Joo, S. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393.
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; ul Haq Ali Shah, A.; Mian, S.A.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. C J. Carbon Res. 2019, 5, 3.
- Liu, L.-P.; Yin, Z.-J.; Yang, Z.-S. A l-cysteine sensor based on Pt nanoparticles/poly(o-aminophenol) film on glassy carbon electrode. Bioelectrochemistry 2010, 79, 84–89.
- Soylemez, S.; Yoon, B.; Toppare, L.; Swager, T. Quaternized polymer-single-walled carbon nanotube scaffolds for a chemire sistive glucose sensor. ACS Sens. 2017, 2, 1123–1127.
- Liu, S.F.; Petty, A.R.; Sazama, G.T.; Swager, T.M. Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chem. Int. Ed. 2015, 54, 6554–6557.
- Münzer, A.; Melzer, K.; Heimgreiter, M.; Scarpa, G. Random CNT network and regioregular poly(3-hexylthiophen) FETs for pH sensing applications: A comparison. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4353–4358.
- Rodríguez-Pérez, L.; Herranz, M.; Martín, N. The chemistry of pristine graphene. Chem. Commun. 2013, 49, 3721–3735.
- Bolotin, K.; Sikes, K.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355.
- Ikhsan, N.I.; Pandikumar, A. Doped-Graphene Modified Electrochemical Sensors. In Graphene-Based Electrochemical Sensors for Biomolecules; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 67–87. ISBN 9780128153949.
- Yang, Z.J.; Yang, C.Y.; Han, J.Y.; Zhao, W.S.; Shao, S.X.; Li, S.Y.; Gao, H.W.; Xie, H.J.; Zhang, X.F. Boosting electrochemical CO2 reduction to formate using SnO2/graphene oxide with amide linkages. J. Mater. Chem. A 2021, 9, 19681–19686.
- Li, N.; Liu, G.; Zhen, C.; Li, F.; Zhang, L.; Cheng, H.-M. Battery Performance and Photocatalytic Activity of Mesoporous Anatase TiO2 Nanospheres/Graphene Composites by Template-Free Self-Assembly. Adv. Funct. Mater. 2011, 21, 1717–1722.




