Food is a necessity in people’s lives. Equally importantly, alcoholic beverages are also highly demanded globally due to the indispensable role they play in cultural, social, and ritual events. However, the production of food and alcoholic beverages suffers from a variety of contaminants, such as toxins, pesticides, antibiotic residues, and heavy metals, which are seriously harmful to human beings. These urgent threats have raised the awareness of the need to improve product quality and safety via developing effective, rapid, and economical monitoring and detecting methods. Fortunately, due to their numerous advantages, including high sensitivity, short response time, low cost, and easy portability, electrochemistry sensors have made huge contributions to ensuring the quality of food and alcoholic beverages.
- carbon-based materials
- electrochemical sensor
- food safety
- alcoholic beverage safety
1. Introduction
2. Electrochemical Techniques
Electrochemical sensors have become a widespread analysis technique with the merits of low cost, high accuracy, fast response, and good reliability, and have drawn substantial attention for the qualitative or/and quantitative analysis of foods and alcoholic beverages [21][22][28,29]. The design of high-quality electrochemical sensors needs a full understanding of their basic detection principles. Figure 1a shows that samples without pretreatment can be first added into the electrochemical cell. Then, upon applying electricity, the electrochemical sensor converts the physical and chemical response into recognizable electrochemical response signals. Through analyzing the correlation between the detected substances and corresponding electronic signals, the contaminants in foods and alcoholic beverages can be quickly identified. As shown in Figure 1b, a typical three-electrode electrochemical system includes a working electrode (WE), a reference electrode (RE) (e.g., Ag/AgCl), and a counter electrode (CE) (e.g., carbon rod, platinum). Recently, functional carbon-based nanomaterials such as CDs, CNTs, graphene oxide (GO), and porous carbon were selected as candidates for the working electrode (WE) due to their excellent conductivity and durability [23][24][30,31]. Of note, the selectivity, linearity, sensitivity, stability, detection limit, dynamic range, and response time of the resultant electrochemical sensors can be dramatically improved by further regulating the composition and the structure of adopted carbon nanomaterials [25][26][27][32,33,34]. Additionally, versatile electrochemical analysis methods are also required to be established for efficiently extracting the unique information from different analytes in food and alcoholic beverages. Specifically, the current-type (generating an amperometric current), potential-type (generating a potentiometric potential), conductivity-type (measurably altering the conductive properties of a medium between electrodes), and impedimetric-type (measuring impedance through an electrochemical impedance spectroscopy method) are the four types of golden protocols [28][29][30][31][35,36,37,38].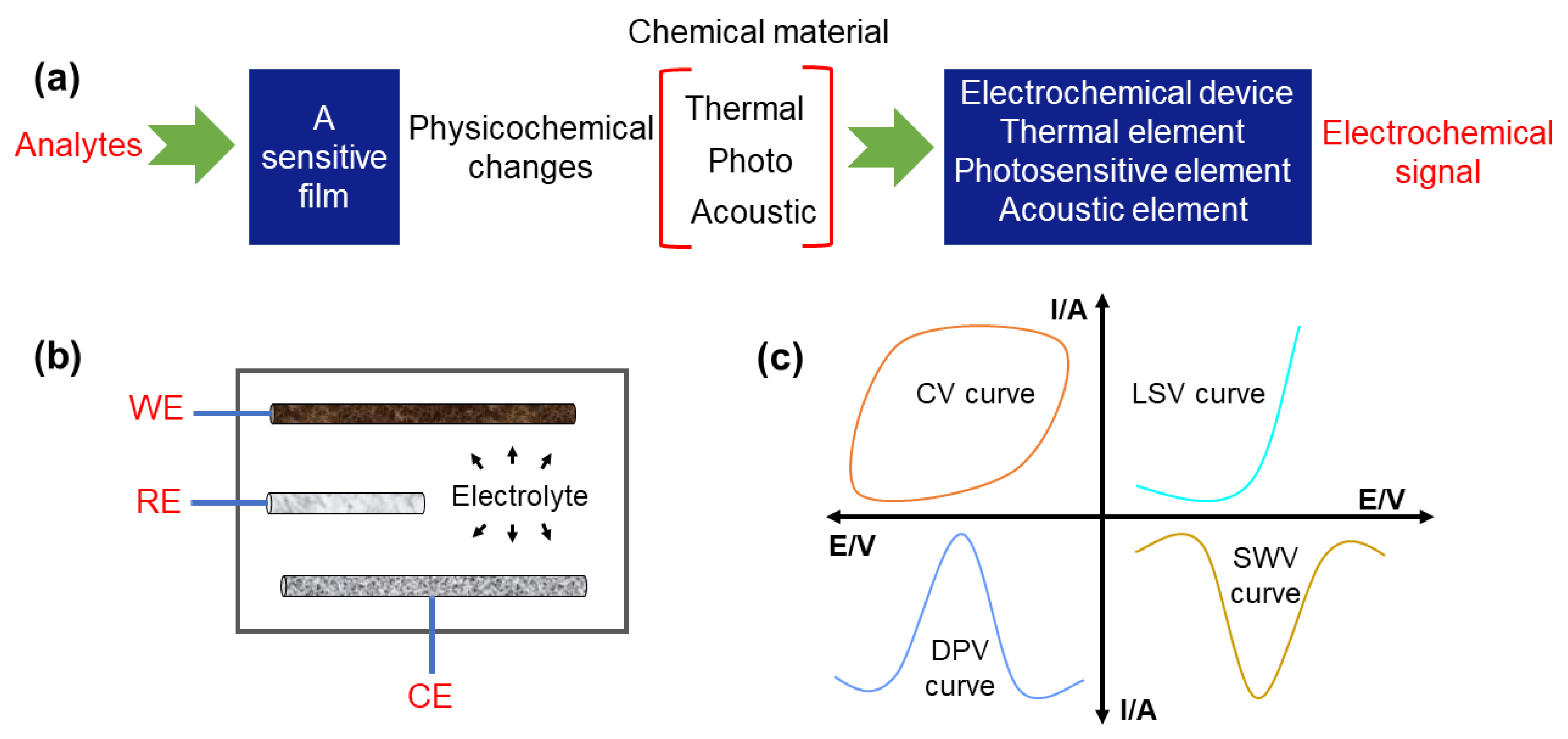
3. Carbon Nanomaterial-Based Electrochemical Sensors
Carbon is one of the most abundant elements in nature, and carbon-based materials have been continuously studied and utilized in different fields. Generally speaking, according to the dimension of the carbon-based materials, they can be divided into zero-dimensional materials (their dimensions in all directions are in the order of nanometers, e.g., CDs), one-dimensional materials (their dimensions in two directions are in the order of nanometers, e.g., CNTs and carbon nanofibers), and two-dimensional materials (their dimension in only one direction is in the order of nanometers, e.g., graphene and GO) [32][33][34][35][36][37][38][39][48,49,50,51,52,53,54,55]. As early as the 18th century, it was known that graphite was formed by sp2 and diamond was formed by sp3 hybridized carbon atoms. Furthermore, the fullerenes, such as C60 and C70, and CNTs, were discovered in 1985 and 1991, respectively [40][41][56,57]. These studies not only expanded the scope of the carbon material family, but also marked the start of a new era for the research of carbon nanomaterials. In particular, the discovery of graphene in 2004 triggered a new wave of research [42][58]. Due to their excellent electrical conductivity, wide potential window, high electrocatalytic activity, and chemical modifiability, carbon-based materials have been accepted as one of the best candidates for the construction of electrochemical sensors [43][59].In the following section, we summarize the exploration and application of typical carbon-based materials, including CDs, CNTs, and graphene, as electrochemical sensors in food and beverage safety due to their low cost, good conductivity, and facile fabrication process.
3.1. CDs
In 2004, Xu et al. discovered that CDs can present fluorescence during the process of electrophoresis [40][56]. CDs are defined as zero-dimensional nanoparticles having a size of less than 10 nm that are mainly composed of carbon elements. CDs have been widely used in electroluminescence, medical imaging, environmental monitoring, chemical analysis, photocatalysis, and energy conversion due to their colorful optical properties, good water solubility, low toxicity, environmental friendliness, abundant raw source, low cost, and good biocompatibility [44][45][60,61]. Regarding CDs’ preparation, a variety of methods, including arc discharge, laser ablation, electrochemical oxidation, acidic oxidation, microwave, ultrasonic, calcination, hydrothermal, template synthesis, and hydrosol condensation polymerization, have been reported [46][47][48][62,63,64]. Acting as a neuromodulator of ionotropic synapses, DA sets a threshold for the striatal activity involved in many diseases and drug addiction. In addition, DA is available as an intravenous medication acting on the sympathetic nervous system. Hence, the determination of DA in vivo/vitro becomes increasingly important in practice [49][50][65,66]. Zhu et al. [51][67] prepared a new type of N-doped CDs by a one-step microwave irradiation method. The N-doped CDs obtained by this method had a highly sensitive electrochemical response to DA, with a linear range of 0.05~8 μM and a detection limit of 1.2 nM. Moreover, in contrast to traditional detection methods requiring complex pretreatment and producing a large quantity of organic waste, the developed electrochemical sensing detection can be performed by directly adding DA to the detection system for qualitative and quantitative analysis. Huang et al. [52][68] designed a new type of DA sensor based on a Au@CDs-CS/glassy carbon electrode (GCE), which has high sensitivity and excellent performance stability and can suppress the background interference currents from ascorbic acid (AA) and uric acid (UA). Under the optimum experimental conditions, the linear range of 0.01~100.0 μM and the detection limit of 0.1 nM (S/N = 3) were obtained. Dai and co-workers [53][69] reported that CDs and chitosan were combined to construct a composite electrode. It was found that the signal was significantly higher than that of the bare electrode. Moreover, the composite electrode has higher sensitivity for the detection of triclosan, and also shows good results for the detection of actual samples such as toothpaste and gargle daily water, with the linear range of 10~1.0 mM and the detection limit of 0.92 nM.3.2. CNTs
CNTs, also known as bucky tubes, are one-dimensional tubular nanomaterials with special structures. CNTs are mainly coaxial round tubes with a single to dozens of layers composed of sp2 hybridized carbon atoms [54][55][75,76]. In theory, CNTs can be regarded as hollow tubes rolled by graphene sheets. According to the number of rolled layers of the graphene sheets, CNTs can be subdivided into single-walled carbon nanotubes (SWCNT) formed by a hexagonal grid structure, and multi-walled carbon nanotubes (MWCNT) assembled from several to dozens of concentric cylinders with regular layer spacing [56][57][77,78]. Because their unique structure is completely different from that of bulk carbon materials, CNTs can exhibit excellent properties, such as unique electrical, special magnetic, and strong light absorption properties. Because of their excellent electrical conductivity, large specific surface area, good biocompatibility, easily functionalization, and abundant active sites, CNTs are also an advantageous option in the design of electrochemical sensors [58][59][60][79,80,81].3.3. Graphene
Graphene is a 2D honeycomb lattice structure composed of a single layer of sp2 hybridized carbon atoms. Graphene-based materials have been widely examined by researchers in experiments and theoretical studies due to, among other factors, their high chemical stability, thermal conductivity, and electron mobility [61][62][95,96]. GAs far as we know, graphene has shown great application potential in the fields of electronics, optics, magnetism, biomedicine, catalysis, and sensors [63][64][97,98]. In particular, graphene is very suitable for the development of sensing materials in electrochemical sensors by taking advantage of its large specific surface area and high electron mobility. Due to the 2D planar structure of graphene, the fabricated detection system can fully contact the detected substances, which is conducive to accelerating mass transfer and therefore realizing rapid detection. In addition, the detection requirements of different pollutants can be rationally met via grafting organic functional groups or compounding different metal nanomaterials. Electrochemical sensors based on graphene composite materials have been widely studied in daily food safety and drug monitoring. In order to maximize the detection efficiency and accuracy, porous structures such as Co3O4, TiO2, and MnO2 were loaded onto the surface of graphene to exhibit greater analytic performance in food and alcohol safety [65][119]. Graphene is an excellent candidate in the design of electrochemical sensors due to its excellent electrical conductivity, large specific surface area, good biocompatibility, easy functionalization, and abundant active sites. Benefiting from the development of functionalization strategies, highly efficient electrochemical sensors can be constructed by doping heteroatoms on graphene. The heteroatom-doped graphene with an accurate structure helps to explore the sensing mechanism at the molecular level. In addition, metal nanoparticles or metal oxide particles are usually loaded on the surface of graphene to improve the sensitivity of electrochemical sensors. However, the added metal salts will greatly increase the cost and even deteriorate the stability of sensors. In future research, it is hoped that researchers will directly use graphene as a 3D self-supported electrode in electrochemical sensors based on graphene’s robust durability, which will provide technical support for further practical industrial applications.4. Conclusions
In summary, electrochemical sensors constructed by carbon-based composites can acquire a wide linear response range and low detection limit in the detection of food and alcoholic beverage contaminants. CV, LSV, SWV, and DPV are the most widely used electrochemical sensing methods because they can discern the possible intermediates with an extremely low detection limit, decode the nature of coupled chemical reactions, explore the weak adsorption phenomenon, study the mechanism of complex electrode reactions, and suppress the background current, respectively. Due to the good electrical conductivity, wide potential window, and easy chemical modifiability, carbon material (i.e., CDs, CNTs, and graphene)-based composite electrodes have been built with the introduction of metal nanoparticles, polymers, organic acids, doped heteroatoms, metal-oxide nanoparticles, and 2D sulfides to offer high sensitivity, unique selectivity, good durability, and repeatability. The presented electrochemical sensors have been widely used for detecting and monitoring chemical contaminants including dopamine, acetaminophen, H2O2, GA, methyl parathion, Cu2+ and Fe3+, Bisphenol A, sunset yellow FCF, Aflatoxin B1, and amino acids in foods and alcoholic beverages. Furthermore, electrochemical sensors have been practically applied for the detection of chemical contaminants in white wine, red wine, beer, edible oils, water, milk, and medicines.
