Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mingqiu Hu | -- | 1282 | 2022-09-19 09:23:32 | | | |
| 2 | Vivi Li | Meta information modification | 1282 | 2022-09-19 10:59:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mao, Y.; Yang, G.; Li, Y.; Liang, G.; Xu, W.; Hu, M. Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/27291 (accessed on 12 January 2026).
Mao Y, Yang G, Li Y, Liang G, Xu W, Hu M. Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/27291. Accessed January 12, 2026.
Mao, Yifeng, Gaowei Yang, Yingbang Li, Guowu Liang, Wangwang Xu, Mingqiu Hu. "Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer" Encyclopedia, https://encyclopedia.pub/entry/27291 (accessed January 12, 2026).
Mao, Y., Yang, G., Li, Y., Liang, G., Xu, W., & Hu, M. (2022, September 19). Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer. In Encyclopedia. https://encyclopedia.pub/entry/27291
Mao, Yifeng, et al. "Mechanisms Governing the Acquisition of Castration-Resistant Prostate Cancer." Encyclopedia. Web. 19 September, 2022.
Copy Citation
Despite aggressive treatment and androgen-deprivation therapy, most prostate cancer patients ultimately develop castration-resistant prostate cancer (CRPC), which is associated with high mortality rates. However, the mechanisms governing the development of CRPC are poorly understood, and androgen receptor (AR) signaling has been shown to be important in CRPC through AR gene mutations, gene overexpression, co-regulatory factors, AR shear variants, and androgen resynthesis. A growing number of non-AR pathways have also been shown to influence the CRPC progression, including the Wnt and Hh pathways. Moreover, non-coding RNAs have been identified as important regulators of the CRPC pathogenesis.
destructive resistance prostate cancer
androgen receptor
Wnt pathway
Hh pathway
ncRNAs
1. Introduction
Prostate cancer is the second leading cause of cancer-related death among men worldwide [1]. In China, relative to the rates from before 1990, PCA incidence has risen by 98.21% while corresponding mortality rates have fallen by 3.82%. Consistently, PCA incidence continues to rise [2]. At the time of initial diagnosis, most of the PCA patients exhibit progressive or metastatic disease in China, with androgen-deprivation therapy (ADT) being the treatment of choice for most of the patients with metastatic PCA (European Society of Urology; Guidelines for the Treatment of Prostate Cancer, 2020) [3]. While initially highly effective, however, the median time that patients respond well to ADT is just 18–24 months, after which the patient will progress to develop castration-resistant prostate cancer (CRPC), which is generally defined based upon serum testosterone levels (<50 ng/dL or 1.7 nmol/L) and biochemical (prostate-specific antigen (PSA) levels increasing three times in a row within one week, with at least two of these increases being by more than 50% of the lowest levels, PSA > 2 ng/mL) or radiological (such as two or more new bone lesions in bone scans or soft tissue lesions based upon solid tumor response assessment criteria) evidence of progression after castration treatment [4].
2. Androgen Receptor Pathways
The androgen receptor (AR) is a 110 kDa 919 amino acid nuclear receptor (NR) family member encoded on the X chromosome (q11-q12) (Figure 1A). The AR is composed of a central DNA-binding domain (DBD), and C-terminal ligand-binding domain (LBD), an N-terminal structural domain (NTD), and a hinge region linking the LBD and DBD [5][6][7][8]. The AR is encoded by eight exons (Figure 1), with exon 1 encoding the NTD, exons 2,3 encoding the DBD, and exons 4–8 encoding the LBD [9][10][11].
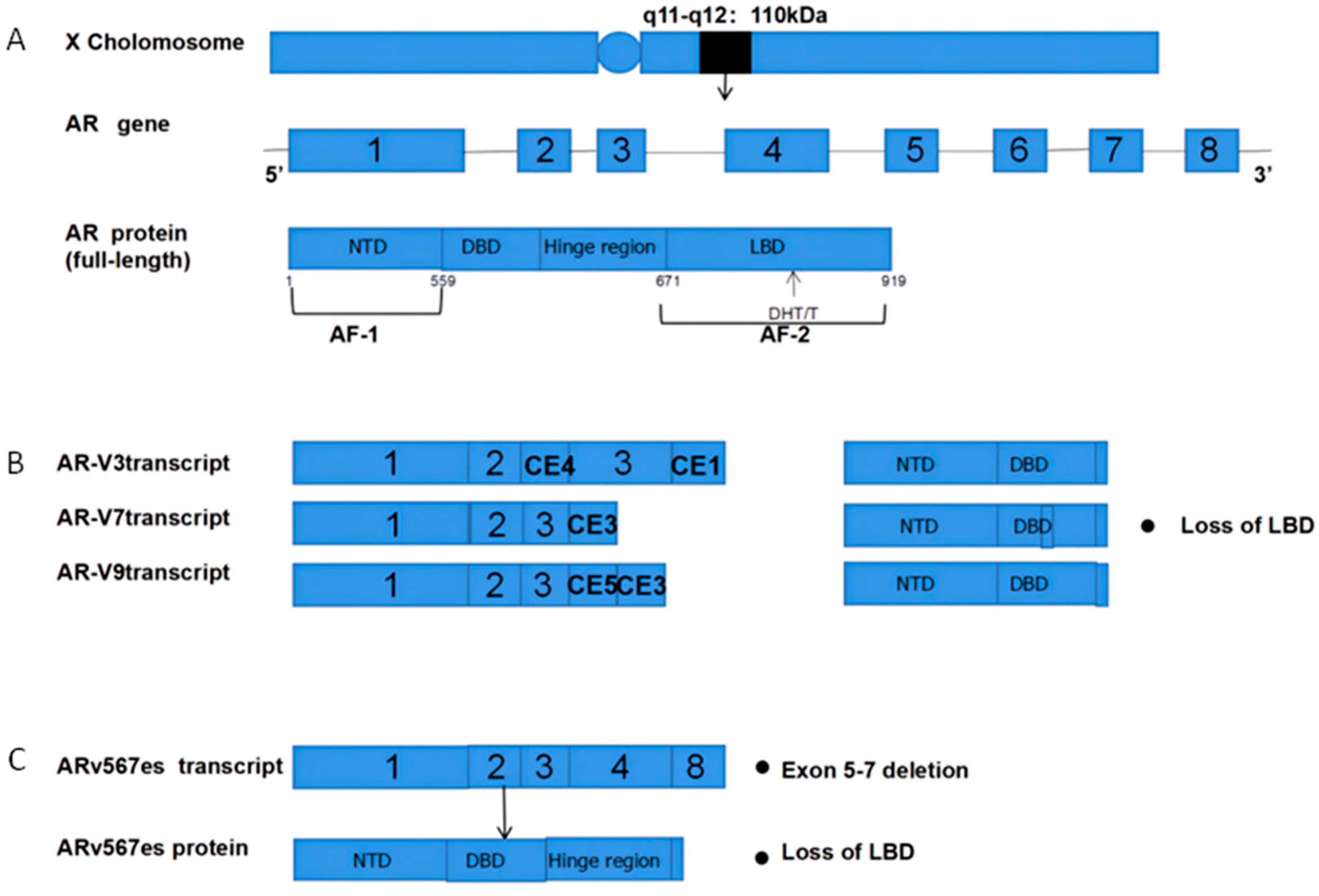
Figure 1. Structural overview of ARs and AR-Vs (AR-V3,7,9 and ARv567es). (A) Structural overview of the AR gene, located on the X chromosome q11-q12, encoding 919 amino acids and consisting of eight exons. The DHT and T ligands to the AR LBD; (B) The mechanisms underlying AR-V3, AR-V7, and AR-V9 production. Exons 4–8 are sheared to produce truncated AR-Vs that lack a LBD and Hinge region; (C) Mechanisms governing the production of ARv567es. Exons 5–7 are missing, resulting in truncated AR proteins and the lack of a LBD. AR = Androgen receptor; AR-Vs = AR variants; NTD = N-terminal transcriptional domain; DBD = DNA-binding domain; LBD = C-terminal ligand-binding domain; CE5 = cryptic exon 5; CE3 = cryptic exon 3; PAS = polyadenylation site; CE4 = cryptic exon.
Several different mechanisms link AR to the development of CRPC, including AR upregulation, de novo androgen synthesis, co-regulatory factor activity, AR gene mutations, and altered splicing (Figure 2).
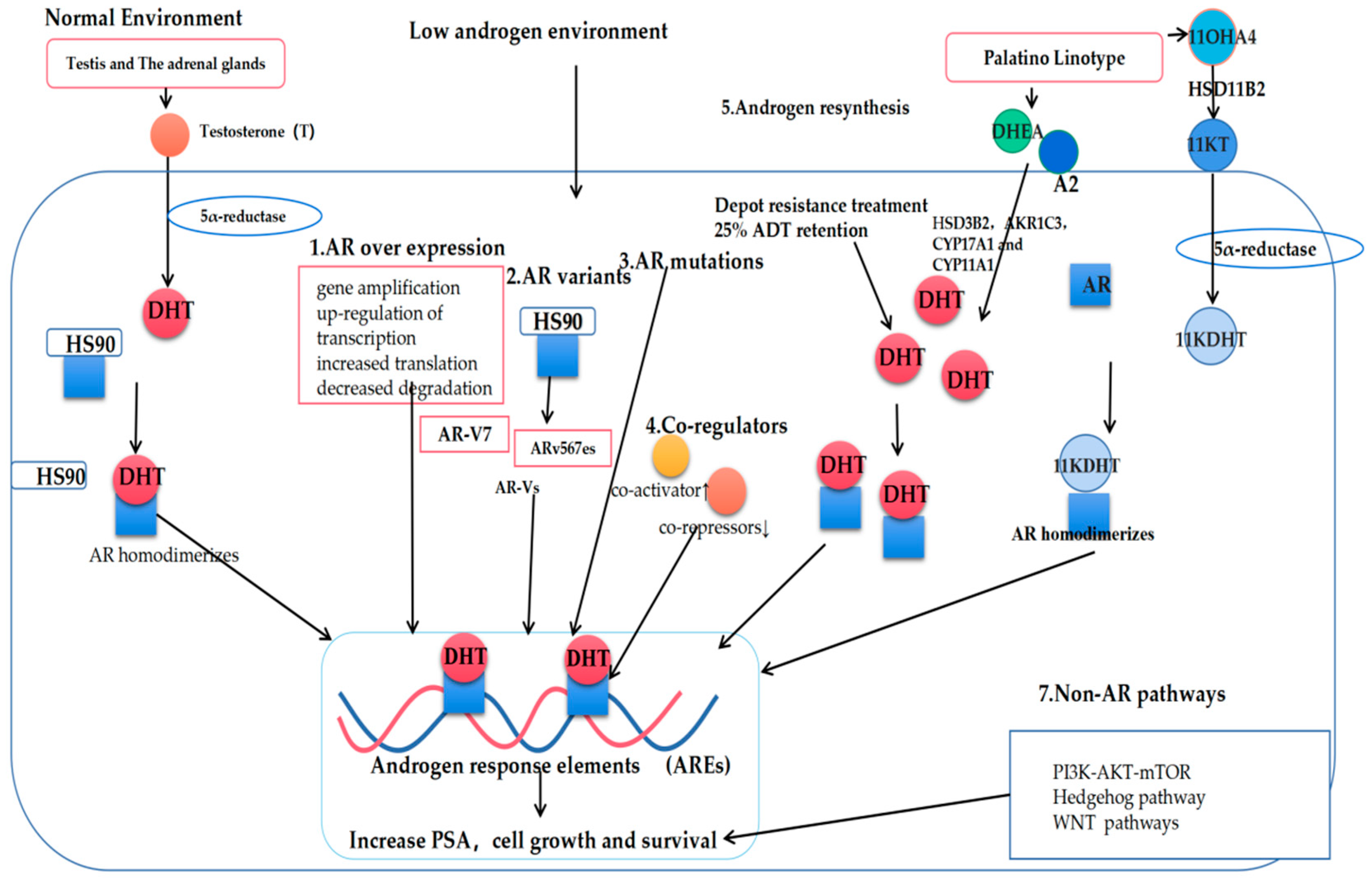
Figure 2. Mechanisms governing the progression of prostate cancer to castration-resistant prostate cancer (CRPC). T: Testosterone; DHT: Dihydrotestosterone; AR: Androgen receptor; AR-Vs: AR variants; DHEA: Dehydroepiandrosterone; A2: androstenedione; 11OXHA4: 11-oxygenated androgens; 11KT: 11-ketotestosterone; 11KDHT: 11-ketodihydrotestosterone.
3. Classic Mechanisms of AR Pathway Enhancement
3.1. AR Overexpression
The AR signaling reactivation most commonly occurs at the level of gene amplification or protein upregulation, with up to 80% of the CRPC patients harboring high AR gene copy numbers, among whom 20–30% of patients exhibit high gene amplification levels [12][13][14]. Such amplification, however, is uncommon among PCA patients that have not undergone hormone therapy. In one fluorescence in situ hybridization (FISH) study, the researchers found AR amplifications to be absent in the benign prostatic hyperplasia (BPH) samples, present in just 2% of the primary PCA tumors, but present in 23.4% of the CRPC tumors [11][15]. At the genetic level, two-fold increases in the AR mRNA expression levels have been reported in the CRPC tumors [16]. The AR overexpression can additionally occur through the enhanced stabilization of AR mRNA or proteins, or through increased transduction rates mediated by heat-shock proteins (HSPs), with HSP40 and HSP70 having been shown to bind the AR NTD and to then interact with the AR LBD, thereby contributing to its overexpression [17][18].
3.2. De Novo Androgen Synthesis
The persistent de novo production of androgens within the CRPC tumors can additionally contribute to enhanced AR activation and the progression of hormone-refractory prostate tumors [17]. Even following ADT, dihydrotestosterone (DHT) levels in the prostate tissue remain at ~25% of baseline; these levels are sufficient to drive altered gene expression and tumor progression through tumor epithelial cell signaling [19][20][21][22][23][24][25]. Nishiyama et al. further conducted a Gleason score analysis of the CRPC patients in which they found that the low levels of DHT in these patients were sufficient to promote AR receptor activation and tumor progression [19][26][27].
When multiple steroids (HSD3B2, AKR1C3, CYP17A1, and CYP11A1) are present [28][29][30][31], adrenal androgen precursors can be used to promote DHT synthesis within tumors through the 5α-diketone pathway, leading to dehydroepiandrosterone (DHEA) and androstenedione conversion into DHT without any requirement for testosterone [32][33][34][35]. Abiraterone is a specific inhibitor of CYP17A1, and it was also the first drug approved to treat CRPC [32][36].
In recent work, 11-oxygenated androgen (11OHA4) was additionally identified as a critical source of intratumoral androgen production [25][37][38], as it can serve as a precursor for the production of the peripherally active androgens, 11KT and 11KDHT. Functionally, 11KT-mediated AR activation has been shown to be similar to that mediated by testosterone, with 11KT and 11KDHT binding to the AR receptors with an affinity comparable to that for testosterone and DHT, respectively [39][40][41][42]. Pretorius et al. consistently found that 1KT and 11KDHT were capable of driving cellular growth through the upregulation of the AR regulatory genes, including KLK3, TMPRSS2, and FKBP5 in the LNCaP and VCaP PCA cell lines [40]. In vitro conversion experiments have also shown that 11KT and 11KDHT can remain present in the LNCaP and VCaP cells for longer than testosterone and DHT [40][43].
3.3. AR Co-Regulatory Proteins
Over 180 such AR co-regulatory proteins have been identified to date, with both co-repressors and co-activators functioning in a synergistic manner to regulate AR transcription [44]. These proteins can modulate transcription, RNA splicing, and epigenetic regulatory mechanisms, including methylation, acetylation, phosphorylation, and ubiquitination, thereby shaping PCA development and progression [45][46][47][48][49][50]. Notably, these co-regulatory proteins can promote the sustained transcriptional activity of AR even under low levels of androgen availability.
The interaction of specific co-activating proteins with AR, including JMJD2C, LSD1, 37CBP/P300, p160/SRC, and SUV39H2, can drive enhanced AR activation and the concomitant upregulation of the AR-dependent genes to augment tumor growth. Consistently, the inhibition of these activators has been linked to reduced AR expression and PCA tumor growth in ex vivo analyses [51][52][53][54]. Askew et al. reported that SUV39H2 can function as a co-activator for AR that enhances its androgen-dependent transcriptional regulatory activity through interactions with MAGE-A11 and AR under androgen-deficient conditions [49]. Moreover, the AEEB1 gene encoding the regulatory protein βArr1 was found to be upregulated in the CRPC tumor tissues, with the βArr1 deletion resulting in impaired PCA tumor growth, invasion, and metastatic progression in vitro and in murine model systems [55]. The AR co-repressors, in contrast, function to counteract the activity of the co-activator proteins [56][57]. Tan et al. reported that the CRPC tumor cells exhibited decreased CKβBP2/CRIF1 expression relative to the levels observed in androgen-dependent PCA tumor cells, with a corresponding increase in the expression of the co-activator STAT3, contributing to synergistic AR signaling enhancement [58]. As such, the downregulation of the co-repressors and the upregulation of the co-activators can spur the CRPC onset and progression [11].
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33.
- Deng, T.; Cai, L.; Chen, Z.; Guo, J. Analysis of the burden of prostate cancer in China in 1990 and 2017. New Med. 2020, 30, 252–259.
- Fang, Y.; Zhou, X. Updates and interpretation of the 2020 guidelines on prostate cancer of European Association of Urology. Chin. J. Endourol. 2020, 14, 401–404.
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; de Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642.
- Dehm, S.M.; Tindall, D.J. Androgen Receptor Structural and Functional Elements: Role and Regulation in Prostate Cancer. Mol. Endocrinol. 2007, 21, 2855–2863.
- Agoulnik, I.U.; Weigel, N.L. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J. Cell. Biochem. 2006, 99, 362–372.
- Zarif, J.C.; Miranti, C.K. The importance of non-nuclear AR signaling in prostate cancer progression and therapeutic resistance. Cell Signal. 2016, 28, 348–356.
- Kahn, B.; Collazo, J.; Kyprianou, N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int. J. Biol. Sci. 2014, 10, 588–595.
- Auwerx, J.; Baulieu, E.; Beato, M.; Becker-Andre, M.; Burbach, P.H.; Camerino, G.; Chambon, P.; Cooney, A.; Dejean, A.; Dreyer, C.; et al. A Unified Nomenclature System for the Nuclear Receptor Superfamily. Cell 1999, 97, 161–163.
- Claessens, F.; Denayer, S.; van Tilborgh, N.; Kerkhofs, S.; Helsen, C.; Haelens, A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl. Recept. Signal. 2008, 6, e008.
- Crowley, F.; Sterpi, M.; Buckley, C.; Margetich, L.; Handa, S.; Dovey, Z. A Review of the Pathophysiological Mechanisms Underlying Castration-resistant Prostate Cancer. Res. Rep. Urol. 2021, 13, 457–472.
- Waltering, K.K.; Urbanucci, A.; Visakorpi, T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 38–43.
- Yang, M.; Li, J.C.; Tao, C.; Wu, S.; Liu, B.; Shu, Q.; Li, B.; Zhu, R. PAQR6 Upregulation Is Associated with AR Signaling and Unfavorite Prognosis in Prostate Cancers. Biomolecules 2021, 11, 1383.
- Koivisto, P.A.; Helin, H.J. Androgen receptor gene amplification increases tissue PSA protein expression in hormone-refractory prostate carcinoma. J. Pathol. 1999, 189, 219–223.
- Bubendorf, L.; Kononen, J.; Kallioniemi, O.-P.; Koivisto, P.; Gasser, T.C.; Schraml, P.; Willi, N.; Sauter, G. High-throughput survey of gene amplifications underlying prostate cancer progression using a novel tissue microarray (“tissue chip”) technology. J. Urol. 1999, 51, 72193671.
- Linja, M.J.; Savinainen, K.J.; Saramäki, O.R.; Tammela, T.L.; Vessella, R.L.; Visakorpi, T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001, 61, 3550–3555.
- Cornford, P.A.; Dodson, A.R.; Parsons, K.F.; Desmond, A.D.; Woolfenden, A.; Fordham, M.; Neoptolemos, J.P.; Ke, Y.; Foster, C.S. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000, 60, 7099–7105.
- Ratajczak, W.; Lubkowski, M.; Lubkowska, A. Heat Shock Proteins in Benign Prostatic Hyperplasia and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 897.
- Nishiyama, T.; Hashimoto, Y.; Takahashi, K. The Influence of Androgen Deprivation Therapy on Dihydrotestosterone Levels in the Prostatic Tissue of Patients with Prostate Cancer. Clin. Cancer Res. 2004, 10, 7121–7126.
- Geller, J. Basis for hormonal management of advanced prostate cancer. Cancer 1993, 71, 1039–1045.
- Geller, J.; Liu, J.; Albert, J.; Fay, W.; Berry, C.C.; Weis, P. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin. Endocrinol. 1987, 26, 155–161.
- Petrylak, D.P. Current state of castration-resistant prostate cancer. Am. J. Manag. Care 2013, 19, S358–S365.
- Deslypere, J.P.; Young, M.; Wilson, J.D.; McPhaul, M.J. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol. Cell. Endocrinol. 1992, 88, 15–22.
- Sharifi, N.; Auchus, R.J. Steroid biosynthesis and prostate cancer. Steroids 2012, 77, 719–726.
- Barnard, M.; Mostaghel, E.A.; Auchus, R.J.; Storbeck, K.-H. The role of adrenal derived androgens in castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2020, 197, 105506.
- Nishiyama, T.; Ikarashi, T.; Hashimoto, Y.; Suzuki, K.; Takahashi, K. Association Between the Dihydrotestosterone Level in the Prostate and Prostate Cancer Aggressiveness Using the Gleason Score. J. Urol. 2006, 176, 1387–1391.
- Nishiyama, T.; Ikarashi, T.; Hashimoto, Y.; Wako, K.; Takahashi, K. The Change in the Dihydrotestosterone Level in the Prostate Before and After Androgen Deprivation Therapy in Connection with Prostate Cancer Aggressiveness Using the Gleason Score. J. Urol. 2007, 178, 1282–1289.
- Xiao, L.; Wang, Y.; Xu, K.; Hu, H.; Xu, Z.; Wu, D.; Wang, Z.; You, W.; Ng, C.-F.; Yu, S.; et al. Nuclear Receptor LRH-1 Functions to Promote Castration-Resistant Growth of Prostate Cancer via Its Promotion of Intratumoral Androgen Biosynthesis. Cancer Res. 2018, 78, 2205–2218.
- Kaipainen, A.; Zhang, A.; Gil da Costa, R.M.; Lucas, J.; Marck, B.; Matsumoto, A.M.; Morrissey, C.; True, L.D.; Mostaghel, E.A.; Nelson, P.S. Testosterone accumulation in prostate cancer cells is enhanced by facilitated diffusion. Prostate 2019, 79, 1530–1542.
- Hertzog, J.R.; Zhang, Z.; Bignan, G.; Connolly, P.J.; Heindl, J.E.; Janetopoulos, C.J.; Rupnow, B.A.; McDevitt, T.M. AKR1C3 mediates pan-AR antagonist resistance in castration-resistant prostate cancer. Prostate 2020, 80, 1223–1232.
- Barnard, M.; Quanson, J.L.; Mostaghel, E.; Pretorius, E.; Snoep, J.L.; Storbeck, K.-H. 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): Implications for castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 2018, 183, 192–201.
- Mostaghel, E.A.; Zhang, A.; Hernandez, S.; Marck, B.T.; Zhang, X.; Tamae, D.; Biehl, H.E.; Tretiakova, M.; Bartlett, J.; Burns, J.; et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 426–439.
- Xu, Z.; Ma, T.; Zhou, J.; Gao, W.; Li, Y.; Yu, S.; Wang, Y.; Chan, F.L. Nuclear receptor ERRα contributes to castration-resistant growth of prostate cancer via its regulation of intratumoral androgen biosynthesis. Theranostics 2020, 10, 4201–4216.
- Huhtaniemi, R.; Oksala, R.; Knuuttila, M.; Mehmood, A.; Aho, E.; Laajala, T.D.; Nicorici, D.; Aittokallio, T.; Laiho, A.; Elo, L.; et al. Adrenals Contribute to Growth of Castration-Resistant VCaP Prostate Cancer Xenografts. Am. J. Pathol. 2018, 188, 2890–2901.
- Montgomery, R.B.; Mostaghel, E.A.; Vessella, R.; Hess, D.L.; Kalhorn, T.F.; Higano, C.S.; True, L.D.; Nelson, P.S. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008, 68, 4447–4454.
- Maity, S.N.; Titus, M.A.; Gyftaki, R.; Wu, G.; Lu, J.-F.; Ramachandran, S.; Li-Ning-Tapia, E.M.; Logothetis, C.J.; Araujo, J.C.; Efstathiou, E. Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer. Sci. Rep. 2016, 6, 35354.
- Swart, A.C.; Storbeck, K.-H. 11β-hydroxyandrostenedione: Downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways. Mol. Cell. Endocrinol. 2015, 408, 114–123.
- Turcu, A.F.; Nanba, A.T.; Auchus, R.J. The Rise, Fall, and Resurrection of 11-Oxygenated Androgens in Human Physiology and Disease. Horm. Res. Paediatr. 2018, 89, 284–291.
- Rege, J.; Nakamura, Y.; Satoh, F.; Morimoto, R.; Kennedy, M.R.; Layman, L.C.; Honma, S.; Sasano, H.; Rainey, W.E. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 2013, 98, 1182–1188.
- Pretorius, E.; Africander, D.J.; Vlok, M.; Perkins, M.S.; Quanson, J.; Storbeck, K.-H. 11-Ketotestosterone and 11-Ketodihydrotestosterone in Castration Resistant Prostate Cancer: Potent Androgens Which Can No Longer Be Ignored. PLoS ONE 2016, 11, e0159867.
- Swart, A.C.; Schloms, L.; Storbeck, K.-H.; Bloem, L.M.; du Toit, T.; Quanson, J.L.; Rainey, W.E.; Swart, P. 11β-Hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5α-reductase yielding 11β-hydroxy-5α-androstanedione. J. Steroid Biochem. Mol. Biol. 2013, 138, 132–142.
- Barnard, L.; Schiffer, L.; Louw du-Toit, R.; Tamblyn, J.A.; Chen, S.; Africander, D.; Arlt, W.; Foster, P.A.; Storbeck, K.-H. 11-Oxygenated Estrogens are a Novel Class of Human Estrogens but Do not Contribute to the Circulating Estrogen Pool. Endocrinology 2021, 162, bqaa231.
- Snaterse, G.; Mies, R.; van Weerden, W.M.; French, P.J.; Jonker, J.W.; Houtsmuller, A.B.; van Royen, M.E.; Visser, J.A.; Hofland, J. Androgen receptor mutations modulate activation by 11-oxygenated androgens and glucocorticoids. Prostate Cancer Prostatic Dis. 2022.
- Van de Wijngaart, D.J.; Dubbink, H.J.; van Royen, M.E.; Trapman, J.; Jenster, G. Androgen receptor coregulators: Recruitment via the coactivator binding groove. Mol. Cell. Endocrinol. 2012, 352, 57–69.
- DePriest, A.D.; Fiandalo, M.V.; Schlanger, S.; Heemers, F.; Mohler, J.L.; Liu, S.; Heemers, H.V. Regulators of Androgen Action Resource: A one-stop shop for the comprehensive study of androgen receptor action. Database 2016, 2016, bav125.
- Heemers, H.V.; Tindall, D.J. Androgen Receptor (AR) Coregulators: A Diversity of Functions Converging on and Regulating the AR Transcriptional Complex. Endocr. Rev. 2007, 28, 778–808.
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452.
- Song, T.; Li, J. New Insights into the Binding Mechanism of Co-regulator BUD31 to AR AF2 Site: Structural Determination and Analysis of the Mutation Effect. Curr. Comput. Aided Drug Des. 2020, 16, 45–53.
- Askew, E.B.; Bai, S.; Parris, A.B.; Minges, J.T.; Wilson, E.M. Androgen receptor regulation by histone methyltransferase Suppressor of variegation 3–9 homolog 2 and Melanoma antigen-A11. Mol. Cell. Endocrinol. 2017, 443, 42–51.
- Chmelar, R.; Buchanan, G.; Need, E.F.; Tilley, W.; Greenberg, N.M. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int. J. Cancer 2006, 120, 719–733.
- Gelman, I.H. Androgen receptor activation in castration-recurrent prostate cancer: The role of Src-family and Ack1 tyrosine kinases. Int. J. Biol. Sci. 2014, 10, 620–626.
- Liu, Y.; Karaca, M.; Zhang, Z.; Gioeli, D.; Earp, H.S.; Whang, Y.E. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 2010, 29, 3208–3216.
- De Silva, D.; Zhang, Z.; Liu, Y.; Parker, J.S.; Xu, C.; Cai, L.; Wang, G.G.; Earp, H.S.; Whang, Y.E. Interaction between androgen receptor and coregulator SLIRP is regulated by Ack1 tyrosine kinase and androgen. Sci. Rep. 2019, 9, 18637.
- Gong, J.; Zhu, J.; Goodman, O.B.; Pestell, R.G.; Schlegel, P.N.; Nanus, D.M.; Shen, R. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene 2006, 25, 2011–2021.
- Purayil, H.T.; Zhang, Y.; Black, J.B.; Gharaibeh, R.; Daaka, Y. Nuclear βArrestin1 regulates androgen receptor function in castration resistant prostate cancer. Oncogene 2021, 40, 2610–2620.
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2003, 10, 33–39.
- Senapati, D.; Kumari, S.; Heemers, H.V. Androgen receptor co-regulation in prostate cancer. Asian J. Urol. 2020, 7, 219–232.
- Tan, J.-A.; Bai, S.; Grossman, G.; Titus, M.A.; Ford, O.H.; Pop, E.A.; Smith, G.J.; Mohler, J.L.; Wilson, E.M.; French, F.S. Mechanism of androgen receptor corepression by CKβBP2/CRIF1, a multifunctional transcription factor coregulator expressed in prostate cancer. Mol. Cell. Endocrinol. 2014, 382, 302–313.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
954
Revisions:
2 times
(View History)
Update Date:
19 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




