Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María de Jesús Loera-Arias | -- | 1670 | 2022-09-14 17:50:43 | | | |
| 2 | Dean Liu | -65 word(s) | 1605 | 2022-09-15 03:37:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Martínez-Puente, D.H.; Pérez-Trujillo, J.J.; Zavala-Flores, L.M.; García-García, A.; Villanueva-Olivo, A.; Rodríguez-Rocha, H.; Valdés, J.; Saucedo-Cárdenas, O.; Oca-Luna, R.M.D.; Loera-Arias, M.D.J. Plasmid DNA for Therapeutic Applications in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/27175 (accessed on 01 March 2026).
Martínez-Puente DH, Pérez-Trujillo JJ, Zavala-Flores LM, García-García A, Villanueva-Olivo A, Rodríguez-Rocha H, et al. Plasmid DNA for Therapeutic Applications in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/27175. Accessed March 01, 2026.
Martínez-Puente, David Hernán, José Juan Pérez-Trujillo, Laura Mireya Zavala-Flores, Aracely García-García, Arnulfo Villanueva-Olivo, Humberto Rodríguez-Rocha, Jesús Valdés, Odila Saucedo-Cárdenas, Roberto Montes De Oca-Luna, María De Jesús Loera-Arias. "Plasmid DNA for Therapeutic Applications in Cancer" Encyclopedia, https://encyclopedia.pub/entry/27175 (accessed March 01, 2026).
Martínez-Puente, D.H., Pérez-Trujillo, J.J., Zavala-Flores, L.M., García-García, A., Villanueva-Olivo, A., Rodríguez-Rocha, H., Valdés, J., Saucedo-Cárdenas, O., Oca-Luna, R.M.D., & Loera-Arias, M.D.J. (2022, September 14). Plasmid DNA for Therapeutic Applications in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/27175
Martínez-Puente, David Hernán, et al. "Plasmid DNA for Therapeutic Applications in Cancer." Encyclopedia. Web. 14 September, 2022.
Copy Citation
Plasmid DNA can be developed to treat different diseases, such as infections and cancer. In most cancers, the immune system is limited or suppressed, allowing cancer cells to grow. DNA vaccination has demonstrated its capacity to stimulate the immune system to fight against cancer cells.
cancer
DNA vaccination
gene therapy
tumor-specific antigens
1. Introduction
According to the World Health Organization (WHO), cancer is a leading cause of death worldwide, with nearly 10 million deaths in 2020 [1]. Different conventional methods and treatments are available for cancer, such as chemotherapy, radiotherapy, and surgical resection. However, if some cancer cells escape these treatments, they can lead to more aggressive tumors [2]; thus, these methods are insufficient. Recently, new therapies have been added to the arsenal to fight cancer with promising results, such as targeted therapy, stem cell therapy, nanoparticles, and active or passive immunotherapy [3][4].
An alternative that has shown promising results is the use of deoxyribonucleic acid (DNA) molecules for gene therapy [5]. Over time, the use of DNA for vaccination against cancer began with the characterization of the first tumor-specific antigen [6]. From there, different strategies have been developed to use this technology in cancer treatment.
The most used DNA-based vectors for cancer gene therapy and DNA vaccination are plasmids, small circular molecules originally obtained from bacteria. Furthermore plasmids, other non-plasmid DNA-based platforms for gene delivery have recently been reported. Some examples of this type of platform are minicircle DNA (the unnecessary plasmid backbone is removed by recombination) [7], MIDGE DNA (minimalistic expression constructs) [8], Doggybone DNA (linear, covalently closed, double-stranded molecules) [9], or linear DNA amplicons produced by polymerase chain reaction (PCR) [10].
Plasmid DNA for gene therapy and DNA vaccination offer several advantages over other nucleic acid platforms, such as being easy to design and manufacture, having a low production cost, and having a high stability for transportation and long-term storage [11].
2. Plasmid Design for Cancer Therapy
Plasmids used for cancer gene therapy or DNA vaccination must contain at least one expression cassette that directs the expression of a protein that will induce the therapeutic effect. After DNA uptake by the cell, it needs to reach the nucleus, where the gene will direct the therapeutic protein expression in the same way the cell produces its own proteins (Figure 1).
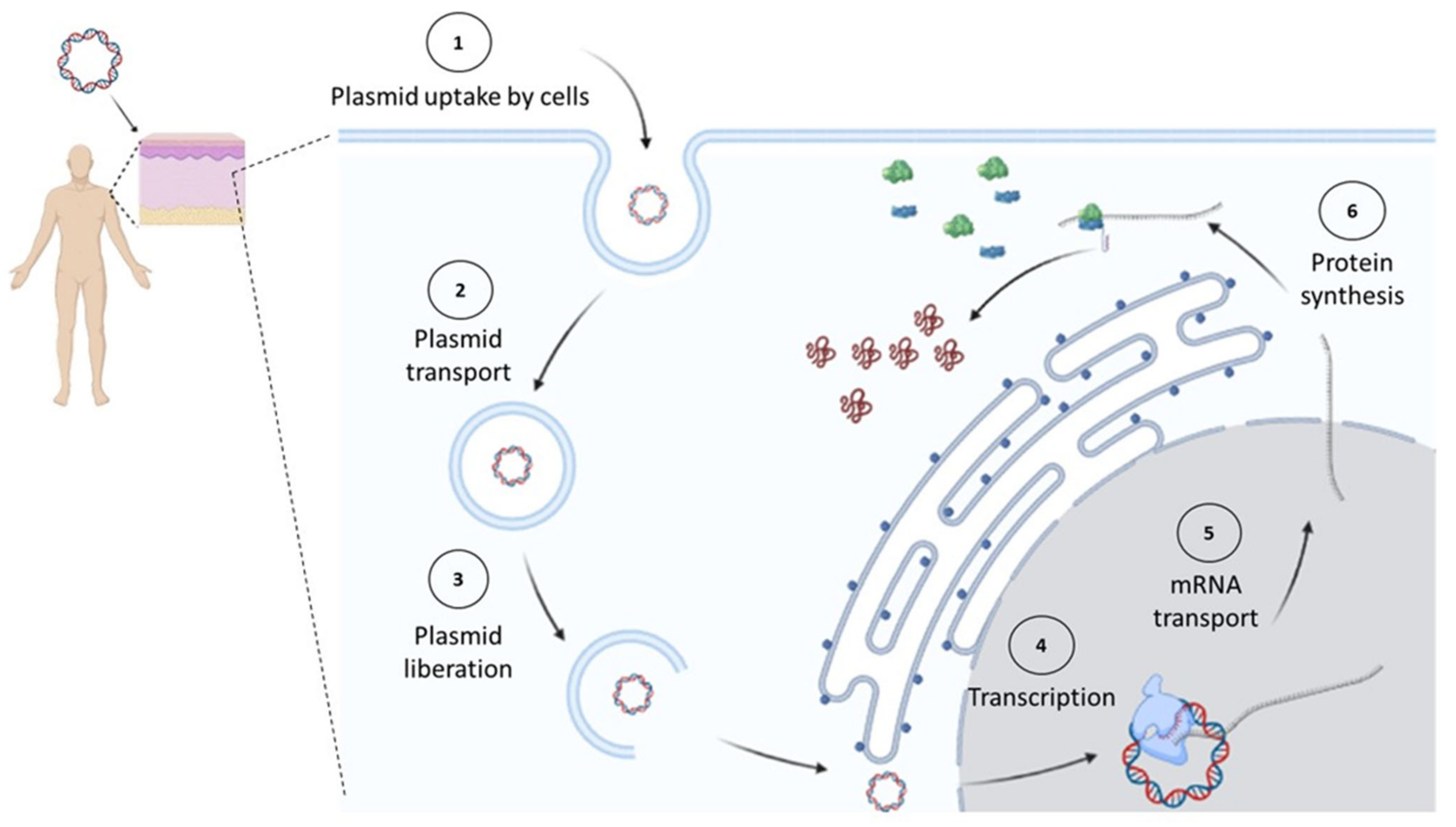
Figure 1. In vivo expression of cancer therapeutic proteins. Once a plasmid enters the cell, it must reach the nucleus, where it will start its transcription by the cell’s machinery. Later, the synthesized messenger RNA (mRNA) will be transported to the cytosol to be decoded by ribosomes into proteins. Figure created in Biorender.com.
For therapy to be effective, the correct design and optimization of the plasmid are crucial (Figure 2). For example, if more than one gene of interest needs to be expressed using a single plasmid, researchers can even express them independently (each gene with its own promoter), in a multicistronic system (two or more genes under the control of the same promoter), or as a fusion protein (a linker sequence between both sequences may be added). For the multicistronic system, an internal ribosome entry site (IRES) or a virus-derived T2A sequence must be placed between the different genes [12][13][14][15].
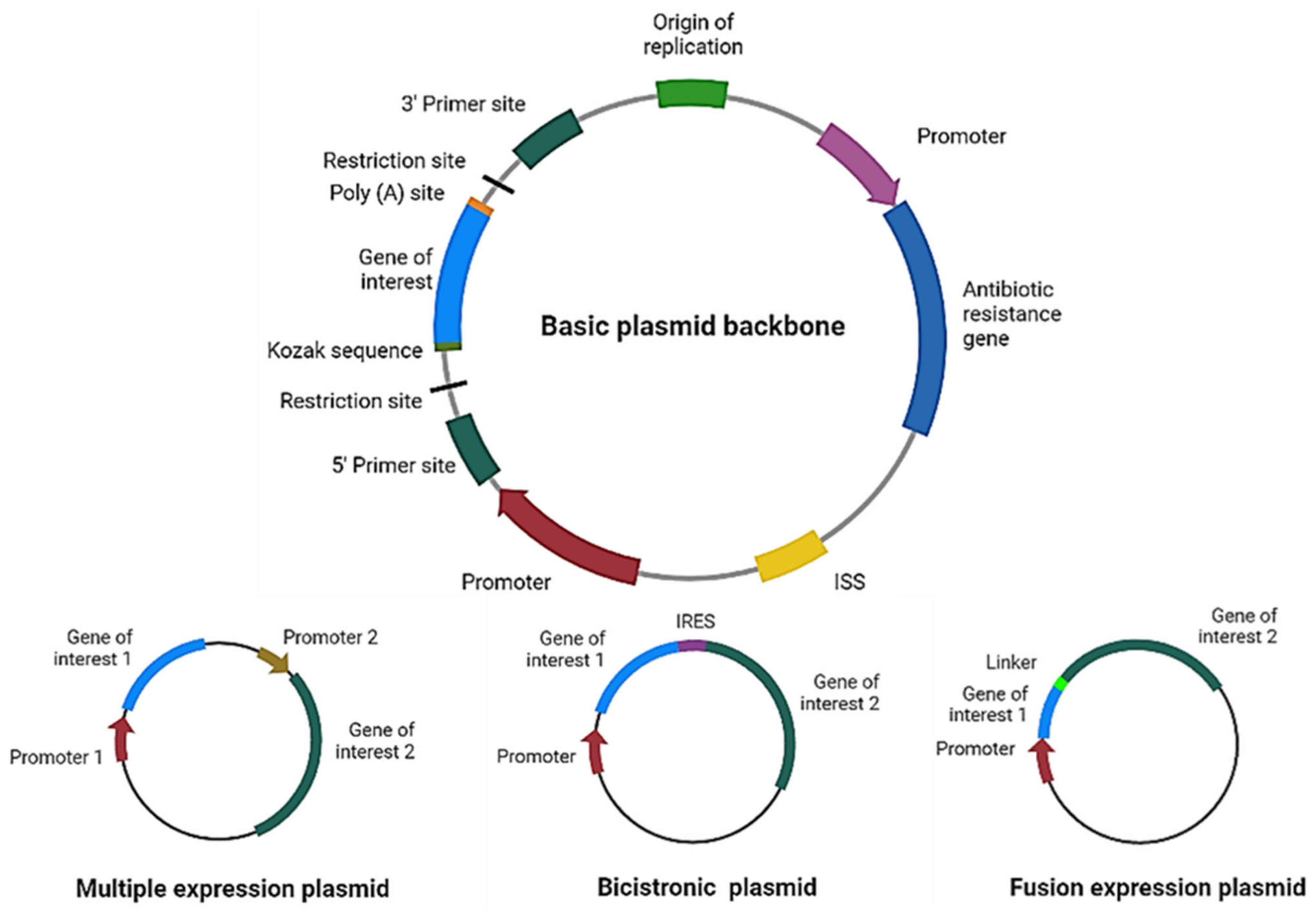
Figure 2. Plasmid design for expression of therapeutic proteins. Schematic representation of the main elements to include in a basic plasmid backbone for cancer therapy and plasmids for expression of multiple proteins. ISS: immunostimulatory sequences; IRES: internal ribosome entry site. Figure created in Biorender.com.
Codon optimization of the gene of interest is highly important, since the richness of guanines and cytosines increases messenger RNA (mRNA) levels [16][17]. Furthermore, the DNA molecule per se may stimulate the immune system through its unmethylated cytosine–phosphate–guanine (CpG) motifs and double-stranded structure [18]. CpG sequences in DNA vaccines have been shown to increase immunogenicity, acting as immunostimulatory sequences (ISS) through recognition by the Toll-like receptor 9 (TLR9) present in antigen-presenting cells (APCs) [19]; however, they may decrease gene expression [20].
Depending on the strategy intended for the plasmid, the gene of interest may encode a therapeutic protein to kill cancer cells directly, for example, a proapoptotic protein [21], an enzyme that activates a prodrug [22][23], a cytotoxic peptide [24], or a bacterial toxin [25][26]. Plasmids encoding specific small interfering RNA (siRNA) molecules may be used for cancer gene therapy [27][28] (Figure 3). In this case, a tumor-specific promoter can direct the transgene expression in cancer cells [29].
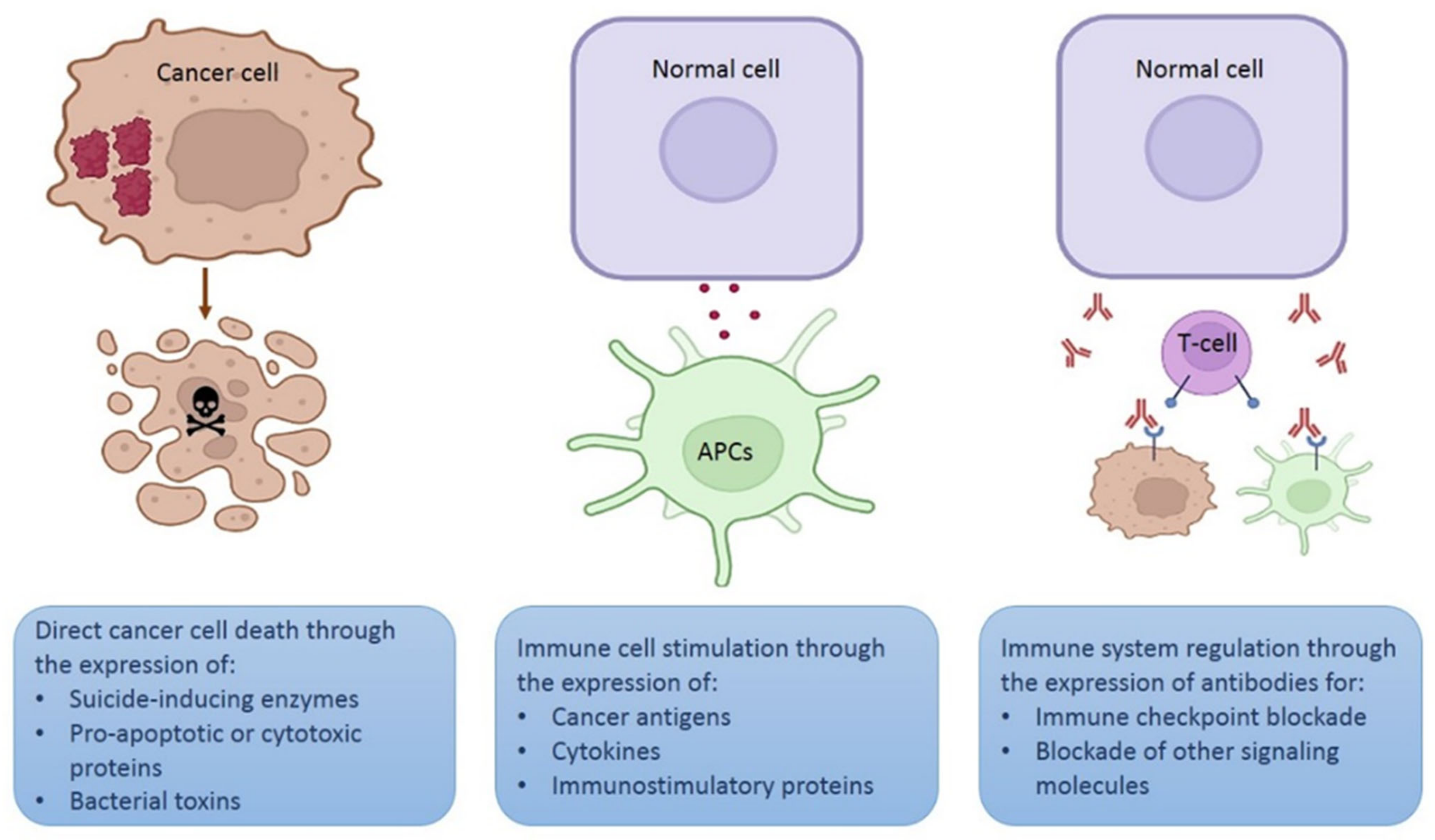
Figure 3. Different strategies using plasmids for therapeutic purposes. Schematic representation of three different strategies involving in vivo expression of therapeutic proteins. Figure created in Biorender.com.
Another option is that the gene of interest encodes an antigen or a cytokine to stimulate immune cells (mainly lymphocytes or APCs) [30][31] to destroy cancer cells. Since a high transgene expression is required for immune stimulation, strong promoters, such as the cytomegalovirus (CMV) promoter, are more suitable for this strategy. Furthermore, expression of the therapeutic protein may be performed by any cell that captures the plasmid. In addition, APC-targeted expression may be achieved using specific promoters [32].
A novel strategy involves using plasmids that encode monoclonal antibodies to block different signaling cascades, such as immune checkpoints or other molecules expressed on the cell surface or secreted in the tumor microenvironment [33].
3. Tumor-Specific Promoters for Gene Therapy
As reseasrchers can find cell- and tissue-specific promoters that regulate the expressions of different genes in normal cells, some promoters also allow for the expression of genes that favor the proliferation of cancer cells. Scientists have taken advantage of the nature of these to allow for the expression of therapeutic genes only in cancer cells. There are promoters functional in cancers of different origin (cancer-specific promoters) but not active in normal cells, and there are specific promoters that are active only in a limited type of cancer cells (tumor-specific promoters) [29]. Herein, reseasrchers mention some of the most widely used cancer-specific promoters, whose antitumoral effects have been analyzed in vivo using non-viral gene therapy.
The promoter of human telomerase reverse transcriptase (hTERT) has null activity in most somatic cells due to the absence of its methylation, which allows for its binding to the repressor. hTERT is a type of promoter active via methylation in different types of tumor tissues, which allows for the high expression of telomerase, an enzyme responsible for increasing telomeres in the proliferation of cancer cells [34][35]. The therapeutic use of this promoter in cancer therapy has been analyzed in different works. A plasmid that encodes the non-metastatic clone 23, isoform H1 (nm23-H1) gene, a metastasis suppressor gene under the control of the hTERT promoter, inhibited tumor growth and distant metastasis when evaluated in a lung cancer xenograft model after intratumoral injection with the vector [36]. In another work, a plasmid that encodes KK-64, a cytotoxic peptide, under the control of hTERT was administered in the form of DNA/liposome complexes to mice previously inoculated with mouse hepatocarcinoma cell line H22, with a reduction in tumor growth observed [37]. A novel version of the hTERT promoter using a VISA (VP16-Gal4-WPRE integrated systemic amplifier) system was reported. The hTERT-VISA system was used to drive the expression of E1A, an adenoviral transcription factor with anticancer properties. Significant antitumor activity was reported in an ovarian cancer xenograft murine model after intravenous delivery of the plasmid/liposomal nanoparticles [38].
The BIRC5 gene is active in different cancers but not in normal tissues. It drives the expression of survivin, an apoptosis inhibitor important for cancer development [39]. This promoter has been used in a minicircle system with potential clinical use for prostate cancer diagnosis and treatment [40]. In another work, the survivin promoter was used in combination with hTERT promoter to form a hybrid promoter to increase its strength of expression in transfected cancer cells. This hybrid promoter directed the expression of Herpes simplex virus-1 thymidine kinase (HSVtk) and the mouse granulocyte-macrophage colony-stimulating factor (GM-CSF). These transfected cancer cells were implanted in mice, and tumor growth inhibition was observed [41].
A candidate promoter for breast cancer is Erb-B2 receptor tyrosine kinase 2 (ERBB2) gene promoter; however, this is expressed in only 20–25% of tumors [42][43][44], and it is also active in prostate, pancreas, colon, and ovary cancer cells [45][46][47]. The ERBB2 gene promoter has been used in some works, as in a clinical trial for breast cancer where the patients received intratumoral injection of a plasmid that encodes the E. coli cytosine deaminase under the control of the ERBB2 gene promoter to activate the prodrug fluorocytosine [48]. In another work, a plasmid containing a minimum version of this promoter directing the expression of HSVtk to confer selective cytotoxicity to ganciclovir was constructed and proved in nude mice bearing human breast cancer cells. The administration of ganciclovir in human breast cancer cells transfected with this plasmid reduced tumor growth [49].
Regarding lung cancer, the thyroid transcription factor-1 (TTF-1) promoter is active in small cell lung carcinoma and adenocarcinoma [50][51]. Low constitutive expression is found in healthy lung cells, such as type II alveolar cells [52]. The use of this promoter to drive the expression of miR-7, a powerful tumor suppressor, was reported. It is showed that the targeting of transgene expression in the tumor cells via a remote hypodermic injection of a plasmid, downregulating tumor growth in a nude mice model of lung cancer [53].
Prostate-specific antigen (PSA) is regulated by the prostate cancer promoter, which has low constitutive expression in the prostate epithelium [54]; however, high levels are detected in patients with metastatic prostate cancer [55]. It is known that the activity of this promoter can be regulated by DNA-binding proteins [55], and this regulation may be androgen dependent or independent [54][56]. A recently published work reported using liposomes with a vector containing the PSA promoter driving the expression of perforin (a protein that makes pores on the plasma membrane) in cancer cells. After intravenous administration of this therapy, a reduced tumor volume was observed in a xenograft model of prostate cancer [57].
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 2 May 2022).
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469.
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 4370.
- Wang, X.; Zhang, H.; Chen, X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160.
- Friedmann, T. A Brief History of Gene Therapy. Nat. Genet. 1992, 2, 93–98.
- Van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A Gene Encoding an Antigen Recognized by Cytolytic T Lymphocytes on a Human Melanoma. Science 1991, 254, 1643–1647.
- Almeida, A.M.; Eusébio, D.; Queiroz, J.A.; Sousa, F.; Sousa, Â. Minicircle DNA Vaccine Purification and E7 Antigen Expression Assessment. In DNA Vaccines; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2021; Volume 2197, pp. 207–222.
- Kobelt, D.; Aumann, J.; Schmidt, M.; Wittig, B.; Fichtner, I.; Behrens, D.; Lemm, M.; Freundt, G.; Schlag, P.M.; Walther, W. Preclinical Study on Combined Chemo- and Nonviral Gene Therapy for Sensitization of Melanoma Using a Human TNF-Alpha Expressing MIDGE DNA Vector. Mol. Oncol. 2014, 8, 609–619.
- Short, C.; Savelyeva, N. Doggybones, DNA Vaccines and Skin-Penetrating Fluids: Whatever It Takes to Win the Fight against Cancer. Biochemist 2021, 43, 22–25.
- Conforti, A.; Salvatori, E.; Lione, L.; Compagnone, M.; Pinto, E.; Shorrock, C.; Hayward, J.A.; Sun, Y.; Liang, B.M.; Palombo, F.; et al. Linear DNA Amplicons as a Novel Cancer Vaccine Strategy. J. Exp. Clin. Cancer Res. 2022, 41, 195.
- Prazeres, D.M.F.; Monteiro, G.A. Plasmid Biopharmaceuticals. Microbiol. Spectr. 2014, 2, 2.6.02.
- Klein, J.S.; Jiang, S.; Galimidi, R.P.; Keeffe, J.R.; Bjorkman, P.J. Design and Characterization of Structured Protein Linkers with Differing Flexibilities. Protein Eng. Des. Sel. 2014, 27, 325–330.
- Chen, X.; Zaro, J.; Shen, W.-C. Fusion Protein Linkers: Property, Design and Functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369.
- Renaud-Gabardos, E.; Hantelys, F.; Morfoisse, F.; Chaufour, X.; Garmy-Susini, B.; Prats, A.-C. Internal Ribosome Entry Site-Based Vectors for Combined Gene Therapy. World J. Exp. Med. 2015, 5, 11–20.
- Kutzler, M.A.; Weiner, D.B. DNA Vaccines: Ready for Prime Time? Nat. Rev. Genet. 2008, 9, 776–788.
- Kudla, G.; Lipinski, L.; Caffin, F.; Helwak, A.; Zylicz, M. High Guanine and Cytosine Content Increases MRNA Levels in Mammalian Cells. PLoS Biol. 2006, 4, e180.
- Mauro, V.P.; Chappell, S.A. A Critical Analysis of Codon Optimization in Human Therapeutics. Trends Mol. Med. 2014, 20, 604–613.
- Coban, C.; Koyama, S.; Takeshita, F.; Akira, S.; Ishii, K.J. Molecular and Cellular Mechanisms of DNA Vaccines. Hum. Vaccin. 2008, 4, 453–456.
- Hanagata, N. Structure-Dependent Immunostimulatory Effect of CpG Oligodeoxynucleotides and Their Delivery System. Int. J. Nanomed. 2012, 7, 2181–2195.
- Sato, Y.; Roman, M.; Tighe, H.; Lee, D.; Corr, M.; Nguyen, M.D.; Silverman, G.J.; Lotz, M.; Carson, D.A.; Raz, E. Immunostimulatory DNA Sequences Necessary for Effective Intradermal Gene Immunization. Science 1996, 273, 352–354.
- Gómez-Navarro, J.; Arafat, W.; Xiang, J. Gene Therapy for Carcinoma of the Breast: Pro-Apoptotic Gene Therapy. Breast Cancer Res. 2000, 2, 32–44.
- Zarogoulidis, P.; Darwiche, K.; Sakkas, A.; Yarmus, L.; Huang, H.; Li, Q.; Freitag, L.; Zarogoulidis, K.; Malecki, M. Suicide Gene Therapy for Cancer—Current Strategies. J. Genet. Syndr. Gene Ther. 2013, 4, 16849.
- Ardiani, A.; Johnson, A.J.; Ruan, H.; Sanchez-Bonilla, M.; Serve, K.; Black, M.E. Enzymes to Die For: Exploiting Nucleotide Metabolizing Enzymes for Cancer Gene Therapy. Curr. Gene Ther. 2012, 12, 77–91.
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the Use of Therapeutic Peptides for Cancer Treatment. J. Biomed. Sci. 2017, 24, 21.
- Pahle, J.; Walther, W. Bacterial Toxins for Oncoleaking Suicidal Cancer Gene Therapy. In Current Strategies in Cancer Gene Therapy; Recent Results in Cancer Research; Springer: Cham, Switzerland, 2016; Volume 209, pp. 95–110.
- Ohana, P.; Matouk, I.; Amit, D.; Gilon, M.; Hochberg, A. Chapter 8—Toxin-Based Cancer Gene Therapy: Under the Control of Oncofetal H19 Regulatory Sequences. In Gene Therapy of Cancer, 3rd ed.; Lattime, E.C., Gerson, S.L., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 107–122. ISBN 978-0-12-394295-1.
- Gawronski, M.; Kopinski, P.; Jankowski, M.; Goede, A.; Szpechcinski, A.; Chorostowska, J. Inhibition of the Effect of Epidermal Growth Factor (EGF) on Lung Cancer Cells. The Use of Plasmids Encoding Specific SiRNA Molecules. Eur. Respir. J. 2015, 46, PA538.
- Wang, S.-L.; Yao, H.-H.; Qin, Z.-H. Strategies for Short Hairpin RNA Delivery in Cancer Gene Therapy. Expert Opin. Biol. Ther. 2009, 9, 1357–1368.
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcón-Hernández, E.; Ibáñez-Hernández, M. Strategies for Targeting Gene Therapy in Cancer Cells with Tumor-Specific Promoters. Front. Oncol. 2020, 10, 605380.
- Wang, X.; Li, X.; Zhong, F.; Li, N.; Han, D.; Pan, S. Strategies for Enhancing DNA Vaccine Potency by Targeting Antigen-Presenting Cells. Front. Agric. China 2009, 3, 478.
- Cáceres-Morgado, P.; Lladser, A. Tumor-Specific CD8+ T-Cell Responses Induced by DNA Vaccination. In DNA Vaccines; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2021; Volume 2197, pp. 225–239.
- Johnson, A.O.; Fowler, S.B.; Webster, C.I.; Brown, A.J.; James, D.C. Bioinformatic Design of Dendritic Cell-Specific Synthetic Promoters. ACS Synth. Biol. 2022, 11, 1613–1626.
- Patel, A.; Bah, M.A.; Weiner, D.B. In Vivo Delivery of Nucleic Acid-Encoded Monoclonal Antibodies. BioDrugs 2020, 34, 273–293.
- Cong, Y.S.; Wen, J.; Bacchetti, S. The Human Telomerase Catalytic Subunit HTERT: Organization of the Gene and Characterization of the Promoter. Hum. Mol. Genet. 1999, 8, 137–142.
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183.
- Fan, Y.; Yao, Y.; Li, L.; Wu, Z.; Xu, F.; Hou, M.; Wu, H.; Shen, Y.; Wan, H.; Zhou, Q. Nm23-H1 Gene Driven by HTERT Promoter Induces Inhibition of Invasive Phenotype and Metastasis of Lung Cancer Xenograft in Mice. Thorac. Cancer 2013, 4, 41–52.
- Lu, Y.; Ma, J.; Lin, J.; Tian, Y.; Ma, Y.; Wang, W.; Li, J.; Zhang, H.; Jiao, P. Cell Membrane Breakage and Triggering T Cell Infiltration Are Involved in Human Telomerase Reverse Transcriptase (HTERT) Promoter-Driven Novel Peptide KK-64 for Liver Cancer Gene Therapy. Bioengineered 2021, 12, 12708–12721.
- Xie, X.; Hsu, J.L.; Choi, M.-G.; Xia, W.; Yamaguchi, H.; Chen, C.-T.; Trinh, B.Q.; Lu, Z.; Ueno, N.T.; Wolf, J.K.; et al. A Novel HTERT Promoter-Driven E1A Therapeutic for Ovarian Cancer. Mol. Cancer Ther. 2009, 8, 2375–2382.
- Li, F.; Aljahdali, I.; Ling, X. Cancer Therapeutics Using Survivin BIRC5 as a Target: What Can We Do after over Two Decades of Study? J. Exp. Clin. Cancer Res. 2019, 38, 368.
- Wang, T.; Chen, Y.; Goodale, D.; Allan, A.L.; Ronald, J.A. A Survivin-Driven, Tumor-Activatable Minicircle System for Prostate Cancer Theranostics. Mol. Ther. Oncolytics 2021, 20, 209–219.
- Alekseenko, I.V.; Pleshkan, V.V.; Sass, A.V.; Filyukova, O.B.; Snezhkov, E.V.; Sverdlov, E.D. A Universal Tumor-Specific Promoter for Cancer Gene Therapy. Dokl. Biochem. Biophys. 2018, 480, 158–161.
- Cordo Russo, R.I.; Chervo, M.F.; Madera, S.; Charreau, E.H.; Elizalde, P.V. Nuclear ErbB-2: A Novel Therapeutic Target in ErbB-2-Positive Breast Cancer? Horm. Cancer 2019, 10, 64–70.
- Mungamuri, S.K.; Murk, W.; Grumolato, L.; Bernstein, E.; Aaronson, S.A. Chromatin Modifications Sequentially Enhance ErbB2 Expression in ErbB2-Positive Breast Cancers. Cell Rep. 2013, 5, 302–313.
- Nami, B.; Ghanaeian, A.; Black, C.; Wang, Z. Epigenetic Silencing of HER2 Expression during Epithelial-Mesenchymal Transition Leads to Trastuzumab Resistance in Breast Cancer. Life 2021, 11, 868.
- Miller, D.; Ingersoll, M.A.; Lin, M.-F. ErbB-2 Signaling in Advanced Prostate Cancer Progression and Potential Therapy. Endocr. Relat. Cancer 2019, 26, R195–R209.
- Vernimmen, D.; Gueders, M.; Pisvin, S.; Delvenne, P.; Winkler, R. Different Mechanisms Are Implicated in ERBB2 Gene Overexpression in Breast and in Other Cancers. Br. J. Cancer 2003, 89, 899–906.
- Hurst, H.C. Update on HER-2 as a Target for Cancer Therapy: The ERBB2 Promoter and Its Exploitation for Cancer Treatment. Breast Cancer Res. 2001, 3, 395–398.
- Pandha, H.S.; Martin, L.A.; Rigg, A.; Hurst, H.C.; Stamp, G.W.; Sikora, K.; Lemoine, N.R. Genetic Prodrug Activation Therapy for Breast Cancer: A Phase I Clinical Trial of ErbB-2-Directed Suicide Gene Expression. J. Clin. Oncol. 1999, 17, 2180–2189.
- Maeda, T.; O-Wang, J.; Matsubara, H.; Asano, T.; Ochiai, T.; Sakiyama, S.; Tagawa, M. A Minimum C-ErbB-2 Promoter-Mediated Expression of Herpes Simplex Virus Thymidine Kinase Gene Confers Selective Cytotoxicity of Human Breast Cancer Cells to Ganciclovir. Cancer Gene Ther. 2001, 8, 890–896.
- Hokari, S.; Tamura, Y.; Kaneda, A.; Katsura, A.; Morikawa, M.; Murai, F.; Ehata, S.; Tsutsumi, S.; Ishikawa, Y.; Aburatani, H.; et al. Comparative Analysis of TTF-1 Binding DNA Regions in Small-Cell Lung Cancer and Non-Small-Cell Lung Cancer. Mol. Oncol. 2020, 14, 277–293.
- Huang, T.-W.; Lin, K.-F.; Lee, C.-H.; Chang, H.; Lee, S.-C.; Shieh, Y.-S. The Role of Thyroid Transcription Factor-1 and Tumor Differentiation in Resected Lung Adenocarcinoma. Sci. Rep. 2017, 7, 14222.
- Kolla, V.; Gonzales, L.W.; Gonzales, J.; Wang, P.; Angampalli, S.; Feinstein, S.I.; Ballard, P.L. Thyroid Transcription Factor in Differentiating Type II Cells: Regulation, Isoforms, and Target Genes. Am. J. Respir. Cell Mol. Biol. 2007, 36, 213–225.
- Lei, L.; Chen, C.; Zhao, J.; Wang, H.; Guo, M.; Zhou, Y.; Luo, J.; Zhang, J.; Xu, L. Targeted Expression of MiR-7 Operated by TTF-1 Promoter Inhibited the Growth of Human Lung Cancer through the NDUFA4 Pathway. Mol. Ther. Nucleic Acids 2017, 6, 183–197.
- Yeung, F.; Li, X.; Ellett, J.; Trapman, J.; Kao, C.; Chung, L.W. Regions of Prostate-Specific Antigen (PSA) Promoter Confer Androgen-Independent Expression of PSA in Prostate Cancer Cells. J. Biol. Chem. 2000, 275, 40846–40855.
- Pang, S.; Taneja, S.; Dardashti, K.; Cohan, P.; Kaboo, R.; Sokoloff, M.; Tso, C.L.; Dekernion, J.B.; Belldegrun, A.S. Prostate Tissue Specificity of the Prostate-Specific Antigen Promoter Isolated from a Patient with Prostate Cancer. Hum. Gene Ther. 1995, 6, 1417–1426.
- Spitzweg, C.; Zhang, S.; Bergert, E.R.; Castro, M.R.; McIver, B.; Heufelder, A.E.; Tindall, D.J.; Young, C.Y.; Morris, J.C. Prostate-Specific Antigen (PSA) Promoter-Driven Androgen-Inducible Expression of Sodium Iodide Symporter in Prostate Cancer Cell Lines. Cancer Res. 1999, 59, 2136–2141.
- Mizutani, K.; Kawakami, K.; Fujita, Y.; Kato, T.; Takai, M.; Kato, D.; Iinuma, K.; Koie, T.; Ito, M. Gene Therapy of Prostate Cancer Using Liposomes Containing Perforin Expression Vector Driven by the Promoter of Prostate-Specific Antigen Gene. Sci. Rep. 2022, 12, 1442.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
15 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





